Solidification and Biotoxicity Assessment of Thermally Treated Municipal Solid Waste Incineration (MSWI) Fly Ash
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Characterization of Raw Fly Ash
3.2. Leaching Concentrations of Heavy Metals
3.3. Mineralogy of Raw and Thermally Treated Fly Ash
3.4. Morphology of Raw and Thermally Treated Fly Ash
3.5. Biotoxicity Assessment
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ma, J.; Hipel, K.W. Exploring social dimensions of municipal solid waste management around the globe—A systematic literature review. Waste Manag. 2016, 56, 3–12. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.M.; Silva Dos Santos, M.J.; Sousa Filho, H.R.; Martins Cardoso, L.A.; de Souza, C.T.; Bezerra, M.A.; Souza, A.S. Multivariate exploratory analysis of metals and phosphorus concentrations of leachates collected monthly from a municipal sanitary landfill. Bull. Environ. Contam. Toxicol. 2015, 95, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, I.; Vazquez, M.A.; Romero-Baena, A.J.; Barba-Brioso, C. Stabilization of fly ash using cementing bacteria. Assessment of cementation and trace element mobilization. J. Hazard. Mater. 2017, 321, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Lou, C.; Cheng, Q.; Zhao, P.; Zhang, X. In situ measurement of alkali metals in an msw incinerator using a spontaneous emission spectrum. Appl. Sci. 2017, 7, 263. [Google Scholar] [CrossRef]
- Lindberg, D.; Molin, C.; Hupa, M. Thermal treatment of solid residues from WtE units: A review. Waste Manag. 2015, 37, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Wey, M.Y.; Liu, K.Y.; Tsai, T.H.; Chou, J.T. Thermal treatment of the fly ash from municipal solid waste incinerator with rotary kiln. J. Hazard. Mater. 2006, 137, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Hiraokab, M. Municipal solid waste incinerator residue recycling by thermal processes. Waste Manag. 2000, 20, 249–258. [Google Scholar] [CrossRef]
- Jie, Y.; Yu, Q.; Limei, J.; Chuan, M.; Nigel, P.; Lushi, S. Removal of toxic and alkali/alkaline earth metals during co-thermal treatment of two types of mswi fly ashes in China. Waste Manag. 2015, 46, 287–297. [Google Scholar] [CrossRef]
- Sorensen, M.A.; Koch, C.B.; Stackpoole, M.M.; Bordia, R.K.; Benjamin, M.M.; Christensen, T.H. Effects of thermal treatment on mineralogy and heavy metal behaviour in iron oxide stabilization pollution control residues. Environ. Sci. Technol. 2000, 34, 4620–4627. [Google Scholar] [CrossRef]
- Chen, B.Y.; Lin, K.L. Biotoxicity assessment on reusability of municipal solid waste incinerator (MSWI) ash. J. Hazard. Mater. 2006, 136, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Phoungthong, K.; Zhang, H.; Shao, L.M.; He, P.J. Variation of the phytotoxicity of municipal solid waste incinerator bottom ash on wheat (Triticum aestivum L.) seed germination with leaching conditions. Chemosphere 2016, 146, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Tsai, J.L.; Liao, M.H. Cytotoxicity of municipal solid waste incinerator ash wastes toward mammalian kidney cell lines. Chemosphere 2008, 71, 1860–1865. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.W.; Liu, Z.S.; Wun, M.J.; Kuo, T.C. Cellular mutagenicity and heavy metal concentrations of leachates extracted from the fly and bottom ash derived from municipal solid waste incineration toxicity. Int. J. Environ. Res. Public Health 2016, 13, 1078. [Google Scholar] [CrossRef] [PubMed]
- China EPA. HJ/T300-2007: Solid Waste-Extraction Procedure for Leaching Toxicity-Acetic Acid Buffer Solution Method; China Environmental Science Press: Beijing, China, 2007. [Google Scholar]
- Mei, S.; Wang, H.; Wang, W.; Tong, L.; Pan, H.; Ruan, C.; Ma, Q.; Liu, M.; Yang, H.; Zhang, L. Antibacterial effects and biocompatibility of titanium surfaces with graded silver incorporation in titania nanotubes. Biomaterials 2014, 35, 4255. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Method 3050B: Acid Digestion of Sediments, Sludges, and Soils 1.0 Scope and Application; US EPA: Washinton, DC, USA, 1996.
- Wan, X.; Wang, W.; Ye, T.; Guo, Y.; Gao, X. A study on the chemical and mineralogical characterization of mswi fly ash using a sequential extraction procedure. J. Hazard. Mater. 2006, 134, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Sabbas, T.; Polettini, A.; Pomi, R.; Astrup, T.; Hjelmar, O.; Mostbauer, P.; Cappai, G.; Magel, G.; Salhofer, S.; Speiser, C.; et al. Management of municipal solid waste incineration residues. Waste Manag. 2003, 23, 61–88. [Google Scholar] [CrossRef]
- Hu, H.; Luo, G.; Liu, H.; Qiao, Y.; Xu, M.; Yao, H. Fate of chromium during thermal treatment of municipal solid waste incineration (MSWI) fly ash. Proc. Combust. Inst. 2013, 34, 2795–2801. [Google Scholar] [CrossRef]
- Sandeep, P.; Sahu, S.K.; Kothai, P.; Pandit, G.G. Leaching behavior of selected trace and toxic metals in coal fly ash samples collected from two thermal power plants, India. Bull. Environ. Contam. Toxicol. 2016, 97, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.S.; Chiang, K.Y.; Lin, K.L.; Sun, C.J. Effects of a water-extraction process on heavy metal behavior in municipal solid waste incinerator fly ash. Hydrometallurgy 2001, 62, 73–81. [Google Scholar] [CrossRef]
- Ribe, V.; Nehrenheim, E.; Odlare, M. Assessment of mobility and bioavailability of contaminants in MSW incineration ash with aquatic and terrestrial bioassays. Waste Manag. 2014, 34, 1871–1876. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Liu, X.; Cheng, Q.Y.; Xiao, S.; Xia, L.X.; Yuan, B.; Feng, Y.Q. Heavy metals induce decline of derivatives of 5-methycytosine in both DNA and rna of stem cells. ACS Chem. Biol. [CrossRef] [PubMed]
- Chen, B.Y.; Lin, K.L. Dose-mortality assessment on municipal solid waste incinerator (MSWI) ash toxicity. J. Hazard. Mater. 2007, 139, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-L.; Peng, T.-H. Evaluation of heavy metal distribution and biological toxicity in agglomeration bed material during artificial waste incineration in fluidized bed. Korean J. Chem. Eng. 2012, 29, 627–635. [Google Scholar] [CrossRef]
- Kaneko, H. Evaluation of municipal waste incinerator fly ash toxicity and the role of cadmium by two aquatic toxicity tests. Waste Manag. 1996, 16, 555–559. [Google Scholar] [CrossRef]
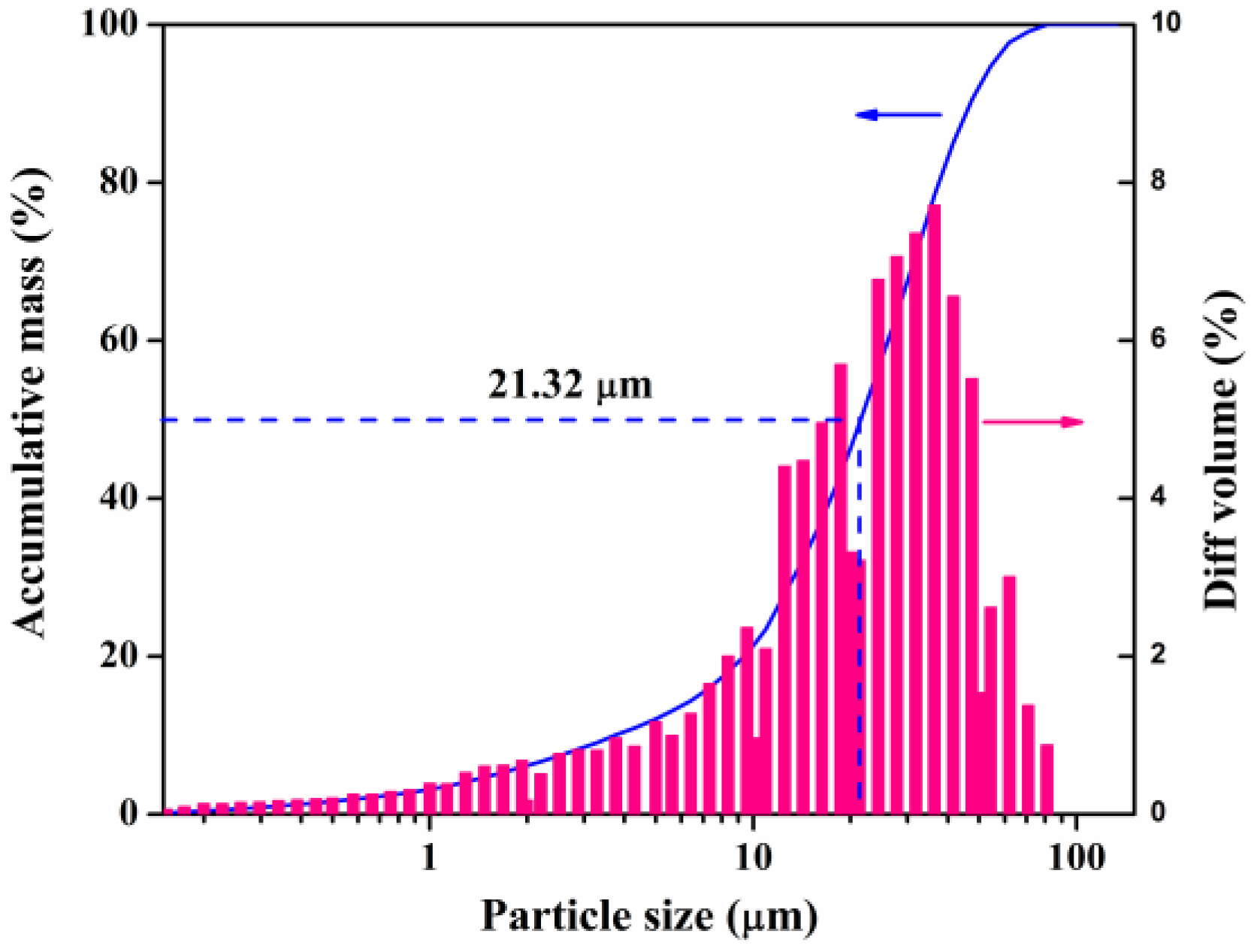

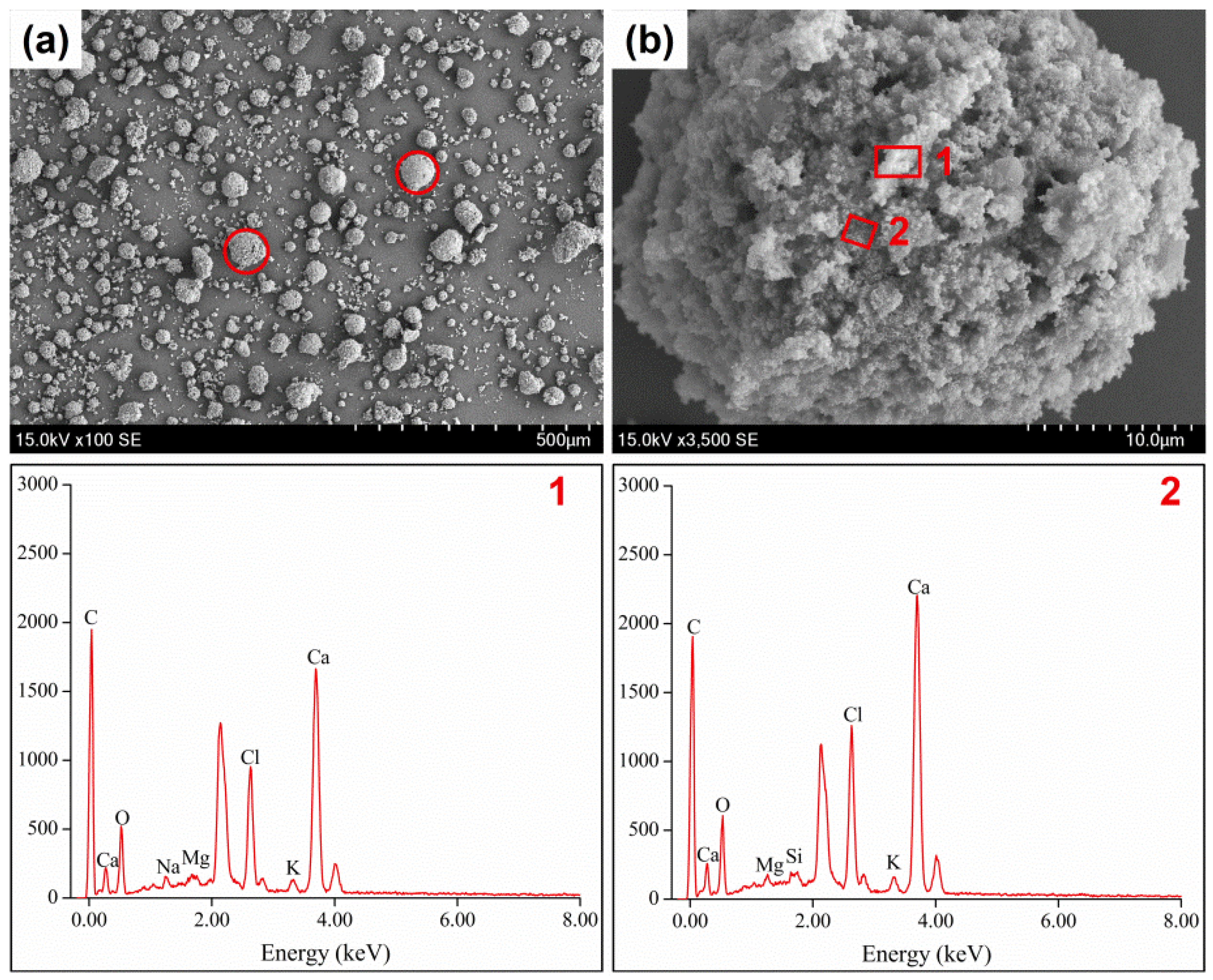
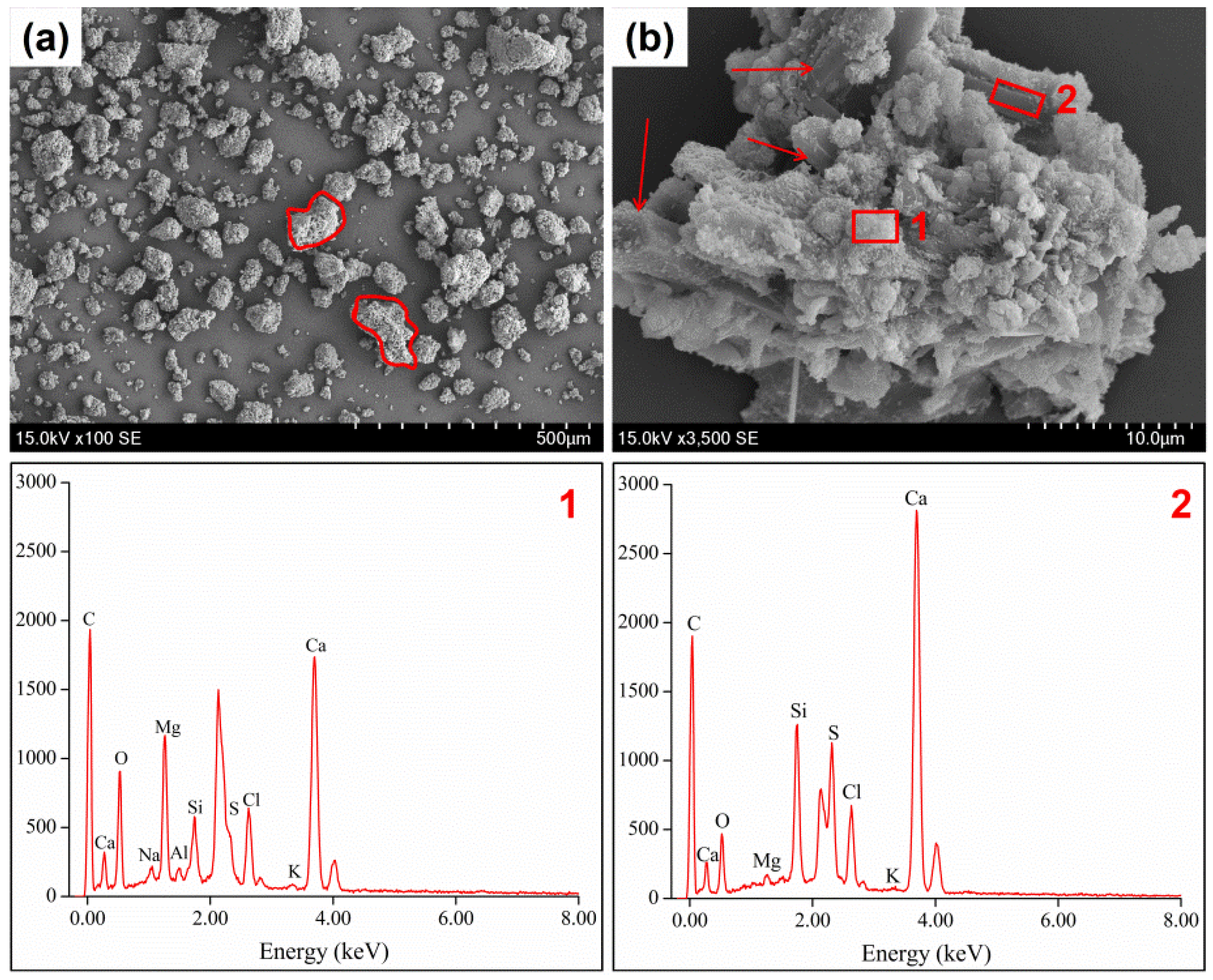

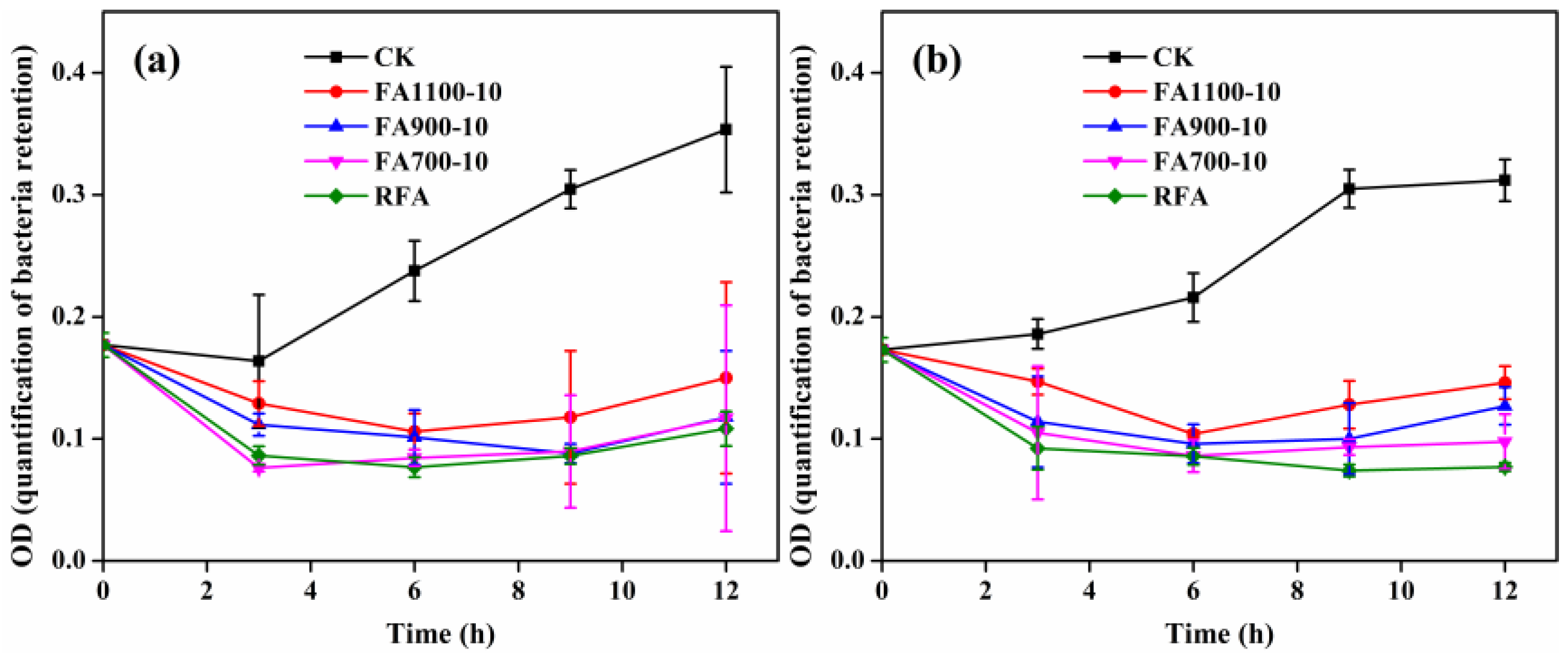
| Composition | CaO | NaO | SO3 | SiO2 | MgO | Fe2O3 | Al2O3 | TiO2 | P2O5 | Cl |
|---|---|---|---|---|---|---|---|---|---|---|
| Ratio (%) | 53.0 | 7.7 | 5.9 | 3.9 | 3.8 | 1.9 | 1.0 | 0.5 | 0.3 | 19.9 |
| Samples | Leaching Concentrations (mg/L) | |||
|---|---|---|---|---|
| Pb | Cr | Cu | Zn | |
| Raw Fly Ash (RFA) | 8.08 | 0.10 | 0.12 | 0.39 |
| FA700-10 | 1.12 | 0.73 | 0.04 | 0.17 |
| FA900-10 | 0.30 | 0.70 | 0.013 | 0.09 |
| FA1100-10 | 0.16 | 1.28 | 0.017 | 0.10 |
| Limits in S1 | 5 | 5 | 100 | 100 |
| Limits in S2 | 0.25 | 1.5 | 40 | 100 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, B.; Deng, Y.; Yang, Y.; Tan, S.N.; Liu, Q.; Yang, W. Solidification and Biotoxicity Assessment of Thermally Treated Municipal Solid Waste Incineration (MSWI) Fly Ash. Int. J. Environ. Res. Public Health 2017, 14, 626. https://doi.org/10.3390/ijerph14060626
Gong B, Deng Y, Yang Y, Tan SN, Liu Q, Yang W. Solidification and Biotoxicity Assessment of Thermally Treated Municipal Solid Waste Incineration (MSWI) Fly Ash. International Journal of Environmental Research and Public Health. 2017; 14(6):626. https://doi.org/10.3390/ijerph14060626
Chicago/Turabian StyleGong, Bing, Yi Deng, Yuanyi Yang, Swee Ngin Tan, Qianni Liu, and Weizhong Yang. 2017. "Solidification and Biotoxicity Assessment of Thermally Treated Municipal Solid Waste Incineration (MSWI) Fly Ash" International Journal of Environmental Research and Public Health 14, no. 6: 626. https://doi.org/10.3390/ijerph14060626






