Endocrine Disrupting Chemicals Mediated through Binding Androgen Receptor Are Associated with Diabetes Mellitus
Abstract
:1. Diabetes Mellitus
2. Target Receptors of Endocrine Disrupting Chemicals
3. Androgenic Activity
4. Metabolism of Androgens
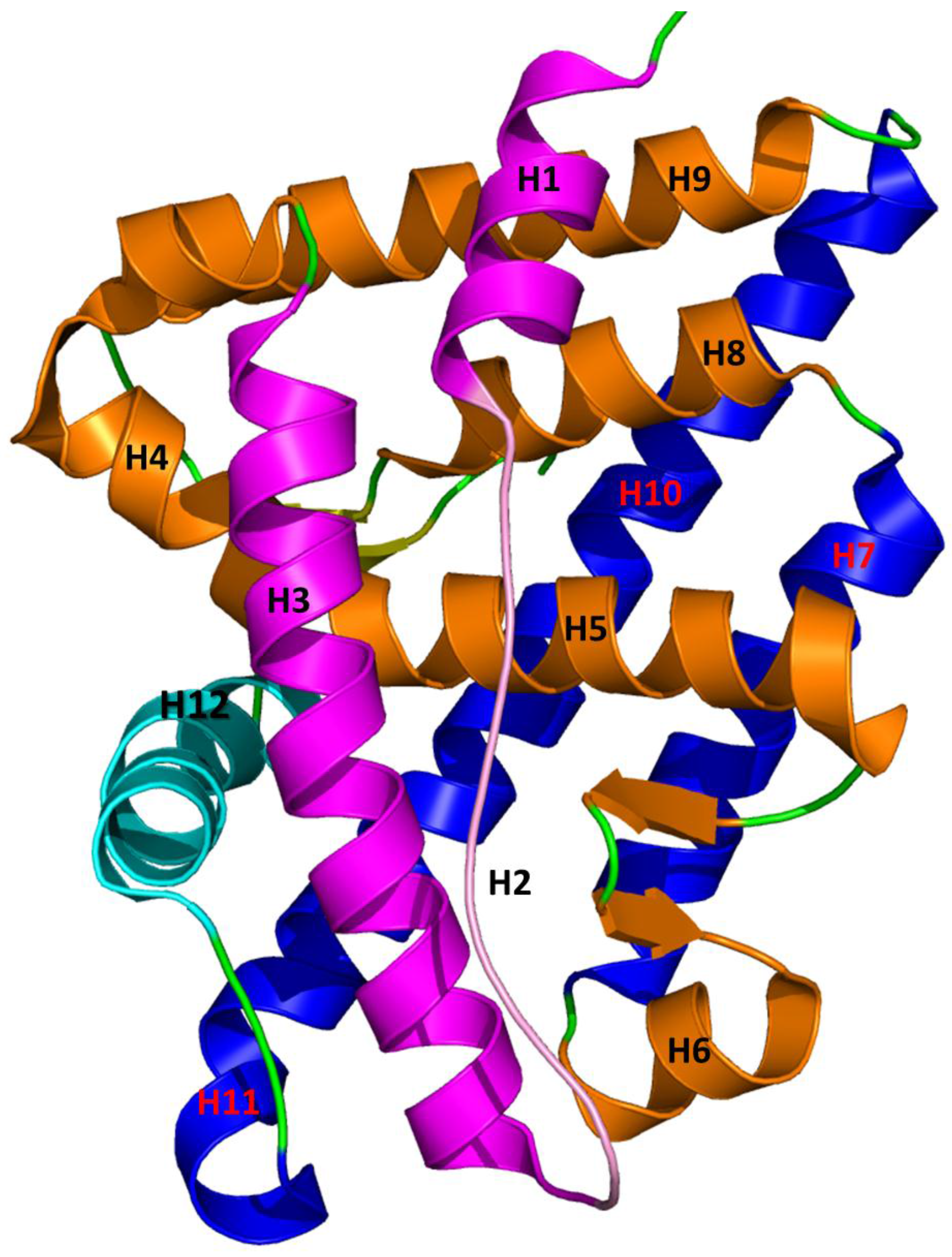
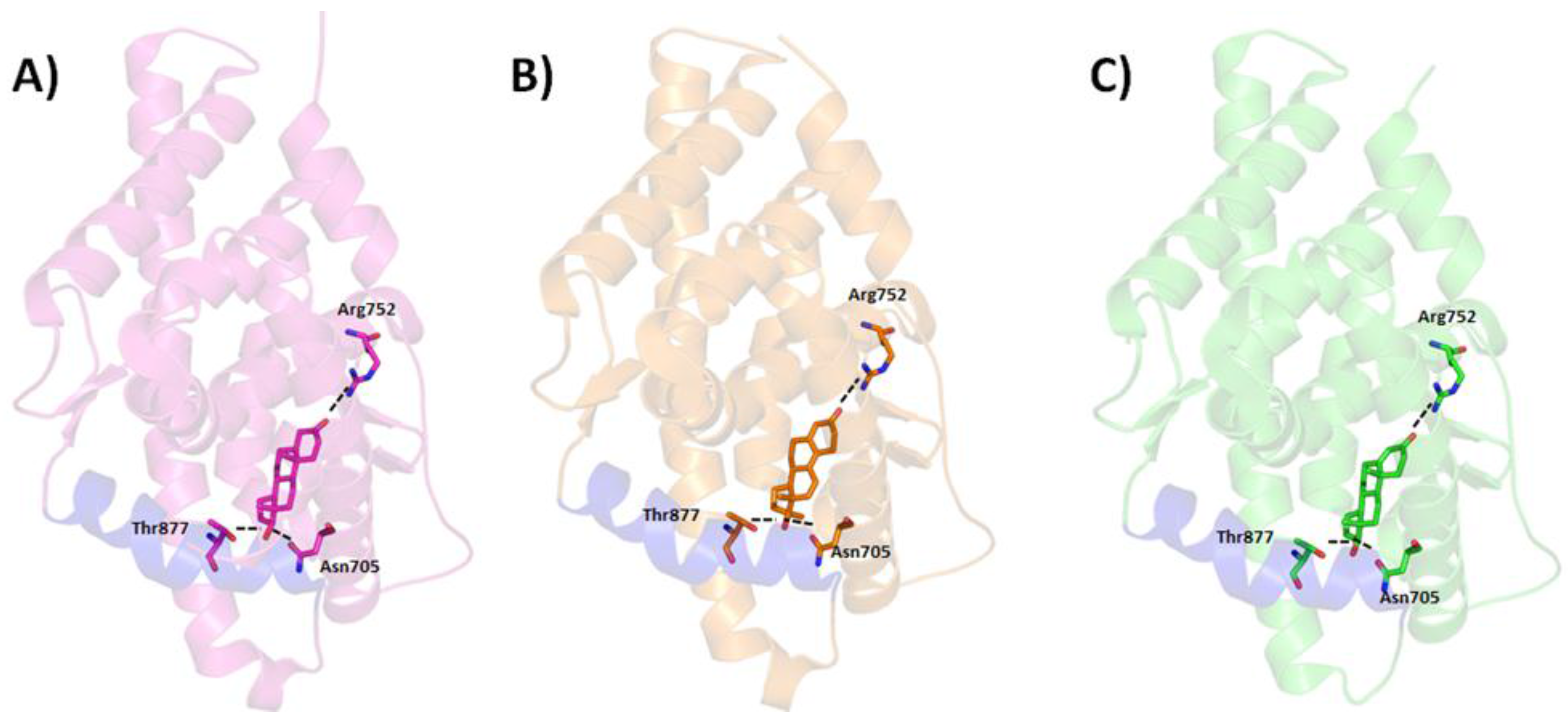
5. Interaction between Agonist/Antagonist and the Androgen Receptor
6. Androgenic Compounds Associated with Diabetes Mellitus
6.1. Androgenic Compounds and Diabetes Mellitus
6.2. Androgenic Compounds and Diabetes Mellitus Experimental
6.3. Androgenic Compounds and Type 1 Diabetes Mellitus
6.4. Androgenic Compounds and Type 2 Diabetes Mellitus
6.5. Androgenic Activity Compounds and Diabetic Nephropathies
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Tuomi, T. Type 1 and type 2 diabetes: What do they have in common? Diabetes 2005, 54 (Suppl. 2), S40–S45. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Davis, E.J.; Lawrence, J.M.; Dabelea, D.; Divers, J.; Isom, S.; Dolan, L.; Imperatore, G.; Linder, B.; Marcovina, S.; Pettitt, D.J. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N. Engl. J. Med. 2017, 376, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Danaei, G.; Finucane, M.M.; Lu, Y.; Singh, G.M.; Cowan, M.J.; Paciorek, C.J.; Lin, J.K.; Farzadfar, F.; Khang, Y.H.; Stevens, G.A.; et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: Systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 2011, 378, 31–40. [Google Scholar] [CrossRef]
- National Diabetes Statistics Report, 2017. Available online: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf (accessed on 18 October 2017).
- American Diabetes Association. Economic costs of diabetes in the US in 2012. Diabetes Care 2013, 36, 1033–1046. [Google Scholar]
- Cnop, M.; Welsh, N.; Jonas, J.-C.; Jörns, A.; Lenzen, S.; Eizirik, D.L. Mechanisms of pancreatic β-cell death in type 1 and type 2 diabetes. Diabetes 2005, 54 (Suppl. 2), S97–S107. [Google Scholar] [CrossRef] [PubMed]
- Rigano, D.; Sirignano, C.; Taglialatela-Scafati, O. The potential of natural products for targeting PPARα. Acta Pharm. Sin. B 2017, 7, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, G.; Ramprasath, T.; Gilles, M.; Swaminathan, K.; Ramasamy, S. Gut microbiota, endocrine-disrupting chemicals, and the diabetes epidemic. Trends Endocrinol. Metab. 2017, 28, 612–625. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Chou, E.L.; Baecker, A.; You, N.C.Y.; Song, Y.; Sun, Q.; Liu, S. Endocrine-disrupting chemicals, risk of type 2 diabetes, and diabetes-related metabolic traits: A systematic review and meta-analysis. J. Diabetes 2016, 8, 516–532. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Deretzi, G.; Zavos, C.; Mantzoros, C.S. The emerging role of endocrine disruptors in pathogenesis of insulin resistance: A concept implicating nonalcoholic fatty liver disease. Curr. Mol. Med. 2012, 12, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.N.; Pandya, K.; Clark, G.J.; Parikh, M.C.; Lau-Cam, C.A. Comparison of taurine and pantoyltaurine as antioxidants in vitro and in the central nervous system of diabetic rats. Exp. Toxicol. Pathol. 2016, 68, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Allard, C.; Xu, W.; Mauvais-Jarvis, F. The role of androgens in metabolism, obesity, and diabetes in males and females. Obesity 2015, 23, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.R.; Meadowcraft, L.M.; Williamson, B. Prevalence, pathophysiology, and management of androgen deficiency in men with metabolic syndrome, type 2 diabetes mellitus, or both. Pharmacotherapy 2015, 35, 780–792. [Google Scholar] [CrossRef] [PubMed]
- Wortham, M.; Sander, M. High T gives β cells a boost. Cell. Metab. 2016, 23, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Laudet, V.; Gronemeyer, H. Introduction to the nuclear receptors. In The Nuclear Receptor FactsBook; Academic Press: London, UK, 2002. [Google Scholar]
- Wurtz, J.M.; Bourguet, W.; Renaud, J.P.; Vivat, V.; Chambon, P.; Moras, D.; Gronemeyer, H. A canonical structure for the ligand-binding domain of nuclear receptors. Nat. Struct. Biol. 1996, 3, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Sakkiah, S.; Ng, H.W.; Tong, W.; Hong, H. Structures of androgen receptor bound with ligands: Advancing understanding of biological functions and drug discovery. Expert Opin. Ther. Targets 2016, 20, 1267–1282. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.; Perkins, R.; Tong, W.; Hong, H. Versatility or promiscuity: The estrogen receptors, control of ligand selectivity and an update on subtype selective ligands. Int. J. Environ. Res. Public Health 2014, 11, 8709–8742. [Google Scholar] [CrossRef] [PubMed]
- Deroo, B.J.; Korach, K.S. Estrogen receptors and human disease. J. Clin Invest. 2006, 116, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Galluzzo, P.; Ascenzi, P. Estrogen signaling multiple pathways to impact gene transcription. Curr. Genomics 2006, 7, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Xu, L.; Fang, H.; Hong, H.; Perkins, R.; Harris, S.; Bearden, E.D.; Shi, L.; Tong, W. The EDKB: An established knowledge base for endocrine disrupting chemicals. BMC Bioinform. 2010, 11 (Suppl. 6). [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Branham, W.S.; Dial, S.L.; Moland, C.L.; Fang, H.; Shen, J.; Perkins, R.; Sheehan, D.; Tong, W. Rat α-Fetoprotein binding affinities of a large set of structurally diverse chemicals elucidated the relationships between structures and binding affinities. Chem. Res. Toxicol. 2012, 25, 2553–2566. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Xu, L.; Fang, H.; Richard, A.M.; Bray, J.D.; Judson, R.S.; Zhou, G.; Colatsky, T.J.; Aungst, J.L.; Teng, C.; et al. EADB: An estrogenic activity database for assessing potential endocrine activity. Toxicol. Sci. 2013, 135, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Branham, W.S.; Ng, H.W.; Moland, C.L.; Dial, S.L.; Fang, H.; Perkins, R.; Sheehan, D.; Tong, W. Human sex hormone-binding globulin binding affinities of 125 structurally diverse chemicals and comparison with their binding to androgen receptor, estrogen receptor, and α-fetoprotein. Toxicol. Sci. 2015, 143, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Fang, H.; Xie, Q.; Perkins, R.; Sheehan, D.M.; Tong, W. Comparative molecular field analysis (CoMFA) model using a large diverse set of natural, synthetic and environmental chemicals for binding to the androgen receptor. SAR QSAR Environ. Res. 2003, 14, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Tong, W.; Xie, Q.; Fang, H.; Perkins, R. An in silico ensemble method for lead discovery: Decision forest. SAR QSAR Environ. Res. 2005, 16, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, W.; Fang, H.; Perkins, R.; Tong, W.; Hong, H. Homology modeling, molecular docking, and molecular dynamics simulations elucidated α-fetoprotein binding modes. BMC Bioinform. 2013, 14 (Suppl. 14). [Google Scholar] [CrossRef] [PubMed]
- Ng, H.W.; Doughty, S.W.; Luo, H.; Ye, H.; Ge, W.; Tong, W.; Hong, H. Development and validation of decision forest model for estrogen receptor binding prediction of chemicals using large data sets. Chem. Res. Toxicol. 2015, 28, 2343–2351. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.W.; Shu, M.; Luo, H.; Ye, H.; Ge, W.; Perkins, R.; Tong, W.; Hong, H. Estrogenic activity data extraction and in silico prediction show the endocrine disruption potential of bisphenol a replacement compounds. Chem. Res. Toxicol. 2015, 28, 1784–1795. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Rua, D.; Sakkiah, S.; Selvaraj, C.; Ge, W.; Tong, W. Consensus modeling for prediction of estrogenic activity of ingredients commonly used in sunscreen products. Int J. Environ. Res. Public Health 2016, 13. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Harvey, B.G.; Palmese, G.R.; Stanzione, J.F.; Ng, H.W.; Sakkiah, S.; Tong, W.; Sadler, J.M. Experimental data extraction and in silico prediction of the estrogenic activity of renewable replacements for bisphenol A. Int J. Environ. Res. Public Health 2016, 13. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Shen, J.; Ng, H.W.; Sakkiah, S.; Ye, H.; Ge, W.; Gong, P.; Xiao, W.; Tong, W. A rat α-fetoprotein binding activity prediction model to facilitate assessment of the endocrine disruption potential of environmental chemicals. Int. J. Environ. Res. Public Health 2016, 13. [Google Scholar] [CrossRef] [PubMed]
- Sakkiah, S.; Selvaraj, C.; Gong, P.; Zhang, C.; Tong, W.; Hong, H. Development of estrogen receptor beta binding prediction model using large sets of chemicals. Oncotarget 2017, 8, 92989–93000. [Google Scholar] [CrossRef] [PubMed]
- MacGregor, J.I.; Jordan, V.C. Basic guide to the mechanisms of antiestrogen action. Pharmacol. Rev. 1998, 50, 151–196. [Google Scholar] [PubMed]
- Chambon, P. The nuclear receptor superfamily: A personal retrospect on the first two decades. Mol. Endocrinol. 2005, 19, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Heine, P.A.; Taylor, J.A.; Iwamoto, G.A.; Lubahn, D.B.; Cooke, P.S. Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc. Natl. Acad. Sci. USA 2000, 97, 12729–12734. [Google Scholar] [CrossRef] [PubMed]
- Naaz, A.; Zakroczymski, M.; Heine, P.; Taylor, J.; Saunders, P.; Lubahn, D.; Cooke, P.S. Effect of ovariectomy on adipose tissue of mice in the absence of estrogen receptor alpha (ERα): A potential role for estrogen receptor beta (ERβ). Horm. Metab. Res. 2002, 34, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.E.; Neinast, M.D.; Sun, K.; Skiles, W.M.; Bills, J.D.; Zehr, J.A.; Zeve, D.; Hahner, L.D.; Cox, D.W.; Gent, L.M.; et al. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol. Metab. 2013, 2, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Bluher, M. Adipose tissue dysfunction in obesity. Exp. Clin. Endocrinol. Diabetes 2009, 117, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Barros, R.P.; Machado, U.F.; Gustafsson, J.A. Estrogen receptors: New players in diabetes mellitus. Trends Mol. Med. 2006, 12, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Heindel, J.J.; Newbold, R.; Schug, T.T. Endocrine disruptors and obesity. Nat. Rev. Endocrinol. 2015, 11, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Giordano Attianese, G.M.; Desvergne, B. Integrative and systemic approaches for evaluating PPARβ/δ (PPARD) function. Nucl. Recept. Signal. 2015, 13. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Waki, H.; Murakami, K.; Motojima, K.; Komeda, K.; Ide, T.; Kubota, N.; Terauchi, Y.; Tobe, K.; et al. The mechanisms by which both heterozygous peroxisome proliferator-activated receptor γ (PPARγ) deficiency and PPARγ agonist improve insulin resistance. J. Biol. Chem. 2001, 276, 41245–41254. [Google Scholar] [CrossRef] [PubMed]
- Lapinskas, P.J.; Brown, S.; Leesnitzer, L.M.; Blanchard, S.; Swanson, C.; Cattley, R.C.; Corton, J.C. Role of PPARα in mediating the effects of phthalates and metabolites in the liver. Toxicology 2005, 207, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Casals-Casas, C.; Feige, J.N.; Desvergne, B. Interference of pollutants with PPARs: Endocrine disruption meets metabolism. Int. J. Obes. 2008, 32 (Suppl. 6), S53–S61. [Google Scholar] [CrossRef] [PubMed]
- Hurst, C.H.; Waxman, D.J. Activation of PPARα and PPARγ by environmental phthalate monoesters. Toxicol. Sci. 2003, 74, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Funder, J.W. Glucocorticoid and mineralocorticoid receptors: Biology and clinical relevance. Annu. Rev. Med. 1997, 48, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.U.; Hitman, G.A.; Kopelman, P.G. An association between a Bc1I restriction fragment length polymorphism of the glucocorticoid receptor locus and hyperinsulinaemia in obese women. J. Mol. Endocrinol. 1992, 9, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Munck, A.; Mendel, D.B.; Smith, L.I.; Orti, E. Glucocorticoid receptors and actions. Am. Rev. Respir. Dis. 1990, 141, S2–S10. [Google Scholar] [PubMed]
- Fuller, P.J.; Young, M.J. Mechanisms of mineralocorticoid action. Hypertension 2005, 46, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Delyani, J.A. Mineralocorticoid receptor antagonists: the evolution of utility and pharmacology. Kidney Int. 2000, 57, 1408–1411. [Google Scholar] [CrossRef] [PubMed]
- Barnes, B.J.; Howard, P.A. Eplerenone: A selective aldosterone receptor antagonist for patients with heart failure. Ann. Pharmacother. 2005, 39, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Newton, K.M.; Knopp, R.H. Gestational diabetes and the incidence of type 2 diabetes: A systematic review. Diabetes Care 2002, 25, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- Al-Trad, B.; Ashankyty, I.M.; Alaraj, M. Progesterone ameliorates diabetic nephropathy in streptozotocin-induced diabetic rats. Diabetol. Metab. Syndr. 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Bellance, C.; Khan, J.A.; Meduri, G.; Guiochon-Mantel, A.; Lombès, M.; Loosfelt, H. Progesterone receptor isoforms PRA and PRB differentially contribute to breast cancer cell migration through interaction with focal adhesion kinase complexes. Mol. Biol. Cell 2013, 24, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Satta, E.; Magno, C.; Galì, A.; Inferrera, A.; Granese, R.; Aloisi, C.; Buemi, M.; Bellinghieri, G.; Santoro, D. Sexual dysfunction in women with diabetic kidney. Int. J. Endocrinol. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Bargiota, A.; Dimitropoulos, K.; Tzortzis, V.; Koukoulis, G.N. Sexual dysfunction in diabetic women. Hormones 2011, 10, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Monami, M.; Rastrelli, G.; Aversa, A.; Sforza, A.; Lenzi, A.; Forti, G.; Mannucci, E.; Maggi, M. Type 2 diabetes mellitus and testosterone: A meta-analysis study. Int. J. Androl. 2011, 34, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Chandra, V.; Rastinejad, F. Structural overview of the nuclear receptor superfamily: Insights into physiology and therapeutics. Annu. Rev. Physiol. 2010, 72, 247–272. [Google Scholar] [CrossRef] [PubMed]
- Lubahn, D.B.; Joseph, D.R.; Sullivan, P.M.; Willard, H.F.; French, F.S.; Wilson, E.M. Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science 1988, 240, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.; Faber, P.W.; van Rooij, H.C.; van der Korput, J.A.; Ris-Stalpers, C.; Klaassen, P.; Trapman, J.; Brinkmann, A.O. Structural organization of the human androgen receptor gene. J. Mol. Endocrinol. 1989, 2, R1–R4. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.H.; Li, J.; Xu, H.E.; Melcher, K.; Yong, E.L. Androgen receptor: Structure, role in prostate cancer and drug discovery. Acta Pharmacol. Sin. 2015, 36, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Centenera, M.M.; Harris, J.M.; Tilley, W.D.; Butler, L.M. The contribution of different androgen receptor domains to receptor dimerization and signaling. Mol. Endocrinol. 2008, 22, 2373–2382. [Google Scholar] [CrossRef] [PubMed]
- Bohl, C.E.; Gao, W.; Miller, D.D.; Bell, C.E.; Dalton, J.T. Structural basis for antagonism and resistance of bicalutamide in prostate cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 6201–6206. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Kemppainen, J.A.; Voegel, J.J.; Gronemeyer, H.; Wilson, E.M. Activation function 2 in the human androgen receptor ligand binding domain mediates interdomain communication with the NH2-terminal domain. J. Biol. Chem. 1999, 274, 37219–37225. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, C.; Watanabe, H.; Tanaka, S. An interpretation of positional displacement of the helix12 in nuclear receptors: Preexistent swing-up motion triggered by ligand binding. Biochim. Biophys. Acta 2010, 1804, 1832–1840. [Google Scholar] [CrossRef] [PubMed]
- Grosdidier, S.; Carbo, L.R.; Buzon, V.; Brooke, G.; Nguyen, P.; Baxter, J.D.; Bevan, C.; Webb, P.; Estebanez-Perpina, E.; Fernandez-Recio, J. Allosteric conversation in the androgen receptor ligand-binding domain surfaces. Mol. Endocrinol. 2012, 26, 1078–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cano, L.Q.; Lavery, D.N.; Bevan, C.L. Mini-review: Foldosome regulation of androgen receptor action in prostate cancer. Mol. Cell. Endocrinol. 2013, 369, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Prescott, J.; Coetzee, G.A. Molecular chaperones throughout the life cycle of the androgen receptor. Cancer Lett. 2006, 231, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Stocco, D.M.; Clark, B.J. Regulation of the acute production of steroids in steroidogenic cells. Endocr. Rev. 1996, 17, 221–244. [Google Scholar] [PubMed]
- Baulieu, E.E.; Lasnitzki, I.; Robel, P. Metabolism of testosterone and action of metabolites on prostate glands grown in organ culture. Nature 1968, 219, 1155–1156. [Google Scholar] [CrossRef] [PubMed]
- Jasuja, R.; Ulloor, J.; Yengo, C.M.; Choong, K.; Istomin, A.Y.; Livesay, D.R.; Jacobs, D.J.; Swerdloff, R.S.; Miksovska, J.; Larsen, R.W.; et al. Kinetic and thermodynamic characterization of dihydrotestosterone-induced conformational perturbations in androgen receptor ligand-binding domain. Mol. Endocrinol. 2009, 23, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Estébanez-Perpiñá, E.; Arnold, L.A.; Nguyen, P.; Rodrigues, E.D.; Mar, E.; Bateman, R.; Pallai, P.; Shokat, K.M.; Baxter, J.D.; Guy, R.K.; et al. A surface on the androgen receptor that allosterically regulates coactivator binding. Proc. Natl. Acad. Sci. USA 2007, 104, 16074–16079. [Google Scholar] [CrossRef] [PubMed]
- Matias, P.M.; Donner, P.; Coelho, R.; Thomaz, M.; Peixoto, C.; Macedo, S.; Otto, N.; Joschko, S.; Scholz, P.; Wegg, A.; et al. Structural evidence for ligand specificity in the binding domain of the human androgen receptor: Implications for pathogenic gene mutations. J. Biol. Chem. 2000, 275, 26164–26171. [Google Scholar] [CrossRef] [PubMed]
- Pereira de Jésus-Tran, K.; Côté, P.-L.; Cantin, L.; Blanchet, J.; Labrie, F.; Breton, R. Comparison of crystal structures of human androgen receptor ligand-binding domain complexed with various agonists reveals molecular determinants responsible for binding affinity. Protein Sci. 2006, 15, 987–999. [Google Scholar] [CrossRef] [PubMed]
- Axerio-Cilies, P.; Lack, N.A.; Nayana, M.R.; Chan, K.H.; Yeung, A.; Leblanc, E.; Guns, E.S.; Rennie, P.S.; Cherkasov, A. Inhibitors of androgen receptor activation function-2 (AF2) site identified through virtual screening. J. Med. Chem. 2011, 54, 6197–6205. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.H.E.; Li, J.; Xu, H.E.; Melcher, K.; Yong, E.-L. Androgen receptor: Structure, role in prostate cancer and drug discovery. Acta Pharmacol. Sin. 2015, 36, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Ryu, B.J.; Kim, N.; Kim, J.T.; Koo, T.S.; Yoo, S.E.; Jeong, S.H.; Kim, S.H.; Kang, N.S. Discovery of non-LBD inhibitor for androgen receptor by structure-guide design. Bioorg. Med. Chem. Lett. 2013, 23, 3887–3890. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Clegg, N.J.; Scher, H.I. Anti-androgens and androgen-depleting therapies in prostate cancer: New agents for an established target. Lancet Oncol. 2009, 10, 981–991. [Google Scholar] [CrossRef]
- Singh, S.M.; Gauthier, S.; Labrie, F. Androgen receptor antagonists (antiandrogens): Structure-activity relationships. Curr. Med. Chem. 2000, 7, 211–247. [Google Scholar] [CrossRef] [PubMed]
- Lack, N.A.; Axerio-Cilies, P.; Tavassoli, P.; Han, F.Q.; Chan, K.H.; Feau, C.; LeBlanc, E.; Guns, E.T.; Guy, R.K.; Rennie, P.S.; et al. Targeting the binding function 3 (BF3) site of the human androgen receptor through virtual screening. J. Med. Chem. 2011, 54, 8563–8573. [Google Scholar] [CrossRef] [PubMed]
- Sopiko, K.; Keti, T.; Salome, K.; Natia, C.; Dimitri, K.; Manana, K. Are androgens valuable in management of diabetes? Curr. Res. Diabetes Obes. J. 2017, 2, 1–4. [Google Scholar]
- Oh, J.Y.; Barrett-Connor, E.; Wedick, N.M.; Wingard, D.L. Endogenous sex hormones and the development of type 2 diabetes in older men and women: The Rancho Bernardo study. Diabetes Care 2002, 25, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Mannucci, E.; Petrone, L.; Ricca, V.; Balercia, G.; Mansani, R.; Chiarini, V.; Giommi, R.; Forti, G.; Maggi, M. Association of hypogonadism and type II diabetes in men attending an outpatient erectile dysfunction clinic. Int. J. Impot. Res. 2006, 18, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Goodwin, E.; Channer, K.S.; Jones, T.H. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur. J. Endocrinol. 2006, 154, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Fukui, M.; Soh, J.; Tanaka, M.; Kitagawa, Y.; Hasegawa, G.; Yoshikawa, T.; Miki, T.; Nakamura, N. Low serum testosterone concentration in middle-aged men with type 2 diabetes. Endocr. J. 2007, 54, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Selvin, E.; Feinleib, M.; Zhang, L.; Rohrmann, S.; Rifai, N.; Nelson, W.G.; Dobs, A.; Basaria, S.; Golden, S.H.; Platz, E.A. Androgens and diabetes in men: Results from the Third National Health and Nutrition Examination Survey (NHANES III). Diabetes Care 2007, 30, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Stanworth, R.D.; Jones, T.H. Testosterone in obesity, metabolic syndrome and type 2 diabetes. Front. Horm. Res. 2009, 37, 74–90. [Google Scholar] [PubMed]
- Yeap, B.B.; Hyde, Z.; Almeida, O.P.; Norman, P.E.; Chubb, S.A.; Jamrozik, K.; Flicker, L.; Hankey, G.J. Lower testosterone levels predict incident stroke and transient ischemic attack in older men. J. Clin. Endocrinol. Metab. 2009, 94, 2353–2359. [Google Scholar] [CrossRef] [PubMed]
- Ding, E.L.; Song, Y.; Malik, V.S.; Liu, S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA 2006, 295, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- Lage, M.J.; Barber, B.L.; Markus, R.A. Association between androgen-deprivation therapy and incidence of diabetes among males with prostate cancer. Urology 2007, 70, 1104–1108. [Google Scholar] [CrossRef] [PubMed]
- Starling, A.P.; Umbach, D.M.; Kamel, F.; Long, S.; Sandler, D.P.; Hoppin, J.A. Pesticide use and incident diabetes among wives of farmers in the Agricultural Health Study. Occup. Environ. Med. 2014, 71, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Saldana, T.M.; Basso, O.; Hoppin, J.A.; Baird, D.D.; Knott, C.; Blair, A.; Alavanja, M.C.; Sandler, D.P. Pesticide exposure and self-reported gestational diabetes mellitus in the Agricultural Health Study. Diabetes Care 2007, 30, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.P.; Kamel, F.; Saldana, T.M.; Alavanja, M.C.; Sandler, D.P. Incident diabetes and pesticide exposure among licensed pesticide applicators: Agricultural Health Study, 1993–2003. Am. J. Epidemiol. 2008, 167, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Gram, D.X.; Ahren, B.; Nagy, I.; Olsen, U.B.; Brand, C.L.; Sundler, F.; Tabanera, R.; Svendsen, O.; Carr, R.D.; Santha, P.; et al. Capsaicin-sensitive sensory fibers in the islets of Langerhans contribute to defective insulin secretion in Zucker diabetic rat, an animal model for some aspects of human type 2 diabetes. Eur. J. Neurosci. 2007, 25, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Kobori, M.; Masumoto, S.; Akimoto, Y.; Takahashi, Y. Dietary quercetin alleviates diabetic symptoms and reduces streptozotocin-induced disturbance of hepatic gene expression in mice. Mol. Nutr. Food Res. 2009, 53, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Palsamy, P.; Subramanian, S. Resveratrol, a natural phytoalexin, normalizes hyperglycemia in streptozotocin-nicotinamide induced experimental diabetic rats. Biomed. Pharmacother. 2008, 62, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Bodin, J.; Bolling, A.K.; Becher, R.; Kuper, F.; Lovik, M.; Nygaard, U.C. Transmaternal bisphenol A exposure accelerates diabetes type 1 development in NOD mice. Toxicol. Sci. 2014, 137, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Riachy, R.; Vandewalle, B.; Belaich, S.; Kerr-Conte, J.; Gmyr, V.; Zerimech, F.; d’Herbomez, M.; Lefebvre, J.; Pattou, F. Beneficial effect of 1,25 dihydroxyvitamin D3 on cytokine-treated human pancreatic islets. J. Endocrinol. 2001, 169, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Silver, M.K.; O’Neill, M.S.; Sowers, M.R.; Park, S.K. Urinary bisphenol A and type-2 diabetes in U.S. adults: Data from NHANES 2003–2008. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Ae Park, S.; Choi, M.S.; Cho, S.Y.; Seo, J.S.; Jung, U.J.; Kim, M.J.; Sung, M.K.; Park, Y.B.; Lee, M.K. Genistein and daidzein modulate hepatic glucose and lipid regulating enzyme activities in C57BL/KsJ-db/db mice. Life Sci. 2006, 79, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, J.; Ungvari, Z.; Zhang, C. Resveratrol improves endothelial function: Role of TNFα and vascular oxidative stress. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Ramadori, G.; Gautron, L.; Fujikawa, T.; Vianna, C.R.; Elmquist, J.K.; Coppari, R. Central administration of resveratrol improves diet-induced diabetes. Endocrinology 2009, 150, 5326–5333. [Google Scholar] [CrossRef] [PubMed]
- Catanuto, P.; Doublier, S.; Lupia, E.; Fornoni, A.; Berho, M.; Karl, M.; Striker, G.E.; Xia, X.; Elliot, S. 17 β-estradiol and tamoxifen upregulate estrogen receptor β expression and control podocyte signaling pathways in a model of type 2 diabetes. Kidney Int. 2009, 75, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Anjaneyulu, M.; Kulkarni, S.K.; Chopra, K. Resveratrol, a polyphenolic phytoalexin, attenuates diabetic nephropathy in rats. Pharmacology 2006, 76, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Tong, W.; Branham, W.S.; Moland, C.L.; Dial, S.L.; Hong, H.; Xie, Q.; Perkins, R.; Owens, W.; Sheehan, D.M. Study of 202 natural, synthetic, and environmental chemicals for binding to the androgen receptor. Chem. Res. Toxicol. 2003, 16, 1338–1358. [Google Scholar] [CrossRef] [PubMed]
- Stoker, T.E.; Laws, S.C.; Guidici, D.L.; Cooper, R.L. The effect of atrazine on puberty in male wistar rats: An evaluation in the protocol for the assessment of pubertal development and thyroid function. Toxicol. Sci. 2000, 58, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Rylander, L.; Rignell-Hydbom, A.; Hagmar, L. A cross-sectional study of the association between persistent organochlorine pollutants and diabetes. Environ. Health 2005, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Pesatori, A.C.; Zocchetti, C.; Guercilena, S.; Consonni, D.; Turrini, D.; Bertazzi, P.A. Dioxin exposure and non-malignant health effects: A mortality study. Occup. Environ. Med. 1998, 55, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Remillard, R.B.; Bunce, N.J. Linking dioxins to diabetes: Epidemiology and biologic plausibility. Environ. Health Perspect. 2002, 110, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lind, P.M.; Jacobs, D.R., Jr.; Salihovic, S.; van Bavel, B.; Lind, L. Polychlorinated biphenyls and organochlorine pesticides in plasma predict development of type 2 diabetes in the elderly: the prospective investigation of the vasculature in Uppsala Seniors (PIVUS) study. Diabetes Care 2011, 34, 1778–1784. [Google Scholar] [CrossRef] [PubMed]
- Kogevinas, M. Human health effects of dioxins: Cancer, reproductive and endocrine system effects. Hum. Reprod Update 2001, 7, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Enan, E.; Liu, P.C.; Matsumura, F. TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin) causes reduction in glucose uptake through glucose transporters on the plasma membrane of the guinea pig adipocyte. J. Environ. Sci. Health B 1992, 27, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Pournourmohammadi, S.; Farzami, B.; Ostad, S.N.; Azizi, E.; Abdollahi, M. Effects of malathion subchronic exposure on rat skeletal muscle glucose metabolism. Environ. Toxicol. Pharmacol. 2005, 19, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.; Donyavi, M.; Pournourmohammadi, S.; Saadat, M. Hyperglycemia associated with increased hepatic glycogen phosphorylase and phosphoenolpyruvate carboxykinase in rats following subchronic exposure to malathion. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2004, 137, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Abu-Basha, E.A.; Yibchok-Anun, S.; Hopper, D.L.; Hsu, W.H. Effects of the pesticide amitraz and its metabolite BTS 27271 on insulin and glucagon secretion from the perfused rat pancreas: Involvement of α2D-adrenergic receptors. Metabolism 1999, 48, 1461–1469. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Kim, Y.S.; Ryu, S.Y.; Cha, M.R.; Yon, G.H.; Yang, H.J.; Kim, M.J.; Kang, S.; Park, S. Capsiate improves glucose metabolism by improving insulin sensitivity better than capsaicin in diabetic rats. J. Nutr. Biochem. 2013, 24, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Makishima, M.; Hashimoto, Y. Development of silicon-containing bis-phenol derivatives as androgen receptor antagonists: Selectivity switching by C/Si exchange. Bioorg. Med. Chem. 2013, 21, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Kaeding, J.; Belanger, J.; Caron, P.; Verreault, M.; Belanger, A.; Barbier, O. Calcitrol (1α,25-dihydroxyvitamin D3) inhibits androgen glucuronidation in prostate cancer cells. Mol. Cancer Ther. 2008, 7, 380–390. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Report on Diabetes. Available online: http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf (accessed on 19 October 2017).
- American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care 2015, 38, S8–S16. [Google Scholar]
- Nishizaki, Y.; Ishimoto, Y.; Hotta, Y.; Hosoda, A.; Yoshikawa, H.; Akamatsu, M.; Tamura, H. Effect of flavonoids on androgen and glucocorticoid receptors based on in vitro reporter gene assay. Bioorg. Med. Chem. Lett. 2009, 19, 4706–4710. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, N.; Barma, S.; Konwar, N.; Dewanjee, S.; Manna, P. Mechanistic insight of diabetic nephropathy and its pharmacotherapeutic targets: An update. Eur. J. Pharmacol. 2016, 791, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Elmarakby, A.A.; Sullivan, J.C. Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovasc. Ther. 2012, 30, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.S.; Ko, G.J.; Lee, M.H.; Song, H.K.; Han, S.Y.; Han, K.H.; Kim, H.K.; Han, J.Y.; Cha, D.R. Effect of eplerenone, enalapril and their combination treatment on diabetic nephropathy in type II diabetic rats. Nephrol. Dial. Transplant. 2009, 24, 73–84. [Google Scholar] [CrossRef] [PubMed]


| Cluster | MESH ID | Diabetes Mellitus Type | Description | Chemical [reference*] |
|---|---|---|---|---|
| 1 | D003920 | Diabetes mellitus | Production of excess glucose level in the blood for a long term | 2,4,5-Trichlorophenoxyacetic acid [92,93], aldrin [94], atrazine [93,94], dieldrin [92,94], heptachlor [94] |
| 2 | D003921 | Diabetes mellitus, experimental | Experimentally induces diabetes mellitus by various diabetogenic agents | Capsaicin [95], quercetin [96], resveratrol [97] |
| 3 | D003922 | Diabetes mellitus, type 1 | Insulin-dependent diabetes mellitus—failed to produce enough insulin | Bisphenol A [98], calcitriol [99] |
| 4 | D003924 | Diabetes mellitus, type 2 | Non-insulin-dependent diabetes mellitus—Resistance to insulin production | Bisphenol A [100], genistein [101], resveratrol [102,103] |
| 5 | D003928 | Diabetic nephropathies | Diabetes mellitus leads to kidney failure | Estradiol [104], resveratrol [105] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakkiah, S.; Wang, T.; Zou, W.; Wang, Y.; Pan, B.; Tong, W.; Hong, H. Endocrine Disrupting Chemicals Mediated through Binding Androgen Receptor Are Associated with Diabetes Mellitus. Int. J. Environ. Res. Public Health 2018, 15, 25. https://doi.org/10.3390/ijerph15010025
Sakkiah S, Wang T, Zou W, Wang Y, Pan B, Tong W, Hong H. Endocrine Disrupting Chemicals Mediated through Binding Androgen Receptor Are Associated with Diabetes Mellitus. International Journal of Environmental Research and Public Health. 2018; 15(1):25. https://doi.org/10.3390/ijerph15010025
Chicago/Turabian StyleSakkiah, Sugunadevi, Tony Wang, Wen Zou, Yuping Wang, Bohu Pan, Weida Tong, and Huixiao Hong. 2018. "Endocrine Disrupting Chemicals Mediated through Binding Androgen Receptor Are Associated with Diabetes Mellitus" International Journal of Environmental Research and Public Health 15, no. 1: 25. https://doi.org/10.3390/ijerph15010025





