The Impact of a Nickel-Copper Smelter on Concentrations of Toxic Elements in Local Wild Food from the Norwegian, Finnish, and Russian Border Regions
Abstract
:1. Introduction
2. Materials and Methods
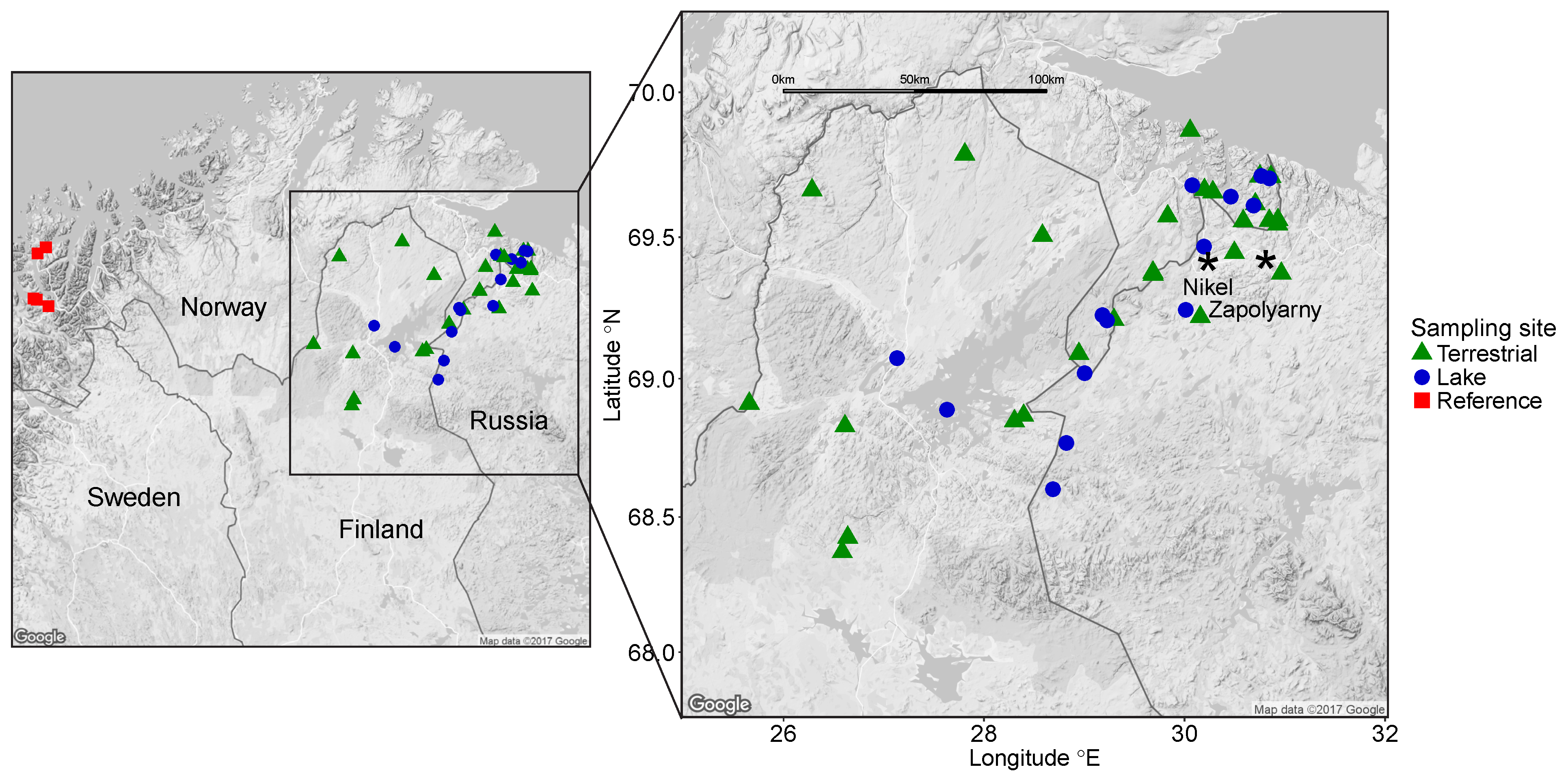
2.1. Sampling Sites and Sample Collection
2.2. Chemical Analyses
2.3. Data Treatment and Statistical Analyses
3. Results
3.1. Spatial Trends in Local Wild Foods
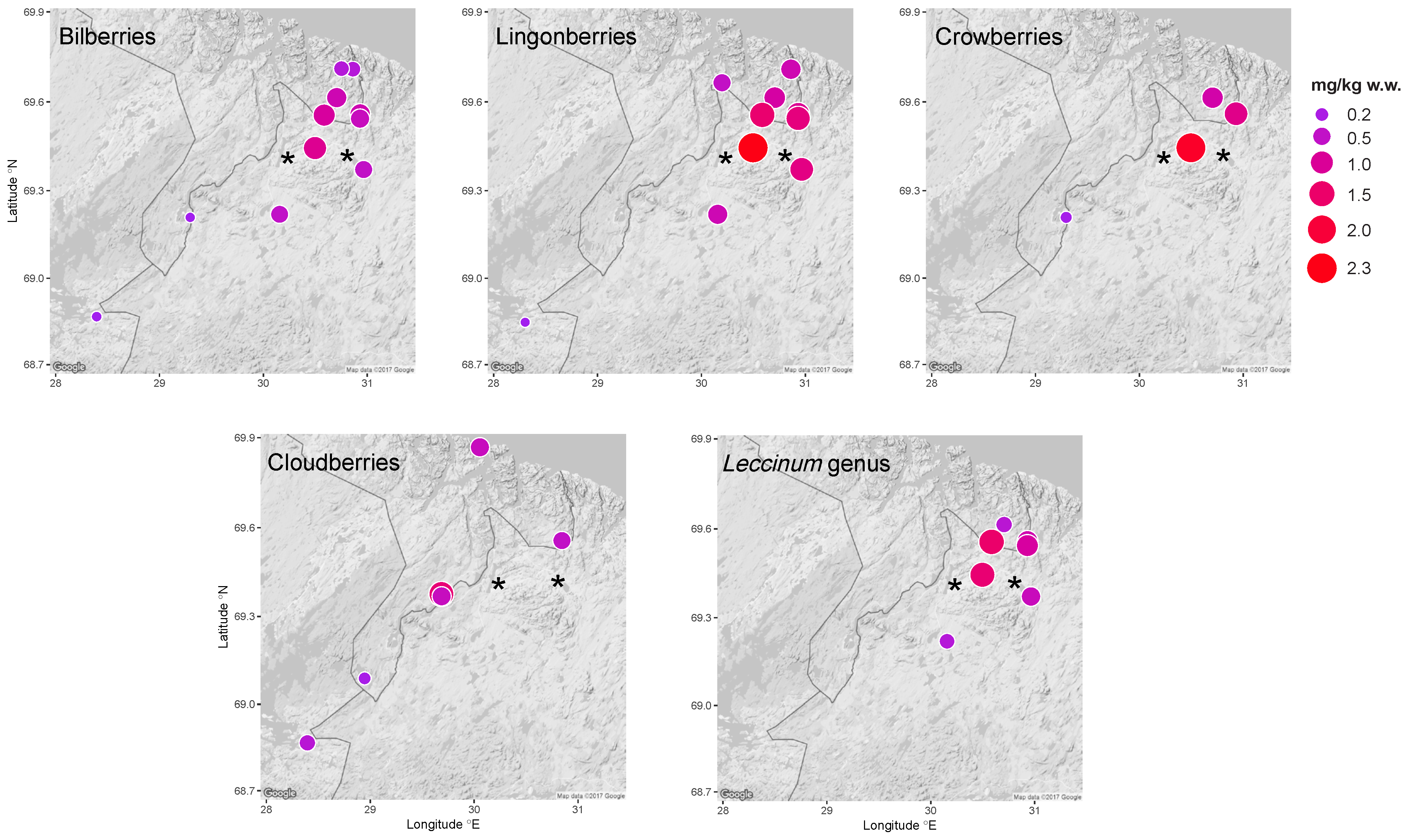
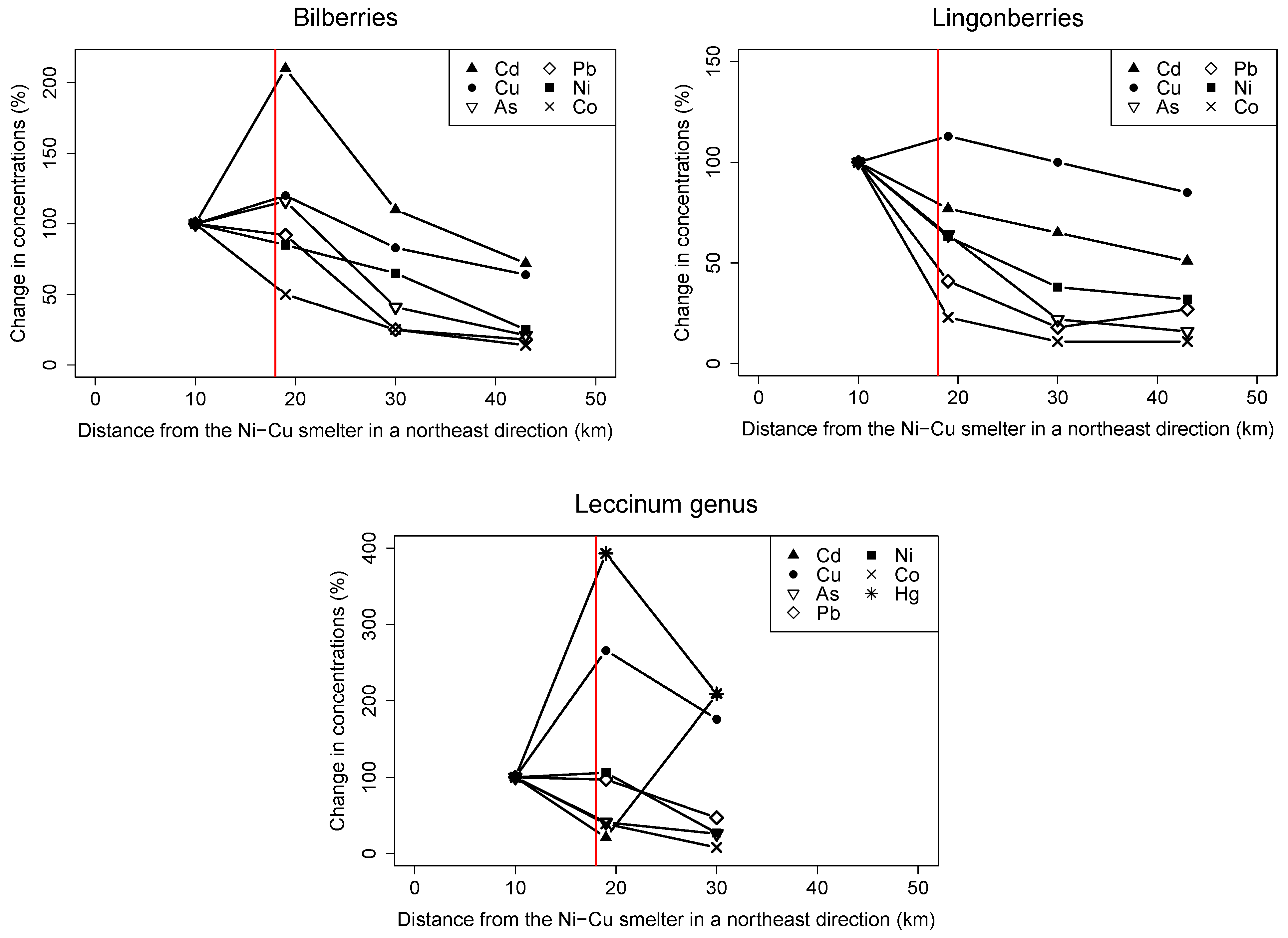
3.1.1. Spatial Trends in Berries and Mushrooms
3.1.2. Spatial Trends in Fish Muscle Tissue
3.1.3. Spatial Trends in Game Muscle Tissue
3.2. Assessment of the Concentration of Toxic Elements in Food Groups
4. Discussion
4.1. Spatial Trends in Local Wild Foods
4.2. Assessment of the Concentration of Toxic Elements in Food Groups
4.3. Considerations of Risk by Wild Food Consumptions
4.4. Limitations of the Study
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Berglen, T.F.; Dauge, F.; Andersen, E.; Nilsson, L.O.; Svendby, T.M.; Tønnesen, D.; Vadset, M.; Våler, R.L. Air Quality Monitoring in the Border Areas of Norway and Russia-Progress Report April 2015–March 2016; 16/2016; NILU-Norsk Institut for Luftforskning: Kjeller, Norway, 2016; p. 112. [Google Scholar]
- Dauvalter, V. Heavy metals in lake-sediments of the Kola-Peninsula, Russia. Sci. Total Environ. 1994, 158, 51–61. [Google Scholar] [CrossRef]
- Rognerud, S.; Dauvalter, V.A.; Fjeld, E.; Skjelkvale, B.L.; Christensen, G.; Kashulin, N. Spatial trends of trace-element contamination in recently deposited lake sediment around the Ni-Cu smelter at Nikel, Kola Peninsula, Russian Arctic. Ambio 2013, 42, 724–736. [Google Scholar] [CrossRef] [PubMed]
- Steinnes, E.; Lukina, N.; Nikonov, V.; Aamlid, D.; Royset, O. A gradient study of 34 elements in the vicinity of a copper-nickel smelter in the Kola Peninsula. Environ. Monit. Assess. 2000, 60, 71–88. [Google Scholar] [CrossRef]
- Dauvalter, V.; Kashulin, N.; Sandimirov, S.; Terentjev, P.; Denisov, D.; Amundsen, P.A. Chemical composition of lake sediments along a pollution gradient in a Subarctic watercourse. J. Environ. Sci. Health A 2011, 46, 1020–1033. [Google Scholar] [CrossRef]
- Ylikörkkö, J.; Christensen, G.N.; Kashulin, N.; Denisov, D.; Andersen, H.J.; Jelkänen, E. Environmental Challenges in the Joint Border Area of Norway, Finland and Russia; 41/2015; Center for Economic Development, Transport and the Environment for Lapland: Rovaniemi, Finland, 2015; p. 165. [Google Scholar]
- Reimann, C.; DeCaritat, P.; Halleraker, J.H.; Finne, T.E.; Boyd, R.; Jaeger, O.; Volden, T.; Kashulina, G.; Bogatyrev, I.; Chekushin, V.; et al. Regional atmospheric deposition patterns of Ag, As, Bi, Cd, Hg, Mo, Sb and Tl in a 188,000 km2 area in the European arctic as displayed by terrestrial moss samples-long-range atmospheric transport vs local impact. Atmos. Environ. 1997, 31, 3887–3901. [Google Scholar] [CrossRef]
- Reimann, C.; Halleraker, J.H.; Kashulina, G.; Bogatyrev, I. Comparison of plant and precipitation chemistry in catchments with different levels of pollution on the Kola Peninsula, Russia. Sci. Total Environ. 1999, 243, 169–191. [Google Scholar] [CrossRef]
- Uhlig, C.; Junttila, O. Airborne heavy metal pollution and its effects on foliar elemental composition of Empetrum hermaphroditum and Vaccinium myrtillus in Sør-Varanger, northern Norway. Environ. Pollut. 2001, 114, 461–469. [Google Scholar] [CrossRef]
- Amundsen, P.-A.; Staldvik, F.J.; Lukin, A.A.; Kashulin, N.A.; Popova, O.A.; Reshetnikov, Y.S. Heavy metal contamination in freshwater fish from the border region between Norway and Russia. Sci. Total Environ. 1997, 201, 211–224. [Google Scholar] [CrossRef]
- Lappalainen, A.; Tammi, J.; Puro-Tahvanainen, A. The effects of nickel smelters on water quality and littoral fish species composition in small watercourses in the border area of Finland, Norway and Russia. Boreal Environ. Res. 2007, 12, 455–466. [Google Scholar]
- Collins, J.F. Copper. In Modern Nutrition in Health and Disease, 11th ed.; Ross, C., Caballero, B., Cousins, R.J., Tucker, K.L., Ziegler, T.R., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2014; pp. 206–216. [Google Scholar]
- Amundsen, P.-A.; Kashulin, N.A.; Terentjev, P.; Gjelland, K.O.; Koroleva, I.M.; Dauvalter, V.A.; Sandimirov, S.; Kashulin, A.; Knudsen, R. Heavy metal contents in whitefish (Coregonus lavaretus) along a pollution gradient in a subarctic watercourse. Environ. Monit. Assess. 2011, 182, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Aamlid, D.; Skogheim, I. Nickel, copper and other metals in berries of cloudberry (Rubus chamaemorus) and bilberry (Vaccinium myrtillus) from South Varanger, north-east Norway, 1992. Skogforsk 1993, 40, 1–15. [Google Scholar]
- Myking, T.; Aarrestad, P.A.; Derome, J.; Bakkestuen, V.; Bierke, J.W.; Gytarsky, M.; Isaeva, L.; Karaban, R.; Korotkov, V.; Lindgren, M.; et al. Effects of air pollution from a Nickel-copper industrial complex on boreal forest vegetation in the joint Russian-Norwegian-Finnish border area. Boreal Environ. Res. 2009, 14, 279–296. [Google Scholar]
- Nguyen, Q.T.; Skov, H.; Sorensen, L.L.; Jensen, B.J.; Grube, A.G.; Massling, A.; Glasius, M.; Nojgaard, J.K. Source apportionment of particles at Station Nord, North East Greenland during 2008–2010 using COPREM and PMF analysis. Atmos. Chem. Phys. 2013, 13, 35–49. [Google Scholar] [CrossRef]
- Kashulin, N.A.; Terentyev, P.M.; Amundsen, P.-A.; Dauvalter, V.A.; Sandimirov, S.S.; Kashulin, A.N. Specific features of accumulation of Cu, Ni, Zn, Cd, and Hg in two whitefish Coregonus lavaretus (L.) morphs inhabiting the Inari-Pasvik lacustrine-riverine system. Inland Water Biol. 2011, 4, 383–392. [Google Scholar] [CrossRef]
- Lukin, A.; Dauvalter, V.; Kashulin, N.; Yakovlev, V.; Sharov, A.; Vandysh, O. Assessment of copper-nickel industry impact on a subarctic lake ecosystem. Sci. Total Environ. 2003, 306, 73–83. [Google Scholar] [CrossRef]
- Mousavi, S.K.; Primicerio, R.; Amundsen, P.-A. Diversity and structure of Chironomidae (Diptera) communities along a gradient of heavy metal contamination in a subarctic watercourse. Sci. Total Environ. 2003, 307, 93–110. [Google Scholar] [PubMed]
- Statistics Norway. Folkemengde Og Befolkningsendringar. Available online: https://www.ssb.no/statistikkbanken/selectvarval/saveselections.asp (accessed on 23 September 2016).
- Regional Council of Lapland. Inari. Available online: http://www.lapland.fi/en/lapland/municipalities/inari (accessed on 23 September 2016).
- Aarrestad, P.A.; Aamlid, D. Vegetation monitoring in South-Varanger, Norway-species composition of ground vegetation and its relation to environmental variables and pollution impact. Environ. Monit. Assess. 1999, 58, 1–21. [Google Scholar] [CrossRef]
- Koptsik, S.; Koptsik, G.; Livantsova, S.; Eruslankina, L.; Zhmelkova, T.; Vologdina, Z. Heavy metals in soils near the nickel smelter: Chemistry, spatial variation, and impacts on plant diversity. J. Environ. Monit. 2003, 5, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Moiseenko, T.I.; Kudryavtseva, L.P. Trace metal accumulation and fish pathologies in areas affected by mining and metallurgical enterprises in the Kola Region, Russia. Environ. Pollut. 2001, 114, 285–297. [Google Scholar] [CrossRef]
- Garmo, Ø.; Skancke, L.B.; Høgåsen, T. Overvåking Av Langtransportert Forurenset Luft Og Nedbør; 6674-2014; NIVA-Norsk Institutt for Vannforskning: Hamar, Norge, 2014; p. 55. [Google Scholar]
- Jensen, H.K.B.; Finne, T.E.; Gwynn, J.; Jensen, L.K. Forurensningsbelastning I Humusprøver Fra Østlige og Indre Finnmark: Tungmetaller, Radioaktive Elementer, Arsen Og Pah16 Og Variasjoner I Perioden 1995–2011; 2012.042; Norges Geologiske Undersøkelse, Statens Strålevern: Trondheim, Norway, 2012; p. 43. [Google Scholar]
- Myking, T. Content of Heavy Metals in Cloudberries and Bilberries in Sør-Varanger, Finnmark 2008; Norsk Institutt for Skog Og Landskap: Fana, Norway, 2009; pp. 1–8. [Google Scholar]
- Anda, E.E.; Nieboer, E.; Dudarev, A.A.; Sandanger, T.M.; Odland, J.O. Intra- and intercompartmental associations between levels of organochlorines in maternal plasma, cord plasma and breast milk, and lead and cadmium in whole blood, for indigenous peoples of Chukotka, Russia. J. Environ. Monit. 2007, 9, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Kahle, D.; Wickham, H. Ggmap: Spatial Visualization with ggplot2. R J. 2013, 5, 144–161. [Google Scholar]
- Newson, R.B. Frequentist q-values for multiple-test procedures. Stata J. 2010, 10, 568–584. [Google Scholar]
- Barcan, V.S.; Kovnatsky, E.F.; Smetannikova, M.S. Absorption of Heavy Metals in Wild Berries and Edible Mushrooms in an Area Affected by Smelter Emissions. Water Air Soil Pollut. 1998, 103, 173–195. [Google Scholar] [CrossRef]
- Steinnes, E.; Uggerud, H.T.; Pfaffhuber, K.A.; Berg, T. Atmospheric Deposition of Heavy Metals in Norway, National Moss Survey 2015; NILU–Norwegian Institute for Air Research: Kjeller, Norway, 2016; p. 57. [Google Scholar]
- Falandysz, J.; Kunito, T.; Kubota, R.; Bielawski, L.; Mazur, A.; Falandysz, J.J.; Tanabe, S. Selected elements in brown birch scaber stalk leccinum scabrum. J. Environ. Sci. Health A 2007, 42, 2081–2088. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.A.; Brustad, M.; Sandanger, T.M. Concentrations and Geographical Variations of Selected Toxic Elements in Meat from Semi-Domesticated Reindeer (Rangifer tarandus tarandus L.) in Mid- and Northern Norway: Evaluation of Risk Assessment. Int. J. Environ Res. Public Health 2012, 9, 1699–1714. [Google Scholar] [CrossRef] [PubMed]
- Arctic Monitoring and Assessment Programme (AMAP). Amap Assessment 2002: Heavy Metals in the Arctic; Arctic Monitoring and Assessment Programme (AMAP): Oslo, Norway, 2005; pp. 1–265. [Google Scholar]
- Kalac, P.; Svoboda, L. A review of trace element concentrations in edible mushrooms. Food Chem. 2000, 69, 273–281. [Google Scholar] [CrossRef]
- Morel, F.M.M.; Kraepiel, A.M.L.; Amyot, M. The chemical cycle and bioaccumulation of mercury. Annu. Rev. Ecol. Syst. 1998, 29, 543–566. [Google Scholar] [CrossRef]
- Jaeger, I.; Hop, H.; Gabrielsen, G.W. Biomagnification of mercury in selected species from an Arctic marine food web in Svalbard. Sci. Total Environ. 2009, 407, 4744–4751. [Google Scholar] [CrossRef] [PubMed]
- European Commission (Ed.) Setting Maximum Levels for Certain Contaminants in Foodstuffs; Commision Regulation (EC) No 1881/2006; European Commission: Brussel, Belgium, 2006. [Google Scholar]
- European Commission (Ed.) Amending Regulation (EC) No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs; Commision Regulation (EC) No 629/2008; European Commision: Brussel, Belgium, 2008. [Google Scholar]
- European Commission (Ed.) Amending Regulation (Ec) No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs; Commision Regulation (EU) No 420/2011; European Commision: Brussel, Belgium, 2011. [Google Scholar]
- European Commission (Ed.) Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Cadmium in Foodstuffs; Commision Regulation (EU) No 488/2014; European Commision: Brussel, Belgium, 2014. [Google Scholar]
- European Food Safety Authority. Cadmium in food-scientific opinion of the panel on contaminants in the food chain. EFSA J. 2009, 7, 980. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain. Scientific opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA J. 2012, 10, 2985. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain. Scientific opinion on the risks to public health related to the presence of nickel in food and drinking water. EFSA J. 2015, 13, 4002. [Google Scholar] [CrossRef]
- Norwegian Food Safety Authority. The Norwegian Directorate of Health; University of Oslo. Norwegian Food Composition Database 2016. Available online: http://www.matvaretabellen.no/ (accessed on 30 October 2016).
- Farkas, A.; Salanki, J.; Specziar, A. Age- and size-specific patterns of heavy metals in the organs of freshwater fish Abramis brama L. Populating a low-contaminated site. Water Res. 2003, 37, 959–964. [Google Scholar] [CrossRef]
- Rintala, R.; Venalainen, E.R.; Hirvi, T. Heavy-metals in muscle, liver, and kidney from Finnish reindeer in 1990-1991 and 1991–1992. Bull. Environ. Contam. Toxicol. 1995, 54, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Sivertsen, T.; Daae, H.L.; Godal, A.; Sand, G. Ruminant uptake of nickel and other elements from industrial air-pollution in the Norwegian-Russian border area. Environ. Pollut. 1995, 90, 75–81. [Google Scholar] [CrossRef]


| Norway * | Russia | Finland | ||||
|---|---|---|---|---|---|---|
| n Sites | n Samples | n Sites | n Samples | n Sites | n Samples | |
| Berries | 16 | 56 | 4 | 10 | 3 | 9 |
| Bilberry (Vaccínium myrtíllus) | 8 | 20 | 4 | 4 | 1 | 3 |
| Lingonberry (Vaccínium vítis-idaéa) | 6 | 16 | 4 | 4 | 2 | 3 |
| Cloudberry (Rubus chamaemorus) | 7 | 11 | - | - | 1 | 3 |
| Crowberry (Empetrum nigrum) | 3 | 9 | 1 | 1 | - | - |
| Bog bilberry (Vaccínium uliginósum) | - | - | 1 | 1 | - | - |
| Mushrooms | 6 | 24 | 4 | 8 | 2 | 3 |
| Orange birch bolete (Leccinum versipelle) | 4 | 12 | - | - | - | - |
| Orange oak bolete (Leccinum aurantiacum) | - | - | 4 | 4 | - | - |
| Birch bolete (Leccinum scabrum) | - | - | 1 | 1 | - | - |
| Gypsy mushroom (Cortinarius caperatus) | 4 | 12 | - | - | - | - |
| Rufous milkcap (Lactarius rufus) | - | - | - | - | 1 | 1 |
| Bearded milkcap (Lactarius torminosus) | - | - | 1 | 1 | - | - |
| Rollrim milkcap (Lactarius resimus) | - | - | 1 | 1 | - | - |
| Russula (Russulaceae) | - | - | 1 | 1 | - | - |
| Mixed mushrooms | - | - | - | - | 1 | 2 |
| Fish muscle | 7 (lakes) ** | 19 | 5 (lakes) ** | 14 | 2 (lakes) ** | 8 |
| Whitefish (Coregonus lavaretus) | 2 | 2 | 4 | 4 | 1 | 3 |
| Perch (Perca fluviatilis) | 3 | 8 | 4 | 4 | 2 | 4 |
| Pike (Esox lucius) | 2 | 2 | 4 | 4 | 1 | 1 |
| Arctic char (Salvelinus alpinus) | 3 | 3 | 1 | 1 | - | - |
| Brown trout (Salmo trutta) | 4 | 4 | 1 | 1 | - | - |
| Game muscle | 2 (region) ** | 13 | 1 (region) ** | 1 | 6 (region) ** | 9 |
| Reindeer (Rangifer tarandus) | 1 | 10 | - | - | 1 | 3 |
| Moose (Alces alces) | 1 | 3 | 1 | 1 | 3 | 3 |
| Ptarmigan (Lagopus lagopus) | - | - | - | - | 2 | 3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hansen, M.D.; Nøst, T.H.; Heimstad, E.S.; Evenset, A.; Dudarev, A.A.; Rautio, A.; Myllynen, P.; Dushkina, E.V.; Jagodic, M.; Christensen, G.N.; et al. The Impact of a Nickel-Copper Smelter on Concentrations of Toxic Elements in Local Wild Food from the Norwegian, Finnish, and Russian Border Regions. Int. J. Environ. Res. Public Health 2017, 14, 694. https://doi.org/10.3390/ijerph14070694
Hansen MD, Nøst TH, Heimstad ES, Evenset A, Dudarev AA, Rautio A, Myllynen P, Dushkina EV, Jagodic M, Christensen GN, et al. The Impact of a Nickel-Copper Smelter on Concentrations of Toxic Elements in Local Wild Food from the Norwegian, Finnish, and Russian Border Regions. International Journal of Environmental Research and Public Health. 2017; 14(7):694. https://doi.org/10.3390/ijerph14070694
Chicago/Turabian StyleHansen, Martine D., Therese H. Nøst, Eldbjørg S. Heimstad, Anita Evenset, Alexey A. Dudarev, Arja Rautio, Päivi Myllynen, Eugenia V. Dushkina, Marta Jagodic, Guttorm N. Christensen, and et al. 2017. "The Impact of a Nickel-Copper Smelter on Concentrations of Toxic Elements in Local Wild Food from the Norwegian, Finnish, and Russian Border Regions" International Journal of Environmental Research and Public Health 14, no. 7: 694. https://doi.org/10.3390/ijerph14070694







