Biometeorological Assessment of Mortality Related to Extreme Temperatures in Helsinki Region, Finland, 1972–2014
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mortality Data in the Study Area
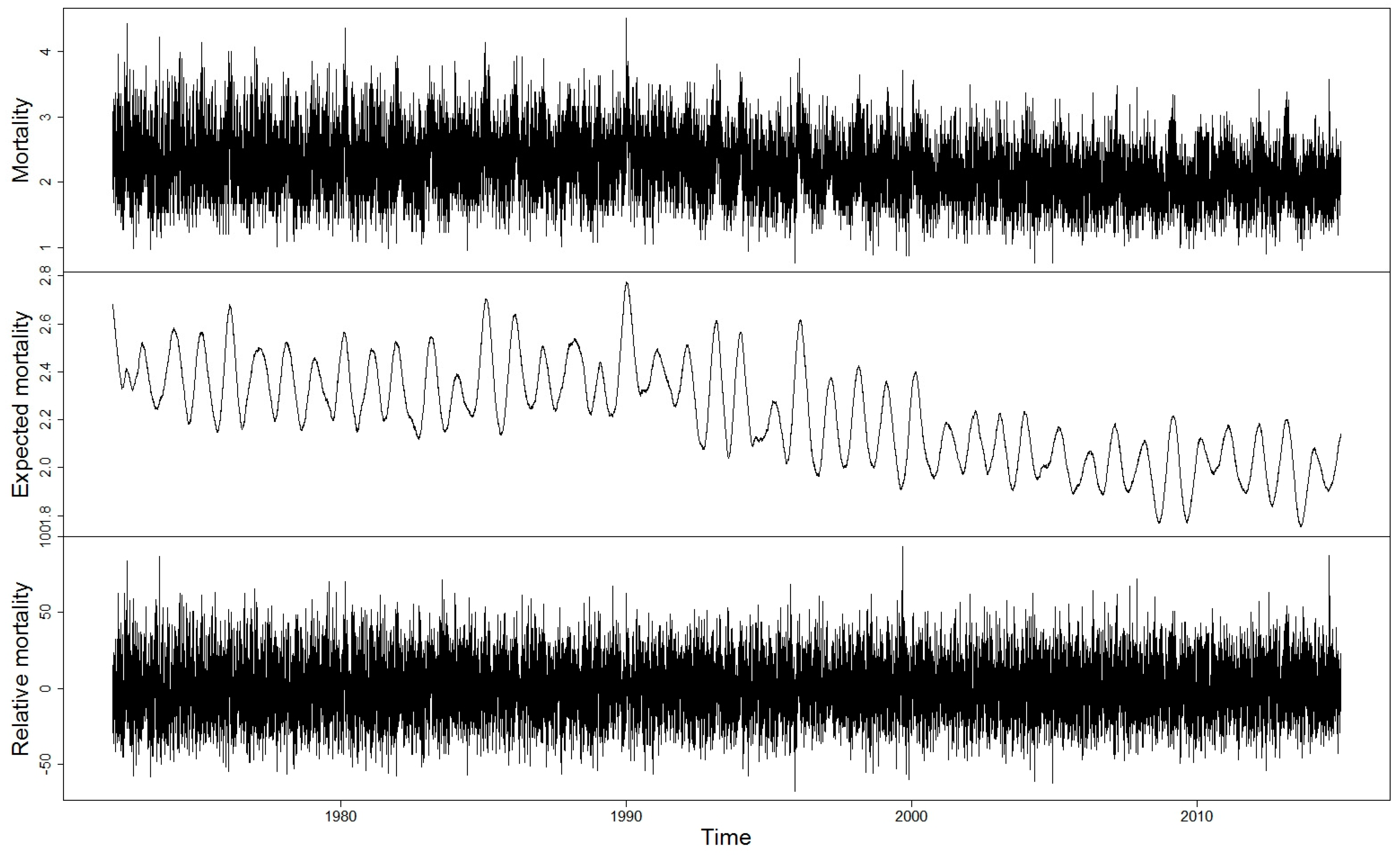
2.2. Expected and Relative Mortality
2.3. Meteorological Data
2.4. Assessing the Relationship between Relative Mortality and Thermal Indices
3. Results
3.1. Trends in the Thermal Indices and Mortality Data
3.2. Relationships between Relative Mortality and Thermal Indices
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Armstrong, B. Models for the relationship between ambient temperature and daily mortality. Epidemiology 2006, 17, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Kovats, R.S.; Hajat, S. Heat stress and public health: A critical review. Annu. Rev. Public Health 2008, 29, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Ballester, J.; Robine, J.-M.; Herrmann, F.R.; Rodó, X. Long-term projections and acclimatization scenarios of temperature-related mortality in Europe. Nat. Commun. 2011, 2, 358. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Samet, J.M. Relation between elevated ambient temperature and mortality: A review of the epidemiologic evidence. Epidemiol. Rev. 2002, 24, 190–202. [Google Scholar] [CrossRef] [PubMed]
- McMichael, A.J.; Woodruff, R.E.; Hales, S. Climate change and human health: Present and future risks. Lancet 2006, 367, 859–869. [Google Scholar] [CrossRef]
- Keatinge, W.R.; Donaldson, G.C.; Cordioli, E.; Martinelli, M.; Kunst, A.E.; Mackenbach, J.P.; Näyhä, S.; Vuori, I. Heat related mortality in warm and cold regions of Europe: Observational study. BMJ 2000, 321, 670–673. [Google Scholar] [CrossRef] [PubMed]
- Näyhä, S. Environmental temperature and mortality. Int. J. Circumpolar Health 2005, 64, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Näyhä, S. Heat mortality in Finland in the 2000s. Int. J. Circumpolar Health 2007, 66, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Robine, J.M.; Cheung, S.L.K.; Le Roy, S.; Van Oyen, H.; Griffiths, C.; Michel, J.P.; Herrmann, F.R. Death toll exceeded 70,000 in Europe during the summer of 2003. C. R. Biol. 2008, 331, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Barriopedro, D.; Fischer, E.M.; Luterbacher, J.; Trigo, R.M.; García-Herrera, R. The hot summer of 2010: Redrawing the temperature record map of Europe. Science 2011, 332, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Kollanus, V.; Lanki, T. 2000-luvun pitkittyneiden helleaaltojen kuolleisuusvaikutukset Suomessa. (Mortality effects of prolonged heat waves in the 2000s in Finland. Summary in English). Duodecim 2014, 130, 983–990. [Google Scholar] [PubMed]
- Gasparrini, A.; Guo, Y.; Hashizume, M.; Lavigne, E.; Zanobetti, A.; Schwartz, J.; Tobias, A.; Tong, S.; Rocklöv, J.; Forsberg, B.; et al. Mortality risk attributable to high and low ambient temperature: A multicountry observational study. Lancet 2015, 386, 369–375. [Google Scholar] [CrossRef]
- Anderson, B.G.; Bell, M.L. Weather-Related Mortality. Epidemiology 2009, 20, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Rocklöv, J.; Forsberg, B. The effect of temperature on mortality in Stockholm 1998–2003: A study of lag structures and heatwave effects. Scand. J. Public Health 2008, 36, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Mengersen, K.; Wang, X.; Ye, X.; Guo, Y.; Pan, X.; Tong, S. Daily average temperature and mortality among the elderly: A meta-analysis and systematic review of epidemiological evidence. Int. J. Biometeorol. 2012, 56, 569–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebi, K.L.; Mills, D. Winter mortality in a warming climate: A reassessment. Wiley Interdiscip. Rev. Clim. Chang. 2013, 4, 203–212. [Google Scholar] [CrossRef]
- Bao, J.; Li, X.; Yu, C. The construction and validation of the heat vulnerability index, a review. Int. J. Environ. Res. Public Health 2015, 12, 7220–7234. [Google Scholar] [CrossRef] [PubMed]
- Carter, T.R.; Fronzek, S.; Inkinen, A.; Lahtinen, I.; Lahtinen, M.; Mela, H.; O’Brien, K.L.; Rosentrater, L.D.; Ruuhela, R.; Simonsson, L.; et al. Characterising vulnerability of the elderly to climate change in the Nordic region. Reg. Environ. Chang. 2016, 16. [Google Scholar] [CrossRef]
- Oudin Åström, D.; Forsberg, B.; Rocklöv, J. Heat wave impact on morbidity and mortality in the elderly population: A review of recent studies. Maturitas 2011, 69, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Bunker, A.; Wildenhain, J.; Vandenbergh, A.; Henschke, N.; Rocklöv, J.; Hajat, S.; Sauerborn, R. Effects of Air Temperature on Climate-Sensitive Mortality and Morbidity Outcomes in the Elderly; a Systematic Review and Meta-analysis of Epidemiological Evidence. EBioMedicine 2016, 6, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Koppe, C.; Jendritzky, G. Inclusion of short-term adaptation to thermal stresses in a heat load warning procedure. Meteorol. Z. 2005, 14, 271–278. [Google Scholar] [CrossRef]
- Lee, M.; Nordio, F.; Zanobetti, A.; Kinney, P.; Vautard, R.; Schwartz, J. Acclimatization across space and time in the effects of temperature on mortality: A time-series analysis. Environ. Health 2014, 13, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oudin Åström, D.; Tornevi, A.; Ebi, K.L.; Rocklöv, J.; Forsberg, B. Evolution of minimum mortality temperature in Stockholm, Sweden, 1901–2009. Environ. Health Perspect. 2016, 124, 740–744. [Google Scholar] [CrossRef]
- Oudin Åström, D.; Forsberg, B.; Ebi, K.L.; Rocklöv, J. Attributing mortality from extreme temperatures to climate change in Stockholm, Sweden. Nat. Clim. Chang. 2013, 3, 1050–1054. [Google Scholar] [CrossRef]
- Smith, K.R.; Woodward, A.; Campbell-Lendrum, D.; Chadee, D.; Honda, Y.; Liu, Q.; Olwoch, J.; Revich, B.; Sauerborn, R.; Dokken, D.; et al. Human Health: Impacts, Adaptation, and Co-Benefits. In Climate Change 2014: Impacts, Adaptation, and Vulnerability; Cambridge University Press: Cambridge, UK, 2014; pp. 709–754. [Google Scholar]
- Huang, C.; Barnett, A.G.; Wang, X.; Vaneckova, P.; FitzGerald, G.; Tong, S. Projecting Future Heat-Related Mortality under Climate Change Scenarios: A Systematic Review. Environ. Health Perspect. 2011, 119, 1681–1690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthers, S.; Matzarakis, A.; Koch, E. Climate change and mortality in Vienna—A human biometeorological analysis based on regional climate modeling. Int. J. Environ. Res. Public Health 2010, 7, 2965–2977. [Google Scholar] [CrossRef] [PubMed]
- Zacharias, S.; Koppe, C.; Mücke, H.-G. Climate Change Effects on Heat Waves and Future Heat Wave-Associated IHD Mortality in Germany. Climate 2015, 3, 100–117. [Google Scholar] [CrossRef]
- Höppe, P.R. Heat balance modelling. Experientia 1993, 49, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Verein Deutscher Ingenieure (VDI). Handbuch Reinhaltung der Luft Band 1: Umweltmeteorologie. In Methoden zur Human-Biometeorologischen Bewertung von Klima und Lufthygiene für die Stadt-und Regionalplanung; Beuth Verlag: Düsseldorf, Germany, 1998; pp. 25–27. [Google Scholar]
- Höppe, P. The physiological equivalent temperature—A universal index for the biometeorological assessment of the thermal environment. Int. J. Biometeorol. 1999, 43, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Matzarakis, A.; Mayer, H.; Iziomon, M.G. Application of a universal thermal index: Physiological equivalent temperature. Int. J. Biometeorol. 1999, 43, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Matzarakis, A.; Mayer, H. Heat stress in Greece. Int. J. Biometeorol. 1997, 41, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Matzarakis, A.; Rocco, M.; Najjar, G. Thermal bioclimate in Strasbourg—The 2003 heat wave. Theor. Appl. Climatol. 2009, 98, 209–220. [Google Scholar] [CrossRef]
- Jendritzky, G.; de Dear, R. Adaptation and Thermal Environment. In Biometeorology for Adaptation to Climate Variability and Change; Ebi, K.L., Burton, I., McGregor, G.R., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 9–32. [Google Scholar] [CrossRef]
- Matzarakis, A.; Endler, C. Climate change and thermal bioclimate in cities: Impacts and options for adaptation in Freiburg, Germany. Int. J. Biometeorol. 2010, 54, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Official Statistics of Finland (OSF). Deaths; Official Statistics of Finland: Helsinki, Finland, 2016; ISSN 1798-2545. Available online: http://tilastokeskus.fi/til/kuol/2015/01/kuol_2015_01_2016-10-28_tie_001_en.html (accessed on 7 March 2017).
- Matzarakis, A.; Rutz, F.; Mayer, H. Modelling radiation fluxes in simple and complex environments—Applications of the RayMan model. Int. J. Biometeorol. 2007, 51, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Matzarakis, A.; Rutz, F.; Mayer, H. Modelling radiation fluxes in simple and complex environments: Basics of the RayMan model. Int. J. Biometeorol. 2010, 54, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Aalto, J.; Pirinen, P.; Jylhä, K. New gridded daily climatology of Finland: Permutation-based uncertainty estimates and temporal trends in climate. J. Geophys. Res. Atmos. 2016, 121, 3807–3823. [Google Scholar] [CrossRef]
- Wood, S. Package ‘mgcv’. 2016. Available online: https://cran.r-project.org/web/packages/mgcv/mgcv.pdf. (accessed on 3 March 2016).
- Gasparrini, A.; Guo, Y.; Hashizume, M.; Kinney, P.L.; Petkova, E.P.; Lavigne, E.; Zanobetti, A.; Schwartz, J.D.; Tobias, A.; Leone, M.; et al. Temporal variation in heat–mortality associations: A multicountry study. Environ. Health Perspect. 2015, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mäkinen, T.M.; Raatikka, V.P.; Rytkönen, M.; Jokelainen, J.; Rintamäki, H.; Ruuhela, R.; Näyhä, S.; Hassi, J. Factors affecting outdoor exposure in winter: Population-based study. Int. J. Biometeorol. 2006, 51, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Mavrogianni, A.; Wilkinson, P.; Davies, M.; Biddulph, P.; Oikonomou, E. Building characteristics as determinants of propensity to high indoor summer temperatures in London dwellings. Build. Environ. 2012, 55, 117–130. [Google Scholar] [CrossRef]
- Taylor, J.; Wilkinson, P.; Davies, M.; Armstrong, B.; Chalabi, Z.; Mavrogianni, A.; Symonds, P.; Oikonomou, E.; Bohnenstengel, S.I. Mapping the effects of urban heat island, housing, and age on excess heat-related mortality in London. Urban Clim. 2015, 14, 517–528. [Google Scholar] [CrossRef]
- Arbuthnott, K.; Hajat, S.; Heaviside, C.; Vardoulakis, S. Changes in population susceptibility to heat and cold over time: Assessing adaptation to climate change. Environ. Health 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, G.C.; Keatinge, W.R.; Näyhä, S. Changes in summer temperature and heat-related mortality since 1971 in North Carolina, South Finland, and Southeast England. Environ. Res. 2003, 91, 1–7. [Google Scholar] [CrossRef]
- De’Donato, F.K.; Leone, M.; Scortichini, M.; De Sario, M.; Katsouyanni, K.; Lanki, T.; Basagaña, X.; Ballester, F.; Åström, C.; Paldy, A.; et al. Changes in the effect of heat on mortality in the last 20 years in nine European cities. Results from the PHASE project. Int. J. Environ. Res. Public Health 2015, 12, 15567–15583. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Sinclair, V.A.; Räisänen, J.; Ruuhela, R. Heat waves in Finland: Present and projected summertime extreme temperatures and their associated circulation patterns. Int. J. Climatol. 2017. [Google Scholar] [CrossRef]
- Ryti, N.R.I.; Mäkikyrö, E.M.S.; Antikainen, H.; Junttila, M.J. Cold spells and ischaemic sudden cardiac death: Effect modification by prior diagnosis of ischaemic heart disease and cardioprotective medication. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Morabito, M.; Crisci, A.; Messeri, A.; Capecchi, V.; Modesti, P.A.; Gensini, G.F.; Orlandini, S. Environmental temperature and thermal indices: What is the most effective predictor of heat-related mortality in different geographical contexts? Sci. World J. 2014, 2014, 961750. [Google Scholar] [CrossRef] [PubMed]


| Percentiles | PET Range | Relative Mortality 1972–2014 (%) | Trend (%/10 years) | t-Test | Relative Mortality 1972–1992 (%) | Relative Mortality 1994–2014 (%) | t-Test | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All | |||||||||||
| 0–1 | −42.0, −25.1 | 2.1 | (−0.2, 4.4) | 0.3 | (−1.6, 2.2) | 2.5 | (−0.6, 5.6) | 1.7 | (−1.7, 5.1) | ||
| 1–2.5 | −25.1, −20.9 | 1.9 | (0.2, 3.6) | −0.6 | (−2.0, 0.9) | 2.7 | (0.3, 5.0) | 0.7 | (−2.0, 3.3) | ||
| 2.5–5 | −20.9, −17.1 | 0.9 | (−0.5, 2.4) | −0.9 | (−2.1, 0.2) | 2.4 | (0.4, 4.3) | −0.4 | (−2.5, 1.7) | ||
| 5–10 | −17.1, −12.8 | 0.2 | (−0.8, 1.2) | 0.5 | (−0.3, 1.3) | −0.4 | (−1.8, 1.0) | 0.9 | (−0.7, 2.4) | ||
| 10–25 | −12.8, −6.9 | 0.4 | (−0.2, 0.9) | 0.1 | (−0.3, 0.6) | 0.3 | (−0.5, 1.2) | 0.4 | (−0.4, 1.2) | ||
| 25–50 | −6.9, 0.4 | −1.5 | (−1.9, −1.1) | 0.1 | (−0.2, 0.5) | −1.5 | (−2.2, −0.9) | −1.6 | (−2.2, −1.0) | ||
| 50–75 | 0.4, 11.3 | −0.9 | (−1.4, −0.5) | 0.3 | (−0.1, 0.6) | −1.3 | (−1.9, −0.7) | −0.6 | (−1.2, −0.1) | ||
| 75–90 | 11.3, 17.3 | 0.0 | (−0.6, 0.6) | −0.3 | (−0.8, 0.2) | 0.3 | (−0.6, 1.2) | −0.2 | (−1.0, 0.6) | ||
| 90–95 | 17.3, 20.4 | 2.5 | (1.5, 3.6) | −0.4 | (−1.2, 0.4) | 3.1 | (1.6, 4.7) | 2.1 | (0.8, 3.4) | ||
| 95–97.5 | 20.4, 22.6 | 4.4 | (2.9, 5.9) | −1.0 | (−2.2, 0.2) | 5.2 | (3.1, 7.4) | 3.5 | (1.4, 5.6) | ||
| 97.5–99 | 22.6, 24.5 | 7.2 | (5.2, 9.3) | −1.5 | (−3.2, 0.1) | 9.5 | (6.3, 12.8) | 5.9 | (3.2, 8.5) | ||
| 99–100 | 24.5, 29.4 | 11.7 | (8.9, 14.5) | −3.9 | (−6.0, −1.8) | *** | 18.3 | (12.4, 24.3) | 8.6 | (5.6, 11.5) | ** |
| Aged ≥75 years | |||||||||||
| 0–1 | −42.0, −25.1 | 5.2 | (2.5, 7.9) | −0.4 | (−2.7, 1.8) | 5.7 | (2.0, 9.3) | 4.7 | (0.6, 8.7) | ||
| 1–2.5 | −25.1, −20.9 | 2.1 | (−0.3, 4.5) | 0.3 | (−1.7, 2.2) | 2.1 | (−1.3, 5.4) | 1.8 | (−1.7, 5.2) | ||
| 2.5–5 | −20.9, −17.1 | 2.6 | (0.7, 4.5) | −2.0 | (−3.5, −0.5) | * | 4.9 | (2.1, 7.7) | 0.4 | (−2.2, 3.0) | * |
| 5–10 | −17.1, −12.8 | 0.0 | (−1.5, 1.4) | 0.6 | (−0.6, 1.7) | −0.9 | (−2.9, 1.2) | 0.9 | (−1.1, 3.0) | ||
| 10–25 | −12.8, −6.9 | 0.1 | (−0.7, 0.9) | 0.2 | (−0.5, 0.9) | 0.3 | (−1.0, 1.6) | 0.0 | (−1.0, 1.1) | ||
| 25–50 | −6.9, 0.4 | −2.3 | (−2.9, −1.7) | 0.3 | (−0.2, 0.8) | −2.5 | (−3.5, −1.6) | −2.2 | (−3.0, −1.4) | ||
| 50–75 | 0.4, 11.3 | −0.8 | (−1.4, −0.2) | 0.6 | (0.1, 1.1) | * | −1.6 | (−2.5, −0.6) | −0.1 | (−0.9, 0.79 | * |
| 75–90 | 11.3, 17.3 | 0.8 | (0.0, 1.6) | −0.4 | (−1.0, 0.3) | 1.4 | (0.1, 2.7) | 0.3 | (−0.8, 1.4) | ||
| 90–95 | 17.3, 20.4 | 3.1 | (1.6, 4.6) | −1.3 | (−2.5, −0.2) | * | 4.7 | (2.2, 7.2) | 1.9 | (0.1, 3.7) | |
| 95–97.5 | 20.4, 22.6 | 4.0 | (1.9, 6.1) | −2.3 | (−4.0, −0.6) | ** | 6.0 | (2.7, 9.3) | 1.8 | (−1.0, 4.5) | * |
| 97.5–99 | 22.6, 24.5 | 7.9 | (4.7, 11.1) | −3.6 | (−6.1, −1.1) | ** | 13.4 | (7.6, 19.3) | 4.6 | (0.9, 8.3) | * |
| 99–100 | 24.5, 29.4 | 14.3 | (10.4, 18.3) | −4.8 | (−7.8, −1.9) | ** | 21.0 | (12.3, 29.7) | 11.1 | (7.1, 15.2) | * |
| Aged 65–74 years | |||||||||||
| 0–1 | −42.0, −25.1 | −0.7 | (−5.3, 3.9) | −1.6 | (−5.5, 2.3) | 1.2 | (−4.8, 7.2) | −2.9 | (−10.2, 4.4) | ||
| 1–2.5 | −25.1, −20.9 | 2.4 | (−1.5, 6.3) | −2.4 | (−5.6, 0.8) | 4.7 | (−0.2, 9.7) | −0.2 | (−6.4, 6.1) | ||
| 2.5–5 | −20.9, −17.1 | −1.6 | (−4.4, 1.1) | −0.4 | (−2.6, 1.8) | 0.0 | (−4.0, 4.0) | −3.2 | (−7.0, 0.7) | ||
| 5–10 | −17.1, −12.8 | 0.1 | (−1.9, 2.1) | 0.3 | (−1.3, 1.9) | 0.0 | (−2.7, 2.7) | −0.2 | (−3.1, 2.7) | ||
| 10–25 | −12.8, −6.9 | 1.8 | (0.6, 2.9) | 0.8 | (−0.2, 1.7) | 0.9 | (−0.7, 2.6) | 2.7 | (1.0, 4.5) | ||
| 25–50 | −6.9, 0.4 | −1.6 | (−2.5, −0.7) | 0.3 | (−0.5, 1.0) | −1.6 | (−2.9, −0.4) | −1.5 | (−2.9, −0.1) | ||
| 50–75 | 0.4, 11.3 | −1.0 | (−1.9, 0.0) | −0.5 | (−1.3, 0.2) | −0.6 | (−1.9, 0.7) | −1.5 | (−2.9, −0.1) | ||
| 75–90 | 11.3, 17.3 | −0.6 | (−1.8, 0.7) | −0.2 | (−1.2, 0.8) | −0.4 | (−2.2, 1.3) | −0.7 | (−2.5, 1.1) | ||
| 90–95 | 17.3, 20.4 | 3.1 | (0.9, 5.2) | 0.7 | (−1.0, 2.4) | 2.5 | (−0.6, 5.5) | 3.9 | (0.8, 7.0) | ||
| 95–97.5 | 20.4, 22.6 | 4.7 | (1.6, 7.8) | −0.2 | (−2.7, 2.3) | 5.3 | (1.4, 9.1) | 4.5 | (−0.2, 9.3) | ||
| 97.5–99 | 22.6, 24.5 | 6.7 | (2.5, 10.8) | 0.3 | (−3.0, 3.6) | 7.2 | (0.9, 13.5) | 6.0 | (0.5, 11.6) | ||
| 99–100 | 24.5, 29.4 | 10.9 | (5.9, 15.9) | −3.7 | (−7.5, 0.1) | 17.7 | (7.9, 27.4) | 7.7 | (2.0, 13.4) | ||
| Aged <65 years | |||||||||||
| 0–1 | −42.0, −25.1 | −1.6 | (−6.2, 3.0) | 2.4 | (−1.4, 6.3) | −1.7 | (−8.0, 4.7) | −1.5 | (−8.2, 5.3) | ||
| 1–2.5 | −25.1, −20.9 | 0.6 | (−2.8, 4.0) | −1.0 | (−3.9, 1.8) | 1.9 | (−2.5, 6.2) | −1.6 | (−7.0, 3.8) | ||
| 2.5–5 | −20.9, −17.1 | 0.6 | (−1.8, 3.0) | −0.1 | (−2.1, 1.8) | 1.1 | (−2.1, 4.3) | 0.1 | (−3.7, 3.8) | ||
| 5–10 | −17.1, −12.8 | 0.8 | (−1.1, 2.6) | 0.7 | (−0.7, 2.2) | 0.2 | (−2.2, 2.6) | 1.6 | (−1.2, 4.5) | ||
| 10–25 | −12.8, −6.9 | −0.1 | (−1.1, 0.9) | −0.5 | (−1.3, 0.3) | 0.0 | (−1.5, 1.4) | −0.7 | (−2.1, 0.7) | ||
| 25–50 | −6.9, 0.4 | 0.0 | (−0.8, 0.8) | 0.1 | (−0.5, 0.8) | 0.0 | (−1.2, 1.1) | 0.1 | (−1.2, 1.3) | ||
| 50–75 | 0.4, 11.3 | −1.5 | (−2.3, −0.7) | 0.4 | (−0.3, 1.0) | −1.5 | (−2.7, −0.4) | −1.2 | (−2.3, 0.0) | ||
| 75–90 | 11.3, 17.3 | −0.8 | (−1.8, 0.3) | −0.5 | (−1.4, 0.3) | −0.7 | (−2.2, 0.8) | −1.1 | (−2.6, 0.4) | ||
| 90–95 | 17.3, 20.4 | 1.7 | (−0.1, 3.5) | −0.1 | (−1.5, 1.4) | 1.7 | (−1.0, 4.4) | 1.3 | (−1.2, 3.8) | ||
| 95–97.5 | 20.4, 22.6 | 5.6 | (2.7, 8.4) | 0.0 | (−2.3, 2.3) | 5.0 | (1.0, 9.0) | 6.0 | (2.1, 10.0) | ||
| 97.5–99 | 22.6, 24.5 | 7.0 | (3.7, 10.4) | 0.3 | (−2.4, 2.9) | 5.8 | (1.5, 10.1) | 7.9 | (3.1, 12.6) | ||
| 99–100 | 24.5, 29.4 | 7.7 | (3.7, 11.7) | −3.1 | (−6.2, −0.1) | * | 15.7 | (8.4, 22.9) | 4.0 | (−0.7, 8.7) | ** |
| Percentiles | Tavg Range | Relative Mortality 1972–2014 (%) | Trend (%/10 years) | t-Test | Relative Mortality 1972–1992 (%) | Relative Mortality 1994–2014 (%) | t-Test | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All | |||||||||||
| 0–1 | −33.4, −18.5 | 0.5 | (−1.7, 2.8) | −0.3 | (−2.2, 1.6) | 1.5 | (−1.5, 4.5) | −0.9 | (−4.3, 2.5) | ||
| 1–2.5 | −18.5, −14.1 | 3.2 | (1.3, 5.0) | −0.7 | (−2.2, 0.8) | 3.3 | (0.8, 5.8) | 2.6 | (−0.1, 5.3) | ||
| 2.5–5 | −14.1, −10.7 | 1.7 | (0.2, 3.1) | −0.8 | (−1.9, 0.4) | 2.8 | (1.0, 4.7) | 0.2 | (−2.2, 2.5) | ||
| 5–10 | −10.7, −6.6 | −0.2 | (−1.2, 0.8) | 0.3 | (−0.5, 1.2) | −0.2 | (−1.6, 1.1) | 0.0 | (−1.5, 1.6) | ||
| 10–25 | −6.6, −0.8 | 0.3 | (−0.1, 1.0) | 0.2 | (−0.2, 0.7) | 0.1 | (−0.8, 0.9) | 0.5 | (−0.3, 1.3) | ||
| 25–50 | −0.8, 4.8 | −1.4 | (−1.7, −0.9) | 0.2 | (−0.2, 0.5) | −1.4 | (−2.0, −0.8) | −1.4 | (−2.0, −0.8) | ||
| 50–75 | 4.8, 12.7 | −1.1 | (−1.6, −0.7) | 0.2 | (−0.2, 0.5) | −1.5 | (−2.2, −0.9) | −0.8 | (−1.4, −0.2) | ||
| 75–90 | 12.7, 16.7 | 0.3 | (−0.3, 0.9) | −0.1 | (−0.6, 0.4) | 0.5 | (−0.4, 1.4) | 0.2 | (−0.6, 1.0) | ||
| 90–95 | 16.7, 18.6 | 1.7 | (0.8, 2.7) | −0.5 | (−1.2, 0.3) | 2.4 | (1.0, 3.9) | 1.1 | (−0.2, 2.4) | ||
| 95–97.5 | 18.6, 19.9 | 4.3 | (2.8, 5.8) | −1.5 | (−2.7, −0.3) | * | 6.0 | (3.7, 8.3) | 2.6 | (0.6, 4.7) | * |
| 97.5–99 | 19.9, 21.4 | 5.6 | (3.5, 7.6) | 0.4 | (−1.2, 2.0) | 4.6 | (1.3, 7.9) | 6.1 | (3.5, 8.6) | ||
| 99–100 | 21.4, 25.4 | 15.2 | (12.5, 18.0) | −4.1 | (−6.0, −2.3) | *** | 22.5 | (17.7, 27.3) | 10.7 | (7.7, 13.8) | *** |
| Aged ≥75 years | |||||||||||
| 0–1 | −33.4, −18.5 | 3.9 | (1.1, 6.8) | −2.0 | (−4.4, 0.4) | 5.9 | (2.0, 9.8) | 0.9 | (−3.1, 5.0) | ||
| 1–2.5 | −18.5, −14.1 | 4.1 | (1.5, 6.7) | 0.1 | (−2.0, 2.2) | 3.5 | (−0.2, 7.2) | 4.5 | (0.9, 8.1) | ||
| 2.5–5 | −14.1, −10.7 | 3.3 | (1.4, 5.3) | −0.8 | (−2.4, 0.7) | 4.4 | (1.8, 7.0) | 2.0 | (−0.9, 4.9) | ||
| 5–10 | −10.7, −6.6 | −0.8 | (−2.2, 0.6) | 0.3 | (−0.9, 1.4) | −0.9 | (−2.9, 1.1) | −0.3 | (−2.3, 1.7) | ||
| 10–25 | −6.6, −0.8 | 0.0 | (−0.8, 0.9) | 0.2 | (−0.5, 0.8) | 0.0 | (−1.3, 1.3) | 0.1 | (−1.0, 1.1) | ||
| 25–50 | −0.8, 4.8 | −2.0 | (−2.6, −1.4) | 0.3 | (−0.2, 0.8) | −2.2 | (−3.1, −1.2) | −1.9 | (−2.7, −1.1) | ||
| 50–75 | 4.8, 12.7 | −1.3 | (−1.9, −0.7) | 0.7 | (0.2, 1.2) | ** | −2.3 | (−3.3, −1.4) | −0.3 | (−1.1, 0.5) | |
| 75–90 | 12.7, 16.7 | 1.0 | (0.2, 1.8) | −0.2 | (−0.9, 0.4) | 1.7 | (0.4, 3.0) | 0.5 | (−0.6, 1.6) | ||
| 90–95 | 16.7, 18.6 | 2.2 | (0.8, 3.6) | −1.2 | (−2.2, −0.1) | * | 3.7 | (1.5, 6.0) | 0.8 | (−0.9, 2.5) | * |
| 95–97.5 | 18.6, 19.9 | 4.7 | (2.5, 6.9) | −3.3 | (−5.0, −1.6) | *** | 8.2 | (4.4, 11.9) | 1.6 | (−1.0, 4.2) | ** |
| 97.5–99 | 19.9, 21.4 | 5.7 | (2.8, 8.7) | −2.5 | (−4.8, −0.1) | * | 8.2 | (2.8, 13.7) | 4.3 | (0.8, 7.9) | |
| 99–100 | 21.4, 25.4 | 18.6 | (14.5, 22.7) | −4.6 | (−7.4, −1.8) | ** | 26.6 | (18.7, 34.4) | 13.7 | (9.2, 18.1) | ** |
| Aged 65–75 years | |||||||||||
| 0–1 | −33.4, −18.5 | −1.8 | (−6.4, 2.7) | 0.9 | (−3.0, 4.8) | −2.3 | (−7.4, 2.8) | −1.2 | (−9.8, 7.4) | ||
| 1–2.5 | −18.5, −14.1 | 2.8 | (−0.9, 6.4) | −3.6 | (−6.6, −0.6) | * | 5.9 | (1.2, 10.6) | −1.2 | (−7.1, 4.7) | |
| 2.5–5 | −14.1, −10.7 | −0.4 | (−3.2, 2.5) | −2.3 | (−4.6, 0.0) | 2.6 | (−1.4, 6.5) | −4.1 | (−8.3, 0.1) | * | |
| 5–10 | −10.7, −6.6 | −0.5 | (−2.4, 1.5) | 0.6 | (−1.0, 2.1) | −0.8 | (−3.4, 1.9) | −0.2 | (−3.1, 2.8) | ||
| 10–25 | −6.6, −0.8 | 1.7 | (0.5, 2.8) | 0.9 | (0.0, 1.9) | 0.6 | (−1.1, 2.2) | 2.7 | (1.0, 4.4) | ||
| 25–50 | −0.8, 4.8 | −1.4 | (−2.3, −0.5) | 0.3 | (−0.4, 1.0) | −1.3 | (−2.5, −0.1) | −1.3 | (−2.7, 0.1) | ||
| 50–75 | 4.8, 12.7 | −1.4 | (−2.3, −0.4) | −0.7 | (−1.4, 0.1) | −0.9 | (−2.2, 0.4) | −2.0 | (−3.4, −0.6) | ||
| 75–90 | 12.7, 16.7 | 0.3 | (−1.0, 1.5) | 0.0 | (−1.0, 0.9) | 0.0 | (−1.7, 1.7) | 0.5 | (−1.3, 2.3) | ||
| 90–95 | 16.7, 18.6 | 2.0 | (−0.1, 4.29 | −0.1 | (−1.8, 1.6) | 2.6 | (−0.4, 5.6) | 1.9 | (−1.1, 5.0) | ||
| 95–97.5 | 18.6, 19.9 | 3.7 | (0.6, 6.8) | 1.1 | (−1.3, 3.5) | 3.1 | (−0.8, 7.0) | 4.2 | (−0.5, 8.9) | ||
| 97.5–99 | 19.9, 21.4 | 4.3 | (0.1, 8.4) | 3.2 | (−0.1, 6.59) | 0.2 | (−5.8, 6.1) | 6.5 | (0.9, 12.2) | ||
| 99–100 | 21.4, 25.4 | 14.4 | (9.6, 19.3) | −5.9 | (−9.2, −2.7) | *** | 24.7 | (16.6, 32.8) | 8.1 | (2.3, 13.9) | ** |
| Aged <65 years | |||||||||||
| 0–1 | −33.4, −18.5 | −3.0 | (−7.3, 1.3) | 0.7 | (−2.9, 4.4) | −1.8 | (−7.4, 3.8) | −4.9 | (−11.7, 2.0) | ||
| 1–2.5 | −18.5, −14.1 | 1.2 | (−2.1, 4.6) | −0.1 | (−2.8, 2.6) | 0.6 | (−3.7, 4.9) | 1.2 | (−4.1, 6.5) | ||
| 2.5–5 | −14.1, −10.7 | 0.0 | (−2.5, 2.5) | −0.5 | (−2.5, 1.5) | 0.8 | (−2.2, 3.8) | −1.4 | (−5.6, 2.8) | ||
| 5–10 | −10.7, −6.6 | 1.3 | (−0.5, 3.1) | 0.3 | (−1.1, 1.8) | 1.6 | (−0.8, 4.0) | 1.1 | (−1.7, 3.8) | ||
| 10–25 | −6.6, −0.8 | −0.1 | (−1.1, 1.0) | −0.2 | (−1.0, 0.6) | 0.0 | (−1.5, 1.4) | −0.4 | (−1.9, 1.0) | ||
| 25–50 | −0.8, 4.8 | −0.3 | (−1.1, 0.5) | 0.1 | (−0.6, 0.7) | −0.3 | (−1.4, 0.8) | −0.2 | (−1.4, 1.0) | ||
| 50–75 | 4.8, 12.7 | −1.0 | (−1.9, −0.2) | 0.1 | (−0.6, 0.7) | −1.0 | (−2.2, 0.2) | −0.9 | (−2.0, 0.3) | ||
| 75–90 | 12.7, 16.7 | −0.9 | (−1.9, 0.2) | −0.1 | (−0.9, 0.8) | −1.1 | (−2.6, 0.4) | −0.8 | (−2.3, 0.7) | ||
| 90–95 | 16.7, 18.6 | 1.2 | (−0.5, 3.0) | 0.1 | (−1.3, 1.4) | 1.0 | (−1.5, 3.5) | 1.2 | (−1.4, 3.7) | ||
| 95–97.5 | 18.6, 19.9 | 5.0 | (2.1, 7.8) | −1.1 | (−3.4, 1.1) | 6.1 | (2.2, 10.0) | 3.5 | (−0.6, 7.6) | ||
| 97.5–99 | 19.9, 21.4 | 7.1 | (3.6, 10.5) | 2.9 | (0.2, 5.6) | * | 3.3 | (−2.1, 8.7) | 9.2 | (4.7, 13.6) | |
| 99–100 | 21.4, 25.4 | 9.7 | (5.7, 13.7) | −3.7 | (−6.4, −1.0) | ** | 16.3 | (10.2, 22.3) | 5.6 | (0.4, 10.8) | ** |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruuhela, R.; Jylhä, K.; Lanki, T.; Tiittanen, P.; Matzarakis, A. Biometeorological Assessment of Mortality Related to Extreme Temperatures in Helsinki Region, Finland, 1972–2014. Int. J. Environ. Res. Public Health 2017, 14, 944. https://doi.org/10.3390/ijerph14080944
Ruuhela R, Jylhä K, Lanki T, Tiittanen P, Matzarakis A. Biometeorological Assessment of Mortality Related to Extreme Temperatures in Helsinki Region, Finland, 1972–2014. International Journal of Environmental Research and Public Health. 2017; 14(8):944. https://doi.org/10.3390/ijerph14080944
Chicago/Turabian StyleRuuhela, Reija, Kirsti Jylhä, Timo Lanki, Pekka Tiittanen, and Andreas Matzarakis. 2017. "Biometeorological Assessment of Mortality Related to Extreme Temperatures in Helsinki Region, Finland, 1972–2014" International Journal of Environmental Research and Public Health 14, no. 8: 944. https://doi.org/10.3390/ijerph14080944







