Mortality Rate Associated with Admission Hyperglycemia in Traumatic Femoral Fracture Patients Is Greater Than Non-Diabetic Normoglycemic Patients but Not Diabetic Normoglycemic Patients
Abstract
:1. Background
2. Methods
2.1. Ethics Statement
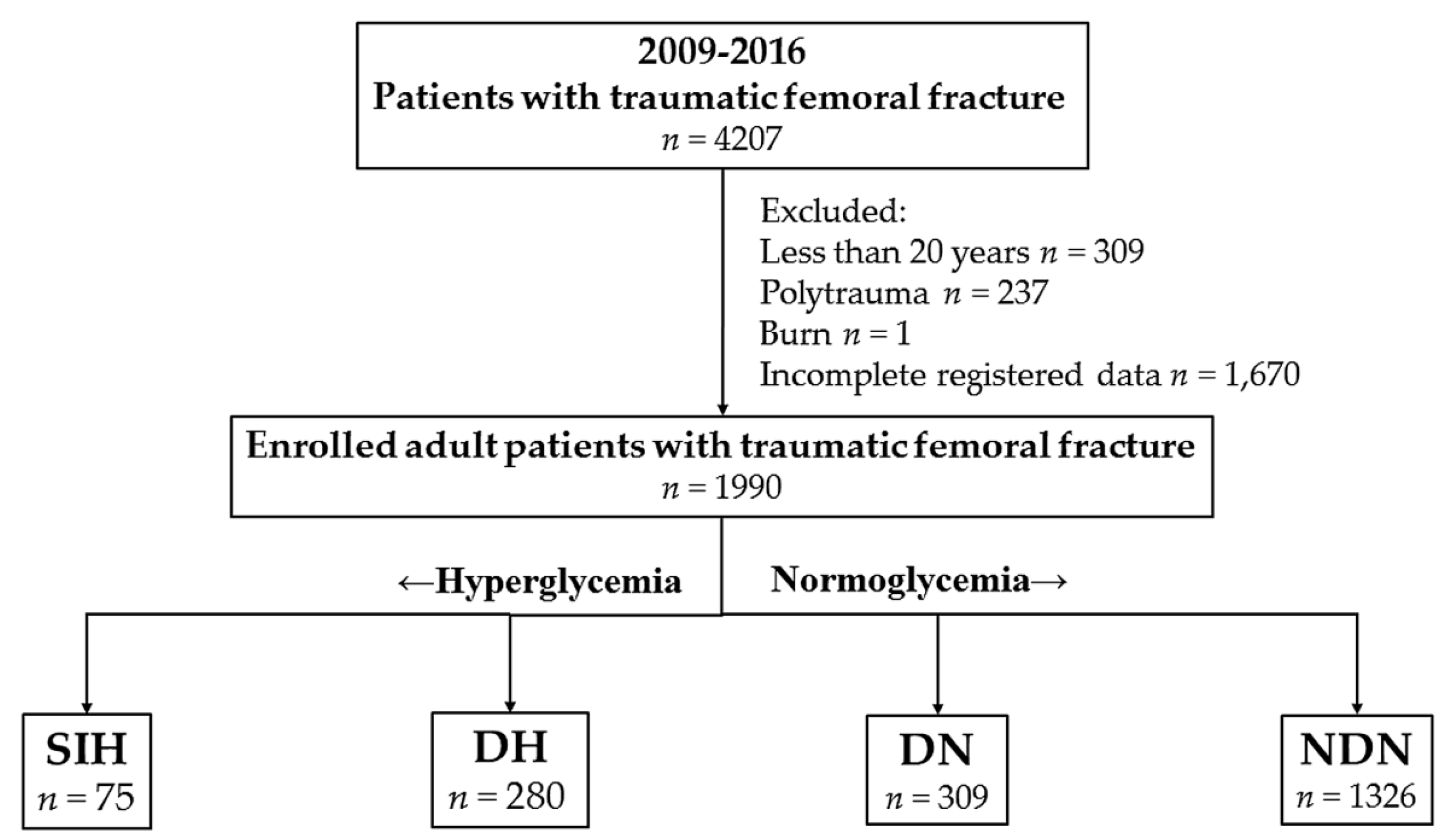
2.2. Study Population
2.3. Study Variables
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Patients with Femoral Fracture with SIH
3.2. Characteristics and Outcomes of Patients with DH
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moore, J.; Carmody, O.; Carey, B.; Harty, J.A.; Reidy, D. The cost and mortality of hip fractures in centenarians. Ir. J. Med. Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Dyer, S.M.; Crotty, M.; Fairhall, N.; Magaziner, J.; Beaupre, L.A.; Cameron, I.D.; Sherrington, C. A critical review of the long-term disability outcomes following hip fracture. BMC Geriatr. 2016, 16, 158. [Google Scholar] [CrossRef] [PubMed]
- Losser, M.R.; Damoisel, C.; Payen, D. Bench-to-bedside review: Glucose and stress conditions in the intensive care unit. Crit. Care 2010, 14, 231. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.; Bochicchio, G.V.; Joshi, M.; Bochicchio, K.; Tracy, K.; Scalea, T.M. Admission hyperglycemia is predictive of outcome in critically ill trauma patients. J. Trauma 2005, 59, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.E.; Kauffmann, R.M.; Zuckerman, S.L.; Obremskey, W.T.; May, A.K. Relationship of hyperglycemia and surgical-site infection in orthopaedic surgery. J. Bone Jt. Surg. Am. 2012, 94, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.E.; Kauffmann, R.M.; Obremskey, W.T.; May, A.K. Stress-induced hyperglycemia as a risk factor for surgical-site infection in nondiabetic orthopedic trauma patients admitted to the intensive care unit. J. Orthop. Trauma 2013, 27, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Mraovic, B.; Suh, D.; Jacovides, C.; Parvizi, J. Perioperative hyperglycemia and postoperative infection after lower limb arthroplasty. J. Diabetes Sci. Technol. 2011, 5, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Leto, R.; Desruelles, D.; Gillet, J.B.; Sabbe, M.B. Admission hyperglycaemia is associated with higher mortality in patients with hip fracture. Eur. J. Emerg. Med. 2015, 22, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Gauglitz, G.G.; Herndon, D.N.; Jeschke, M.G. Insulin resistance postburn: Underlying mechanisms and current therapeutic strategies. J. Burn Care Res. 2008, 29, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Ray, B.; Ludwig, A.; Yearout, L.K.; Thompson, D.M.; Bohnstedt, B.N. Stress-Induced Hyperglycemia after Spontaneous Subarachnoid Hemorrhage and Its Role in Predicting Cerebrospinal Fluid Diversion. World Neurosurg. 2017, 100, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.J.; Liao, W.I.; Wang, J.C.; Tsai, C.L.; Lee, J.T.; Peng, G.S.; Lee, C.H.; Hsu, C.W.; Tsai, S.H. Usefulness of glycated hemoglobin A1c-based adjusted glycemic variables in diabetic patients presenting with acute ischemic stroke. Am. J. Emerg. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Hiesmayr, M.J. Hyperglycemia and outcome after myocardial infarction and cardiac surgery: So what? Semin. Cardiothorac. Vasc. Anesth. 2006, 10, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Smit, J.W.; Romijn, J.A. Acute insulin resistance in myocardial ischemia: Causes and consequences. Semin. Cardiothorac. Vasc. Anesth. 2006, 10, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E.; Bellomo, R. Stress hyperglycemia: An essential survival response! Crit. Care Med. 2013, 41, e93–e94. [Google Scholar] [CrossRef] [PubMed]
- Plummer, M.P.; Deane, A.M. Dysglycemia and Glucose Control During Sepsis. Clin. Chest Med. 2016, 37, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Dungan, K.M.; Braithwaite, S.S.; Preiser, J.C. Stress hyperglycaemia. Lancet 2009, 373, 1798–1807. [Google Scholar] [CrossRef]
- McCowen, K.C.; Malhotra, A.; Bistrian, B.R. Stress-induced hyperglycemia. Crit. Care Clin. 2001, 17, 107–124. [Google Scholar] [CrossRef]
- Bosarge, P.L.; Kerby, J.D. Stress-induced hyperglycemia: Is it harmful following trauma? Adv. Surg. 2013, 47, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, X.; Meng, K.; Zeng, Z.; Ma, B.; Liu, X.; Qi, B.; Cui, S.; Cao, P.; Yang, Y. Stress-induced hyperglycemia after hip fracture and the increased risk of acute myocardial infarction in nondiabetic patients. Diabetes Care 2013, 36, 3328–3332. [Google Scholar] [CrossRef] [PubMed]
- Kreutziger, J.; Wenzel, V.; Kurz, A.; Constantinescu, M.A. Admission blood glucose is an independent predictive factor for hospital mortality in polytraumatised patients. Intensive Care Med. 2009, 35, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Vogelzang, M.; van der Horst, I.C.; Nijsten, M.W. Hyperglycaemic index as a tool to assess glucose control: A retrospective study. Crit. Care 2004, 8, R122–R127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerby, J.D.; Griffin, R.L.; MacLennan, P.; Rue, L.W., 3rd. Stress-induced hyperglycemia, not diabetic hyperglycemia, is associated with higher mortality in trauma. Ann. Surg. 2012, 256, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.H.; Hsu, S.Y.; Hsieh, H.Y.; Chen, Y.C. Differences between the sexes in motorcycle-related injuries and fatalities at a Taiwanese level I trauma center. Biomed. J. 2017, 40, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.H.; Liu, H.T.; Hsu, S.Y.; Hsieh, H.Y.; Chen, Y.C. Motorcycle-related hospitalizations of the elderly. Biomed. J. 2017, 40, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Diagnosis and classification of diabetes mellitus. Diabetes Care 2012, 35 (Suppl. 1), S64–S71. [CrossRef]
- Butcher, N.; Balogh, Z.J. AIS > 2 in at least two body regions: A potential new anatomical definition of polytrauma. Injury 2012, 43, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Neumann, T.; Samann, A.; Lodes, S.; Kastner, B.; Franke, S.; Kiehntopf, M.; Hemmelmann, C.; Lehmann, T.; Muller, U.A.; Hein, G.; et al. Glycaemic control is positively associated with prevalent fractures but not with bone mineral density in patients with Type 1 diabetes. Diabet. Med. 2011, 28, 872–875. [Google Scholar] [CrossRef] [PubMed]
- Srikanthan, P.; Crandall, C.J.; Miller-Martinez, D.; Seeman, T.E.; Greendale, G.A.; Binkley, N.; Karlamangla, A.S. Insulin resistance and bone strength: Findings from the study of midlife in the United States. J. Bone Miner. Res. 2014, 29, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—A meta-analysis. Osteoporos. Int. 2007, 18, 427–444. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Rau, C.S.; Wu, S.C.; Kuo, P.J.; Chen, Y.C.; Hsieh, H.Y.; Hsieh, C.H. Association of Osteoporosis Self-Assessment Tool for Asians (OSTA) Score with Clinical Presentation and Expenditure in Hospitalized Trauma Patients with Femoral Fractures. Int. J. Environ. Res. Public Health 2016, 13, 995. [Google Scholar] [CrossRef] [PubMed]
- Janghorbani, M.; Van Dam, R.M.; Willett, W.C.; Hu, F.B. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am. J. Epidemiol. 2007, 166, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Maffi, P.; Secchi, A. The Burden of Diabetes: Emerging Data. Dev. Ophthalmol. 2017, 60, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tun, N.N.; Arunagirinathan, G.; Munshi, S.K.; Pappachan, J.M. Diabetes mellitus and stroke: A clinical update. World J. Diabetes 2017, 8, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Oei, L.; Rivadeneira, F.; Zillikens, M.C.; Oei, E.H. Diabetes, diabetic complications, and fracture risk. Curr. Osteoporos. Rep. 2015, 13, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.Y.; Kim, Y.S. Peripheral Arterial Disease in Patients with Type 2 Diabetes Mellitus. Diabetes Metab. J. 2015, 39, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Mittal, K.; Katare, D.P. Shared links between type 2 diabetes mellitus and Alzheimer’s disease: A review. Diabetes Metab. Syndr. 2016, 10, S144–S149. [Google Scholar] [CrossRef] [PubMed]
- Wahl, W.L.; Taddonio, M.; Maggio, P.M.; Arbabi, S.; Hemmila, M.R. Mean glucose values predict trauma patient mortality. J. Trauma 2008, 65, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Kutcher, M.E.; Pepper, M.B.; Morabito, D.; Sunjaya, D.; Knudson, M.M.; Cohen, M.J. Finding the sweet spot: Identification of optimal glucose levels in critically injured patients. J. Trauma 2011, 71, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Finfer, S.; Chittock, D.R.; Su, S.Y.; Blair, D.; Foster, D.; Dhingra, V.; Bellomo, R.; Cook, D.; Dodek, P.; Henderson, W.R.; et al. Intensive versus conventional glucose control in critically ill patients. N. Engl. J. Med. 2009, 360, 1283–1297. [Google Scholar] [CrossRef] [PubMed]
| Variables | SIH, n = 75 | DH, n = 280 | DN, n = 309 | NDN, n = 1326 |
|---|---|---|---|---|
| Sex | ||||
| Female, n (%) | 42 (56.0) | 190 (67.9) | 212 (68.6) | 746 (56.3) |
| Male, n (%) | 33 (44.0) | 90 (32.1) | 97 (31.4) | 580 (43.7) |
| Age (years) | 72.6 ± 13.7 | 72.4 ± 10.2 | 74.2 ± 10.2 | 67.1 ± 20.1 |
| Comorbidity | ||||
| HTN, n (%) | 37 (49.3) | 186 (66.4) | 235 (76.1) | 544 (41.0) |
| CAD, n (%) | 4 (5.3) | 31 (11.1) | 32 (10.4) | 89 (6.7) |
| CHF, n (%) | 0 (0.0) | 8 (2.9) | 10 (3.2) | 28 (2.1) |
| CVA, n (%) | 7 (9.3) | 47 (16.8) | 57 (18.4) | 120 (9.0) |
| ESRD, n (%) | 0 (0.0) | 1 (0.4) | 0 (0.0) | 2 (0.2) |
| GCS | 14.4 ± 1.9 | 14.8 ± 0.8 | 14.8 ± 0.9 | 14.8 ± 0.9 |
| ≤8 | 1 (1.3) | 0 (0.0) | 1 (0.3) | 7 (0.5) |
| 9–12 | 5 (6.7) | 6 (2.1) | 6 (1.9) | 28 (2.1) |
| ≥13 | 69 (92.0) | 274 (97.9) | 302 (97.7) | 1291 (97.4) |
| ISS, median (IQR) | 9 (9–9) | 9 (9–9) | 9 (9–9) | 9 (9–9) |
| <16 | 72 (96.0) | 280 (100.0) | 309 (100.0) | 1314 (99.1) |
| 16–24 | 3 (4.0) | 0 (0.0) | 0 (0.0) | 12 (0.9) |
| ≥25 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hospital LOS (days) | 12.7 ± 11.1 | 10.3 ± 9.0 | 9.9 ± 8.1 | 9.7 ± 7.1 |
| ICU admission, n (%) | 9 (12.0) | 24 (8.6) | 19 (6.1) | 102 (7.7) |
| Mortality, n (%) | 3 (4.0) | 5 (1.8) | 9 (2.9) | 4 (0.3) |
| Variables | SIH vs. NDN | SIH vs. DN | DH vs. NDN | DH vs. DN | ||||
|---|---|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | p | Odds Ratio (95% CI) | p | Odds Ratio (95% CI) | p | Odds Ratio (95% CI) | p | |
| Characteristic | ||||||||
| Sex | 0.965 | 0.038 | <0.001 | 0.845 | ||||
| Female, n (%) | 1.0 (0.62–1.58) | 0.6 (0.35–0.98) | 1.6 (1.25–2.16) | 1.0 (0.68–1.37) | ||||
| Male, n (%) | 1.0 (0.63–1.62) | 1.7 (1.03–2.88) | 0.6 (0.46–0.80) | 1.0 (0.73–1.47) | ||||
| Comorbidity | ||||||||
| HTN, n (%) | 1.4 (0.88–2.23) | 0.155 | 0.3 (0.18–0.52) | <0.001 | 2.8 (2.17–3.73) | <0.001 | 0.6 (0.44–0.89) | 0.010 |
| CAD, n (%) | 0.8 (0.28–2.19) | 0.813 | 0.5 (0.17–1.42) | 0.181 | 1.7 (1.13–2.66) | 0.012 | 1.1 (0.64–1.82) | 0.779 |
| CHF, n (%) | - | 0.397 | - | 0.221 | 1.4 (0.62–3.02) | 0.444 | 0.9 (0.34–2.26) | 0.790 |
| CVA, n (%) | 1.0 (0.47–2.30) | 0.934 | 0.5 (0.20–1.04) | 0.057 | 2.0 (1.41–2.92) | <0.001 | 0.9 (0.58–1.36) | 0.598 |
| ESRD, n (%) | ― | 1.000 | - | - | 2.4 (0.21–26.26) | 0.437 | - | 0.475 |
| GCS | ||||||||
| ≤8 | 2.5 (0.31–20.97) | 0.357 | 4.2 (0.26–67.32) | 0.353 | - | 0.613 | - | 1.000 |
| 9–12 | 3.3 (1.24–8.84) | 0.028 | 3.6 (1.07–12.16) | 0.044 | 1.0 (0.42–2.48) | 0.974 | 1.1 (0.35–3.47) | 0.863 |
| ≥13 | 0.3 (0.13–0.77) | 0.019 | 0.3 (0.09–0.82) | 0.025 | 1.2 (0.52–2.97) | 0.632 | 1.1 (0.35–3.19) | 0.919 |
| ISS | ||||||||
| <16 | 0.2 (0.06–0.79) | 0.042 | - | 0.007 | - | 0.241 | - | - |
| 16–24 | 4.6 (1.26–16.53) | 0.042 | - | 0.007 | - | 0.241 | - | - |
| ≥25 | - | - | - | - | - | - | - | - |
| Outcomes | ||||||||
| ICU admission, n (%) | 1.6 (0.79–3.38) | 0.179 | 2.1 (0.90–4.81) | 0.080 | 1.1 (0.71–1.79) | 0.619 | 1.4 (0.77–2.67) | 0.259 |
| Mortality, n (%) | 13.8 (3.03–62.69) | 0.004 | 1.4 (0.37–5.26) | 0.710 | 6.0 (1.60–22.52) | 0.011 | 6.0 (0.20–1.83) | 0.370 |
| Adjusted mortality, n (%) | 9.8 (1.54–62.05) | 0.016 | 0.3 (0.03–2.99) | 0.302 | 5.8 (1.46–22.67) | 0.012 | 0.6 (0.20–1.89) | 0.394 |
| SIH, n = 75 | DH, n = 280 | DN, n = 309 | NDN, n = 1326 | Levene’s Test p | F | p | Median Difference | Post-Hoc p | |||
| Age | 72.6 ± 13.7 | 72.4 ± 10.2 | 74.2 ± 10.2 | 67.1 ± 20.1 | <0.001 | 18.6 | <0.001 | NDN | SIH | −5.5 | 0.008 |
| (years) | DH | −5.3 | <0.001 | ||||||||
| DN | SIH | 1.6 | 0.774 | ||||||||
| DH | 1.8 | 0.142 | |||||||||
| GCS | 14.4 ± 1.9 | 14.8 ± 0.8 | 14.8 ± 0.9 | 14.8 ± 0.9 | <0.001 | 4.8 | 0.002 | NDN | SIH | 0.4 | 0.238 |
| DH | −0.0 | 0.967 | |||||||||
| DN | SIH | 0.4 | 0.236 | ||||||||
| DH | −0.0 | 0.996 | |||||||||
| LOS | 12.7 ± 11.1 | 10.3 ± 9.0 | 9.9 ± 8.1 | 9.7 ± 7.1 | <0.001 | 3.9 | 0.009 | NDN | SIH | −3.0 | 0.104 |
| (days) | DH | −0.6 | 0.675 | ||||||||
| DN | SIH | −2.7 | 0.194 | ||||||||
| DH | −0.4 | 0.953 | |||||||||
| SIH, n = 75 | DH, n = 280 | DN, n = 309 | NDN, n = 1326 | Levene’s Test p | F | Kruskal-Wallis p | Median Difference | Post-Hoc p | |||
| ISS, | 9 (9–9) | 9 (9–9) | 9 (9–9) | 9 (9–9) | N/A | N/A | <0.001 | NDN | SIH | 0 | 0.854 |
| Median (IQR) | DH | 0 | <0.001 | ||||||||
| DN | SIH | 0 | 0.053 | ||||||||
| DH | 0 | 0.630 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rau, C.-S.; Wu, S.-C.; Chen, Y.-C.; Chien, P.-C.; Hsieh, H.-Y.; Kuo, P.-J.; Hsieh, C.-H. Mortality Rate Associated with Admission Hyperglycemia in Traumatic Femoral Fracture Patients Is Greater Than Non-Diabetic Normoglycemic Patients but Not Diabetic Normoglycemic Patients. Int. J. Environ. Res. Public Health 2018, 15, 28. https://doi.org/10.3390/ijerph15010028
Rau C-S, Wu S-C, Chen Y-C, Chien P-C, Hsieh H-Y, Kuo P-J, Hsieh C-H. Mortality Rate Associated with Admission Hyperglycemia in Traumatic Femoral Fracture Patients Is Greater Than Non-Diabetic Normoglycemic Patients but Not Diabetic Normoglycemic Patients. International Journal of Environmental Research and Public Health. 2018; 15(1):28. https://doi.org/10.3390/ijerph15010028
Chicago/Turabian StyleRau, Cheng-Shyuan, Shao-Chun Wu, Yi-Chun Chen, Peng-Chen Chien, Hsiao-Yun Hsieh, Pao-Jen Kuo, and Ching-Hua Hsieh. 2018. "Mortality Rate Associated with Admission Hyperglycemia in Traumatic Femoral Fracture Patients Is Greater Than Non-Diabetic Normoglycemic Patients but Not Diabetic Normoglycemic Patients" International Journal of Environmental Research and Public Health 15, no. 1: 28. https://doi.org/10.3390/ijerph15010028






