Impact of Sexual Dimorphism on Trauma Patterns and Clinical Outcomes of Patients with a High-Risk Score of the Osteoporosis Self-Assessment Tool for Asians: A Propensity Score-Matched Analysis
Abstract
:1. Background
2. Methods
2.1. Ethical Considerations
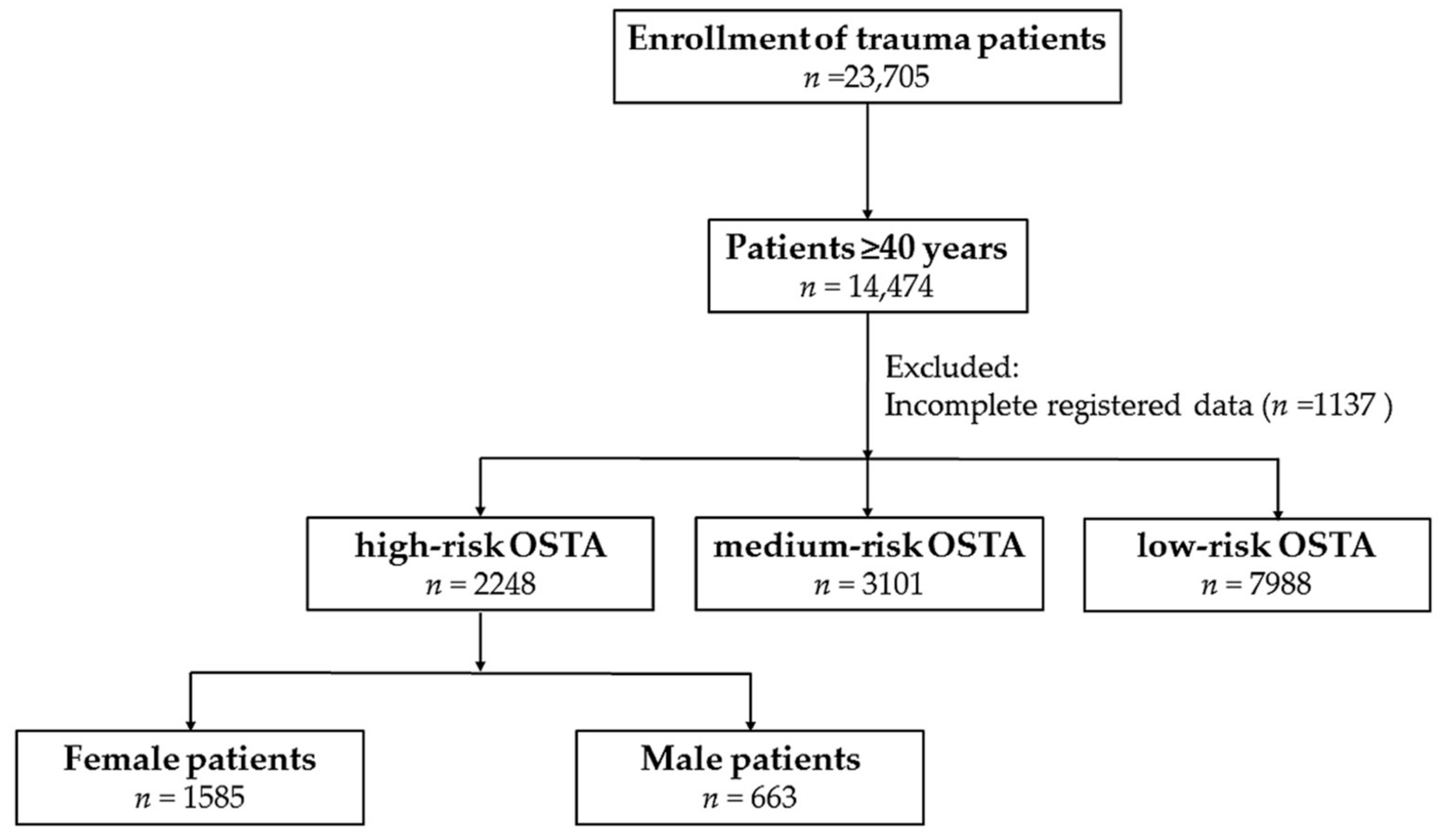
2.2. Study Population
2.3. Statistical Analysis
3. Results
3.1. Characteristics of Patients
3.2. Outcome of Propensity-Score Matched Patients
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AIS | abbreviated injury scale |
| BMI | body mass index |
| CAD | coronary artery disease |
| CHF | congestive heart failure |
| CIs | confidence intervals |
| CVA | cerebrovascular accident |
| DM | diabetes mellitus |
| ED | emergency department |
| GCS | Glasgow coma scale |
| HTN | hypertension |
| ICU | intensive-care unit |
| IQR | interquartile range |
| ISS | injury-severity score |
| LOS | length of stay |
| ORs | odds ratios |
| OSTA | Osteoporosis Self-Assessment Tool for Asians |
Appendix A
| Variables | Female, n = 573 | Male, n = 573 | Odds Ratio (95% CI) | p |
|---|---|---|---|---|
| Head trauma, n (%) | ||||
| Cranial fracture | 15 (2.6) | 23 (4.0) | 0.6 (0.33–1.25) | 0.187 |
| Epidural hematoma (EDH) | 19 (3.3) | 17 (3.0) | 1.1 (0.58–2.18) | 0.735 |
| Subdural hematoma (SDH) | 87 (15.2) | 101 (17.6) | 0.8 (0.61–1.15) | 0.264 |
| Subarachnoid hemorrhage (SAH) | 49 (8.6) | 56 (9.8) | 0.9 (0.58–1.29) | 0.474 |
| Intracerebral hematoma (ICH) | 20 (3.5) | 24 (4.2) | 0.8 (0.45–1.52) | 0.539 |
| Cerebral contusion | 35 (6.1) | 54 (9.4) | 0.6 (0.40–0.97) | 0.036 |
| Cervical vertebral fracture | 3 (0.5) | 8 (1.4) | 0.4 (0.10–1.41) | 0.130 |
| Maxillofacial trauma, n (%) | ||||
| Orbital fracture | 1 (0.2) | 2 (0.3) | 0.5 (0.05–5.52) | 1.000 |
| Nasal fracture | 2 (0.3) | 1 (0.2) | 2.0 (0.18–22.16) | 1.000 |
| Maxillary fracture | 12 (2.1) | 15 (2.6) | 0.8 (0.37–1.72) | 0.559 |
| Mandibular fracture | 2 (0.3) | 0 (0.0) | - | 0.500 |
| Thoracic trauma, n (%) | ||||
| Rib fracture | 33 (5.8) | 45 (7.9) | 0.7 (0.45–1.14) | 0.159 |
| Hemothorax | 5 (0.9) | 9 (1.6) | 0.6 (0.18–1.66) | 0.282 |
| Pneumothorax | 3 (0.5) | 11 (1.9) | 0.3 (0.08–0.97) | 0.031 |
| Hemopneumothorax | 2 (0.3) | 6 (1.0) | 0.3 (0.07–1.65) | 0.287 |
| Thoracic vertebral fracture | 9 (1.6) | 7 (1.2) | 1.3 (0.48–3.49) | 0.615 |
| Abdominal trauma, n (%) | ||||
| Hepatic injury | 2 (0.3) | 3 (0.5) | 0.7 (0.11–4.00) | 1.000 |
| Splenic injury | 4 (0.7) | 0 (0.0) | - | 0.124 |
| Retroperitoneal injury | 1 (0.2) | 3 (0.5) | 0.3 (0.03–3.20) | 0.624 |
| Renal injury | 1 (0.2) | 2 (0.3) | 0.5 (0.05–5.52) | 1.000 |
| Lumbar vertebral fracture | 14 (2.4) | 8 (1.4) | 1.8 (0.74–4.25) | 0.196 |
| Extremity trauma, n (%) | ||||
| Scapular fracture | 2 (0.3) | 6 (1.0) | 0.3 (0.07–1.65) | 0.287 |
| Clavicle fracture | 16 (2.8) | 18 (3.1) | 0.9 (0.45–1.76) | 0.728 |
| Humeral fracture | 23 (4.0) | 15 (2.6) | 1.6 (0.80–3.01) | 0.187 |
| Radial fracture | 52 (9.1) | 19 (3.3) | 2.9 (1.70–4.99) | <0.001 |
| Ulnar fracture | 31 (5.4) | 8 (1.4) | 4.0 (1.84–8.87) | <0.001 |
| Metacarpal fracture | 7 (1.2) | 7 (1.2) | 1.0 (0.35–2.87) | 1.000 |
| Pelvic fracture | 9 (1.6) | 6 (1.0) | 1.5 (0.53–4.26) | 0.436 |
| Femoral fracture | 291 (50.8) | 251 (43.8) | 1.3 (1.05–1.67) | 0.018 |
| Patella fracture | 6 (1.0) | 9 (1.6) | 0.7 (0.23–1.88) | 0.436 |
| Tibia fracture | 18 (3.1) | 14 (2.4) | 1.3 (0.64–2.63) | 0.473 |
| Fibular fracture | 12 (2.1) | 9 (1.6) | 1.3 (0.56–3.21) | 0.509 |
| Calcaneal fracture | 6 (1.0) | 5 (0.9) | 1.2 (0.37–3.96) | 0.762 |
| Male | Motorcycle, n = 132 | Bicycle, n = 53 | Fall, n = 434 | Motorcycle vs. Fall (p) | Bicycle vs. Fall (p) |
|---|---|---|---|---|---|
| Age [range] (years) | 80.6 ± 5.7 [68–94] | 82.4 ± 5.6 [68–98] | 82.7 ± 6.5 [59–99] | 0.001 | 0.770 |
References
- Johnell, O.; Kanis, J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006, 17, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Atik, O.S.; Gunal, I.; Korkusuz, F. Burden of osteoporosis. Clin. Orthop. Relat. Res. 2006, 443, 19–24. [Google Scholar] [CrossRef] [PubMed]
- LK, K. A simple tool to identify Asian women at increased risk of osteoporosis. Osteoporos. Int. 2001, 12, 699–705. [Google Scholar]
- Lin, J.; Yang, Y.; Fei, Q.; Zhang, X.; Ma, Z.; Wang, Q.; Li, J.; Li, D.; Meng, Q.; Wang, B. Validation of three tools for identifying painful new osteoporotic vertebral fractures in older Chinese men: Bone mineral density, Osteoporosis Self-Assessment Tool for Asians, and fracture risk assessment tool. Clin. Interven. Aging 2016, 11, 461–469. [Google Scholar]
- Chan, S.P.; Teo, C.C.; Ng, S.A.; Goh, N.; Tan, C.; Deurenberg-Yap, M. Validation of various osteoporosis risk indices in elderly Chinese females in Singapore. Osteoporos. Int. 2006, 17, 1182–1188. [Google Scholar] [CrossRef] [PubMed]
- Li-Yu, J.T.; Llamado, L.J.; Torralba, T.P. Validation of OSTA among Filipinos. Osteoporos. Int. 2005, 16, 1789–1793. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.M.; Nam, B.H.; Rhee, Y.; Moon, S.H.; Kim, D.Y.; Kang, D.R.; Kim, H.C. Development and validation of osteoporosis risk-assessment model for Korean postmenopausal women. J. Bone Miner. Metab. 2013, 31, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.M.; Song, B.M.; Nam, B.H.; Rhee, Y.; Moon, S.H.; Kim, D.Y.; Kang, D.R.; Kim, H.C. Development and Validation of Osteoporosis Risk-Assessment Model for Korean Men. Yonsei Med. J. 2016, 57, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Su, F.M.; Liu, D.H.; Chen, J.F.; Yu, S.F.; Chiu, W.C.; Hsu, C.Y.; Ko, C.H.; Tsai, C.C.; Cheng, T.T. Development and Validation of an Osteoporosis Self-Assessment Tool for Taiwan (OSTAi) Postmenopausal Women—A Sub-Study of the Taiwan OsteoPorosis Survey (TOPS). PLoS ONE 2015, 10, e0130716. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, B.; Fei, Q.; Meng, Q.; Li, D.; Tang, H.; Li, J.; Su, N. Validation of an osteoporosis self-assessment tool to identify primary osteoporosis and new osteoporotic vertebral fractures in postmenopausal Chinese women in Beijing. BMC Musculoskelet. Disord. 2013, 14, 271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Liu, H.L.; Wang, X.; Chen, W.; Chen, D.; Zhang, Z.Z.; Wang, H.M. Clinical value of self-assessment risk of osteoporosis in Chinese. Open Med. (Wars) 2016, 11, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.H.; Hsu, S.Y.; Hsieh, H.Y.; Chen, Y.C. Differences between the sexes in motorcycle-related injuries and fatalities at a Taiwanese level I trauma center. Biomed. J. 2017, 40, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, P.M. Gender differences in osteoporosis and fractures. Clin. Orthop. Relat. Res. 2011, 469, 1900–1905. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.D.; Ward, P.; Bartle, C.; Truman, W. Killer crashes: Fatal road traffic accidents in the UK. Accid. Anal. Prevent. 2010, 42, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Santamarina-Rubio, E.; Perez, K.; Ricart, I.; Rodriguez-Sanz, M.; Rodriguez-Martos, A.; Brugal, M.T.; Borrell, C.; Ariza, C.; Diez, E.; Beneyto, V.M.; et al. Substance use among road traffic casualties admitted to emergency departments. J. Int. Soc. Child Adolesc. Inj. Prev. 2009, 15, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.; Curtis, K.; Fisher, M. Understanding trauma as a men’s health issue: Sex differences in traumatic injury presentations at a level 1 trauma center in Australia. J. Trauma Nurs. 2012, 19, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Gannon, C.J.; Napolitano, L.M.; Pasquale, M.; Tracy, J.K.; McCarter, R.J. A statewide population-based study of gender differences in trauma: Validation of a prior single-institution study. J. Am. Coll. Surg. 2002, 195, 11–18. [Google Scholar] [CrossRef]
- Rau, C.S.; Kuo, P.J.; Wu, S.C.; Chen, Y.C.; Hsieh, H.Y.; Hsieh, C.H. Association between the Osteoporosis Self-Assessment Tool for Asians Score and Mortality in Patients with Isolated Moderate and Severe Traumatic Brain Injury: A Propensity Score-Matched Analysis. Int. J. Environ. Res. Public Health 2016, 13, 1203. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Rau, C.S.; Wu, S.C.; Kuo, P.J.; Chen, Y.C.; Hsieh, H.Y.; Hsieh, C.H. Association of Osteoporosis Self-Assessment Tool for Asians (OSTA) Score with Clinical Presentation and Expenditure in Hospitalized Trauma Patients with Femoral Fractures. Int. J. Environ. Res. Public Health 2016, 13, 995. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Chen, D.; Cai, Y.; Wei, S. Concordane of OSTA and lumbar spine BMD by DXA in identifying risk of osteoporosis. J. Orthop. Surg. Res. 2006, 1, 14. [Google Scholar] [CrossRef] [PubMed]
- Muslim, D.; Mohd, E.; Sallehudin, A.; Tengku Muzaffar, T.; Ezane, A. Performance of Osteoporosis Self-assessment Tool for Asian (OSTA) for Primary Osteoporosis in Post-menopausal Malay Women. Malays. Orthop. J. 2012, 6, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.A.; Kakaji, M.; Awasthi, A.; Kumar, K.; Mishra, K.; Shukla, M.; Gupta, S.K. Utility of Osteoporosis Self-Assessment Tool as a Screening Tool for Predicting Osteoporosis in Indian Men. J. Clin. Densitom. 2017, 20, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Rau, C.S.; Wu, S.C.; Kuo, P.J.; Chen, Y.C.; Chien, P.C.; Hsieh, H.Y.; Hsieh, C.H. Epidemiology of Bone Fracture in Female Trauma Patients Based on Risks of Osteoporosis Assessed using the Osteoporosis Self-Assessment Tool for Asians Score. Int. J. Environ. Res. Public Health 2017, 14, 1380. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.H.; Liu, H.T.; Hsu, S.Y.; Hsieh, H.Y.; Chen, Y.C. Motorcycle-related hospitalizations of the elderly. Biomed. J. 2017, 40, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974, 2, 81–84. [Google Scholar] [CrossRef]
- Rating the severity of tissue damage. I. The abbreviated scale. JAMA 1971, 215, 277–280.
- Baker, S.P.; O’Neill, B.; Haddon, W., Jr.; Long, W.B. The injury severity score: A method for describing patients with multiple injuries and evaluating emergency care. J. Trauma 1974, 14, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Leijdesdorff, H.A.; Siegerink, B.; Sier, C.F.; Reurings, M.C.; Schipper, I.B. Injury pattern, injury severity, and mortality in 33,495 hospital-admitted victims of motorized two-wheeled vehicle crashes in The Netherlands. J. Trauma Acute Care Surg. 2012, 72, 1363–1368. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.Q.; Gutierrez, C.J.; Mariano, M.C.; Vander Laan, T.; Gaspard, D.J.; Carpenter, C.L.; Stain, S.C. Blunt chest trauma in the elderly patient: how cardiopulmonary disease affects outcome. Am. Surg. 2000, 66, 855–857. [Google Scholar] [PubMed]
- Rau, C.S.; Wu, S.C.; Kuo, P.J.; Chen, Y.C.; Chien, P.C.; Hsieh, H.Y.; Hsieh, C.H. Same Abbreviated Injury Scale Values May Be Associated with Different Risks to Mortality in Trauma Patients: A Cross-Sectional Retrospective Study Based on the Trauma Registry System in a Level I Trauma Center. Int. J. Environ. Res. Public Health 2017, 14, 1552. [Google Scholar] [CrossRef] [PubMed]
- Al-Tarrah, K.; Moiemen, N.; Lord, J.M. The influence of sex steroid hormones on the response to trauma and burn injury. Burns. Trauma 2017, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Albertsmeier, M.; Pratschke, S.; Chaudry, I.; Angele, M.K. Gender-Specific Effects on Immune Response and Cardiac Function after Trauma Hemorrhage and Sepsis. Viszeralmedizin 2014, 30, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Knoferl, M.W.; Diodato, M.D.; Angele, M.K.; Ayala, A.; Cioffi, W.G.; Bland, K.I.; Chaudry, I.H. Do female sex steroids adversely or beneficially affect the depressed immune responses in males after trauma-hemorrhage? Arch. Surg. 2000, 135, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Gurney, E.P.; Nachtigall, M.J.; Nachtigall, L.E.; Naftolin, F. The Women’s Health Initiative trial and related studies: 10 years later: A clinician’s view. J. Steroid Biochem. Mol. Biol. 2014, 142, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, M.M.; Aquilino, A.; Cortese, F.; Scicchitano, P.; Sassara, M.; Mola, E.; Rollo, R.; Caldarola, P.; Giorgino, F.; Pomo, V.; et al. Feasibility and effectiveness of a disease and care management model in the primary health care system for patients with heart failure and diabetes (Project Leonardo). Vasc. Health Risk Manag. 2010, 6, 297–305. [Google Scholar] [CrossRef] [PubMed]


| Variables | Female, n = 1585 | Male, n = 663 | Odds Ratio (95% CI) | p |
|---|---|---|---|---|
| Age [range] (years) | 80.6 ± 6.9 [58–102] | 82.1 ± 6.3 [59–99] | - | <0.001 |
| Body weight (kg) | 48.6 ± 7.1 | 52.7 ± 6.7 | - | <0.001 |
| Body height (cm) | 151.7 ± 5.7 | 162.9 ± 6.1 | - | <0.001 |
| Co-morbidity, n (%) | ||||
| DM | 400 (25.2) | 92 (13.9) | 2.1 (1.64–2.68) | <0.001 |
| HTN | 962 (60.7) | 310 (46.8) | 1.8 (1.47–2.11) | <0.001 |
| CAD | 156 (9.8) | 59 (8.9) | 1.1 (0.82–1.53) | 0.488 |
| CHF | 44 (2.8) | 17 (2.6) | 1.1 (0.62–1.91) | 0.778 |
| CVA | 151 (9.5) | 91 (13.7) | 0.7 (0.50-0.87) | 0.003 |
| Mechanism, n (%) | ||||
| Motor vehicle | 9 (0.6) | 3 (0.5) | 1.3 (0.34–4.66) | 1.000 |
| Motorcycle | 133 (8.4) | 132 (19.9) | 0.4 (0.28–0.48) | <0.001 |
| Bicycle | 74 (4.7) | 53 (8.0) | 0.6 (0.39–0.81) | 0.002 |
| Pedestrian | 56 (3.5) | 18 (2.7) | 1.3 (0.77–2.25) | 0.321 |
| Fall | 1277 (80.6) | 434 (65.5) | 2.2 (1.79–2.68) | <0.001 |
| Penetrating injury | 7 (0.4) | 6 (0.9) | 0.5 (0.16–1.45) | 0.222 |
| Struck by/against | 29 (1.8) | 17 (2.6) | 0.7 (0.39–1.30) | 0.262 |
| BAC ≥ 50 mg/dL, n (%) | 1 (0.1) | 5 (0.8) | 0.1 (0.01–0.71) | 0.010 |
| GCS | 14.4 ± 1.9 | 14.1 ± 2.2 | - | 0.006 |
| ≤8 | 49 (3.1) | 32 (4.8) | 0.6 (0.40–0.99) | 0.044 |
| 9–12 | 63 (4.0) | 40 (6.0) | 0.6 (0.43–0.97) | 0.033 |
| ≥13 | 1473 (92.9) | 591 (89.1) | 1.6 (1.17–2.19) | 0.003 |
| AIS ≥ 3, n (%) | ||||
| Head/Neck | 270 (17.0) | 188 (28.4) | 0.5 (0.42–0.64) | <0.001 |
| Face | 0 (0.0) | 0 (0.0) | - | - |
| Thorax | 34 (2.1) | 40 (6.0) | 0.3 (0.21–0.54) | <0.001 |
| Abdomen | 15 (0.9) | 5 (0.8) | 1.3 (0.46–3.47) | 0.658 |
| Extremity | 991 (62.5) | 327 (49.3) | 1.7 (1.43–2.06) | <0.001 |
| ISS, median (IQR) | 9 (9–9) | 9 (9–13) | - | 0.001 |
| <16 | 1369 (86.4) | 512 (77.2) | 1.9 (1.48–2.36) | <0.001 |
| 16–24 | 161 (10.2) | 118 (17.8) | 0.5 (0.40–0.68) | <0.001 |
| ≥25 | 55 (3.5) | 33 (5.0) | 0.7 (0.44–1.07) | 0.093 |
| Mortality, n (%) | 44 (2.8) | 40 (6.0) | 0.4 (0.29–0.69) | <0.001 |
| LOS in hospital (days) | 9.6 ± 8.3 | 11.2 ± 11.4 | - | 0.001 |
| ICU admission, n (%) | 308 (19.4) | 197 (29.7) | 0.6 (0.46–0.70) | <0.001 |
| LOS in ICU (days) | 7.1 ± 9.7 | 8.6 ± 10.0 | - | 0.097 |
| Variables | Before Matching | After Matching | ||||||
|---|---|---|---|---|---|---|---|---|
| Female, n = 1585 | Male, n = 663 | Odds Ratio (95% CI) | p | Female, n = 573 | Male, n = 573 | Odds Ratio (95% CI) | p | |
| Age (years) | 80.6 ± 6.9 | 82.1 ± 6.3 | - | <0.001 | 81.4 ± 6.3 | 81.8 ± 6.4 | - | 0.402 |
| Co-morbidity, n (%) | ||||||||
| DM | 400 (25.2) | 92 (13.9) | 2.1 (1.64–2.68) | <0.001 | 80 (1.4) | 80 (1.4) | 1.0 (0.72–1.40) | 1.000 |
| HTN | 962 (60.7) | 310 (46.8) | 1.8 (1.47–2.11) | <0.001 | 282 (49.2) | 282 (49.2) | 1.0 (0.79–1.26) | 1.000 |
| CAD | 156 (9.8) | 59 (8.9) | 1.1 (0.82–1.53) | 0.488 | 49 (8.6) | 49 (8.6) | 1.0 (0.66–1.51) | 1.000 |
| CHF | 44 (2.8) | 17 (2.6) | 1.1 (0.62–1.91) | 0.778 | 12 (2.1) | 12 (2.1) | 1.0 (0.45–2.25) | 1.000 |
| CVA | 151 (9.5) | 91 (13.7) | 0.7 (0.50–0.87) | 0.003 | 73 (12.7) | 73 (12.7) | 1.0 (0.71–1.42) | 1.000 |
| Mechanism, n (%) | ||||||||
| Motor vehicle | 9 (0.6) | 3 (0.5) | 1.3 (0.34–4.66) | 1.000 | 1 (0.2) | 1 (0.2) | 1.0 (0.06–16.03) | 1.000 |
| Motorcycle | 133 (8.4) | 132 (19.9) | 0.4 (0.28–0.48) | <0.001 | 80 (14.0) | 80 (14.0) | 1.0 (0.72–1.40) | 1.000 |
| Bicycle | 74 (4.7) | 53 (8.0) | 0.6 (0.39–0.81) | 0.002 | 39 (6.8) | 39 (6.8) | 1.0 (0.63–1.58) | 1.000 |
| Pedestrian | 56 (3.5) | 18 (2.7) | 1.3 (0.77–2.25) | 0.321 | 15 (2.6) | 15 (2.6) | 1.0 (0.48–2.07) | 1.000 |
| Fall | 1277 (80.6) | 434 (65.5) | 2.2 (1.79–2.68) | <0.001 | 427 (74.5) | 427 (74.5) | 1.0 (0.77–1.30) | 1.000 |
| Penetrating injury | 7 (0.4) | 6 (0.9) | 0.5 (0.16–1.45) | 0.222 | 1 (0.2) | 1 (0.2) | 1.0 (0.06–16.03) | 1.000 |
| Struck by/against | 29 (1.8) | 17 (2.6) | 0.7 (0.39–1.30) | 0.262 | 10 (1.7) | 10 (1.7) | 1.0 (0.41–2.42) | 1.000 |
| ISS, median (IQR) | 9 (9–9) | 9 (9–13) | - | 0.001 | 9 (9–13) | 9 (9–13) | - | 0.400 |
| Variables | Propensity-Score Matched Cohort | |||
|---|---|---|---|---|
| Female, n = 573 | Male, n = 573 | Odds Ratio (95% CI) | p | |
| Mortality, n (%) | 19 (3.3) | 36 (6.3) | 0.5 (0.29–0.90) | 0.019 |
| LOS in hospital (days) | 10.4 ± 9.3 | 11.3 ± 11.5 | - | 0.154 |
| ICU admission, n (%) | 143 (25.0) | 172 (30.0) | 0.8 (0.60–1.01) | 0.055 |
| LOS in ICU (days) | 7.6 ± 10.0 | 8.8 ± 10.4 | - | 0.286 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, C.-E.; Liu, H.-T.; Kuo, P.-J.; Chen, Y.-C.; Hsu, S.-Y.; Lin, C.-C.; Hsieh, C.-H. Impact of Sexual Dimorphism on Trauma Patterns and Clinical Outcomes of Patients with a High-Risk Score of the Osteoporosis Self-Assessment Tool for Asians: A Propensity Score-Matched Analysis. Int. J. Environ. Res. Public Health 2018, 15, 418. https://doi.org/10.3390/ijerph15030418
Tang C-E, Liu H-T, Kuo P-J, Chen Y-C, Hsu S-Y, Lin C-C, Hsieh C-H. Impact of Sexual Dimorphism on Trauma Patterns and Clinical Outcomes of Patients with a High-Risk Score of the Osteoporosis Self-Assessment Tool for Asians: A Propensity Score-Matched Analysis. International Journal of Environmental Research and Public Health. 2018; 15(3):418. https://doi.org/10.3390/ijerph15030418
Chicago/Turabian StyleTang, Chien-En, Hang-Tsung Liu, Pao-Jen Kuo, Yi-Chun Chen, Shiun-Yuan Hsu, Chih-Che Lin, and Ching-Hua Hsieh. 2018. "Impact of Sexual Dimorphism on Trauma Patterns and Clinical Outcomes of Patients with a High-Risk Score of the Osteoporosis Self-Assessment Tool for Asians: A Propensity Score-Matched Analysis" International Journal of Environmental Research and Public Health 15, no. 3: 418. https://doi.org/10.3390/ijerph15030418





