Microwave-Hydrothermal Treated Grape Peel as an Efficient Biosorbent for Methylene Blue Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterizations of Grape Peel Biosorbents
2.2. Adsorption Experiments
3. Results and Discussion
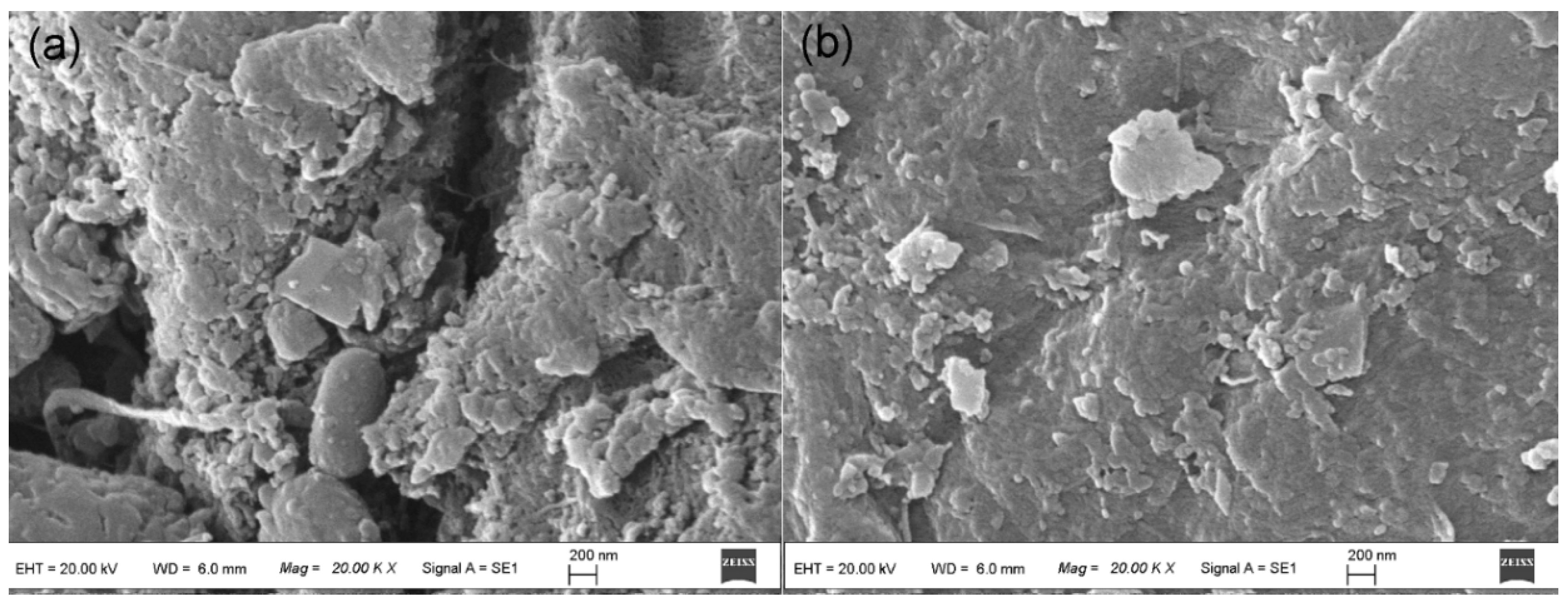
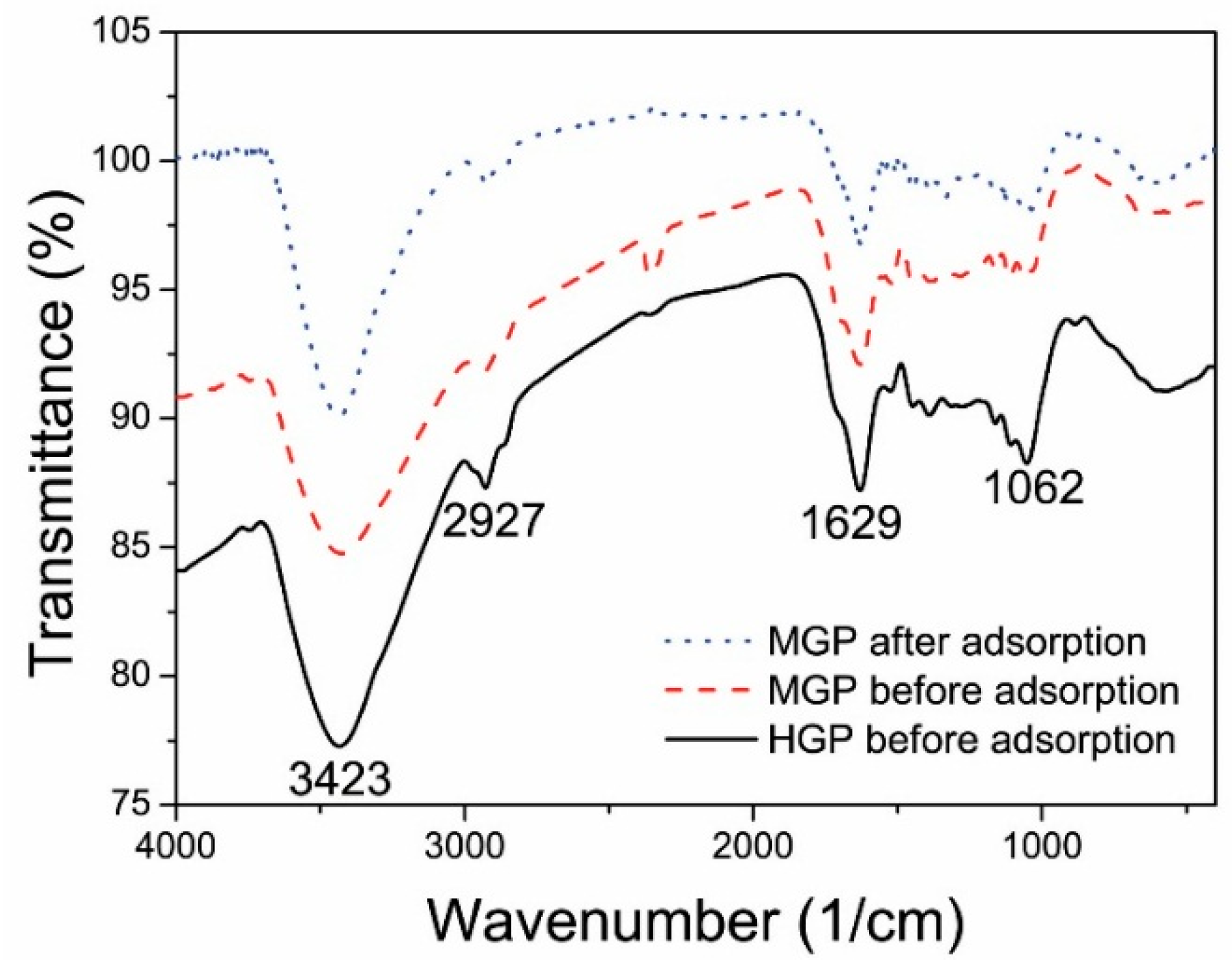
3.1. Characterizations of the Biosorbents
3.2. Adsorption Properties
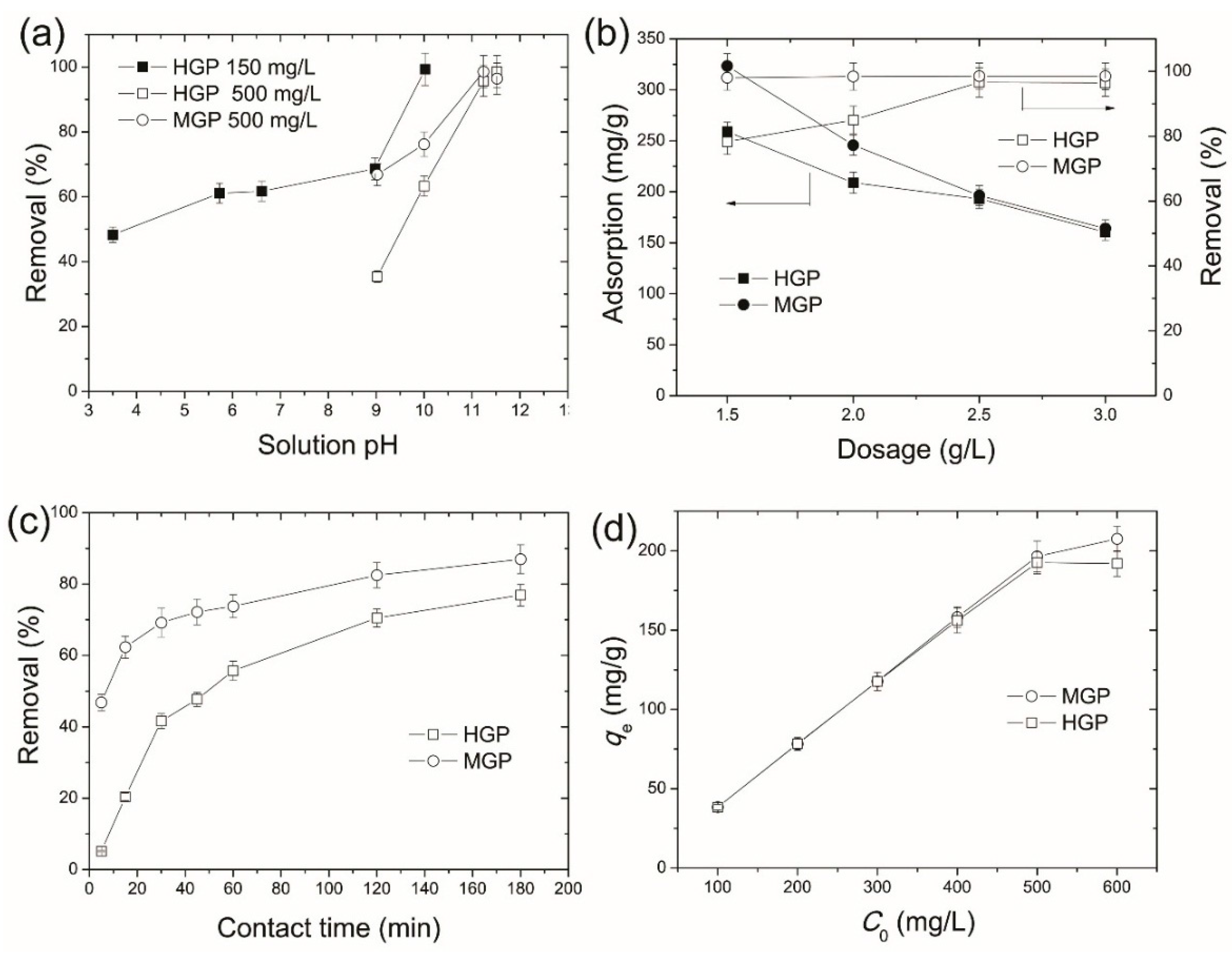
3.2.1. Effect of Solution pH
3.2.2. Effect of Dosage
3.2.3. Effect of Contact Time
3.2.4. Effect of Initial MB Concentration
3.2.5. Adsorption Isotherms
3.2.6. Adsorption Kinetics
3.2.7. Comparison of Various Adsorbents
4. Conclusions
Acknowldgements
Author Contributions
Conflicts of Interest
References
- Bulut, Y.; Aydın, H. A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalination 2006, 194, 259–267. [Google Scholar] [CrossRef]
- Gong, R.; Li, M.; Yang, C.; Sun, Y.; Chen, J. Removal of cationic dyes from aqueous solution by adsorption on peanut hull. J. Hazard. Mater. 2005, 121, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Vadivelan, V.; Kumar, K.V. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J. Colloid Interface Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Kumar, A.; Naushad, M.; Kumar, A.; Al-Muhtaseb, A.A.H.; Dhiman, P.; Ghfar, A.A.; Stadler, F.J.; Khan, M.R. Photoremediation of toxic dye from aqueous environment using monometallic and bimetallic quantum dots based nanocomposites. J. Clean. Prod. 2018, 172, 2919–2930. [Google Scholar] [CrossRef]
- Pathania, D.; Gupta, D.; Al-Muhtaseb, A.A.H.; Sharma, G.; Kumar, A.; Naushad, M.; Ahamad, T.; Alshehri, S.M. Photocatalytic degradation of highly toxic dyes using chitosan-g-poly(acrylamide)/ZnS in presence of solar irradiation. J. Photochem. Photobiol. A 2016, 329, 61–68. [Google Scholar] [CrossRef]
- Kannan, N.; Sundaram, M.M. Kinetics and mechanism of removal of methylene blue by adsorption on various carbons—A comparative study. Dyes Pigm. 2001, 51, 25–40. [Google Scholar] [CrossRef]
- Zou, Z.; Zhang, Y.; Zhang, H.; Jiang, C. A combined H3PO4 activation and boron templating process for easy synthesis of highly porous, spherical activated carbons as a superior adsorbent for rhodamine B. RSC Adv. 2016, 6, 15226–15233. [Google Scholar] [CrossRef]
- Zou, Z.; Jiang, C. Solvothermal polycondensation of novolacs phenolic-resin to nanopowders and their derived activated nanocarbons as efficient adsorbents for water cleaning. J. Porous Mater. 2017, 24, 1555–1564. [Google Scholar] [CrossRef]
- Sharma, G.; Naushad, M.; Kumar, A.; Rana, S.; Sharma, S.; Bhatnagar, A.; Stadler, F.J.; Ghfar, A.A.; Khan, M.R. Efficient removal of coomassie brilliant blue R-250 dye using starch/poly(alginic acid-cl-acrylamide) nanohydrogel. Process Saf. Environ. Protect. 2017, 109, 301–310. [Google Scholar] [CrossRef]
- Naushad, M.; Alothman, Z.A.; Awual, M.R.; Alfadul, S.M.; Ahamad, T. Adsorption of rose Bengal dye from aqueous solution by amberlite Ira-938 resin: Kinetics, isotherms, and thermodynamic studies. Desalination Water Treat. 2016, 57, 13527–13533. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef] [PubMed]
- Ilevbare, I.M.; Probert, D.; Phaal, R. A review of TRIZ, and its benefits and challenges in practice. Technovation 2013, 33, 30–37. [Google Scholar] [CrossRef]
- Ferrero, F. Dye removal by low cost adsorbents: Hazelnut shells in comparison with wood sawdust. J. Hazard. Mater. 2007, 142, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Khattri, S.D.; Singh, M.K. Removal of malachite green from dye wastewater using neem sawdust by adsorption. J. Hazard. Mater. 2009, 167, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Hameed, B.H.; Ahmad, A.A. Batch adsorption of methylene blue from aqueous solution by garlic peel, an agricultural waste biomass. J. Hazard. Mater. 2009, 164, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ali, S.; Jaouali, I.; Souissi-Najar, S.; Ouederni, A. Characterization and adsorption capacity of raw pomegranate peel biosorbent for copper removal. J. Clean. Prod. 2017, 142, 3809–3821. [Google Scholar] [CrossRef]
- Bulut, Y.; Tez, Z. Adsorption studies on ground shells of hazelnut and almond. J. Hazard. Mater. 2007, 149, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Lafka, T.-I.; Sinanoglou, V.; Lazos, E.S. On the extraction and antioxidant activity of phenolic compounds from winery wastes. Food Chem. 2007, 104, 1206–1214. [Google Scholar] [CrossRef]
- Negro, C.; Tommasi, L.; Miceli, A. Phenolic compounds and antioxidant activity from red grape marc extracts. Bioresour. Technol. 2003, 87, 41–44. [Google Scholar] [CrossRef]
- Chand, R.; Narimura, K.; Kawakita, H.; Ohto, K.; Watari, T.; Inoue, K. Grape waste as a biosorbent for removing Cr (VI) from aqueous solution. J. Hazard. Mater. 2009, 163, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Avantaggiato, G.; Greco, D.; Damascelli, A.; Solfrizzo, M.; Visconti, A. Assessment of multi-mycotoxin adsorption efficacy of grape pomace. J. Agric. Food Chem. 2014, 62, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Sayğılı, H.; Sayğılı, G.A.; Güzel, F. Using grape pulp as a new alternative biosorbent for removal of a model basic dye, Asia-Pac. J. Chem. Eng. 2014, 9, 214–225. [Google Scholar]
- Lee, L.Y.; Gan, S.; Tan, M.S.Y.; Lim, S.S.; Lee, X.J.; Lam, Y.F. Effective removal of Acid Blue 113 dye using overripe Cucumis sativus peel as an eco-friendly biosorbent from agricultural residue. J. Clean. Prod. 2016, 113, 194–203. [Google Scholar] [CrossRef]
- Peydayesh, M.; Rahbar-Kelishami, A. Adsorption of methylene blue onto Platanus orientalis leaf powder: Kinetic, equilibrium and thermodynamic studies. J. Ind. Eng. Chem. 2015, 21, 1014–1019. [Google Scholar] [CrossRef]
- Pavan, F.A.; Lima, E.C.; Dias, S.L.P.; Mazzocato, A.C. Methylene blue biosorption from aqueous solutions by yellow passion fruit waste. J. Hazard. Mater. 2008, 150, 703–712. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Brito, S.M.; Andrade, H.M.C.; Soares, L.F.; de Azevedo, R.P. Brazil nut shells as a new biosorbent to remove methylene blue and indigo carmine from aqueous solutions. J. Hazard. Mater. 2010, 174, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.T.; Islam, M.A.; Mahmud, S.; Rukanuzzaman, M. Adsorptive removal of methylene blue by tea waste. J. Hazard. Mater. 2009, 164, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Annadurai, G.; Juang, R.-S.; Lee, D.-J. Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J. Hazard. Mater. 2002, 92, 263–274. [Google Scholar] [CrossRef]
- Franca, A.S.; Oliveira, L.S.; Ferreira, M.E. Kinetics and equilibrium studies of methylene blue adsorption by spent coffee grounds. Desalination 2009, 249, 267–272. [Google Scholar] [CrossRef]
| Biosorbents | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) |
|---|---|---|
| HGP | 4.12 | 0.0335 |
| MGP | 3.01 | 0.0193 |
| Biosorbent | Langmuir Isotherm Constants | Freundlich Isotherm Constants | ||||
|---|---|---|---|---|---|---|
| k3 (L/mg) | qm (mg/g) | R2 | 1/n (g/L) | k4 (mg/g) | R2 | |
| MGP | 0.33 | 215.7 | 0.994 | 0.45 | 6.06 | 0.189 |
| HGP | 0.16 | 192.7 | 0.991 | 0.33 | 5.32 | 0.454 |
| Sample | qe,exp. (mg/g) | Pseudo-First Order | Pseudo-Second Order | ||||
|---|---|---|---|---|---|---|---|
| k5 (1/min) | qe_cal. (mg/g) | R2 | k6 (g/mg/min) | qe_cal. (mg/g) | R2 | ||
| MGP | 208.1 | 0.027 | 203.3 | 0.931 | 4.2 × 10−4 | 217.9 | 0.997 |
| HGP | 183.9 | 0.024 | 182.6 | 0.990 | 7.9 × 10−5 | 240.4 | 0.994 |
| Biosorbent | qm (mg/g) | Ref. |
|---|---|---|
| Grape-peel | 215.7 | This work |
| Platanus orientalis leaf | 114.9 | [25] |
| Tea waste | 85.2 | [28] |
| Peanut hull | 68.0 | [2] |
| Garlic peel | 82.6 | [16] |
| Banana peel | 20.8 | [29] |
| Orange peel | 18.6 | [29] |
| Spent coffee grounds | 18.7 | [30] |
| Rice husk | 40.6 | [3] |
| Grape pulp | 118.3 | [23] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Jiang, C.; Lin, Z.; Zou, Z. Microwave-Hydrothermal Treated Grape Peel as an Efficient Biosorbent for Methylene Blue Removal. Int. J. Environ. Res. Public Health 2018, 15, 239. https://doi.org/10.3390/ijerph15020239
Ma L, Jiang C, Lin Z, Zou Z. Microwave-Hydrothermal Treated Grape Peel as an Efficient Biosorbent for Methylene Blue Removal. International Journal of Environmental Research and Public Health. 2018; 15(2):239. https://doi.org/10.3390/ijerph15020239
Chicago/Turabian StyleMa, Lin, Chunhai Jiang, Zhenyu Lin, and Zhimin Zou. 2018. "Microwave-Hydrothermal Treated Grape Peel as an Efficient Biosorbent for Methylene Blue Removal" International Journal of Environmental Research and Public Health 15, no. 2: 239. https://doi.org/10.3390/ijerph15020239




