Reduced Dietary Selenium Impairs Vascular Function by Increasing Oxidative Stress in Sprague-Dawley Rat Aortas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Surgery, Blood Collection, and Aortic Ring Acquisition
2.3. Measurement of Isometric Tension of Rat Aortic Rings and Assessment of Aortic Ring Reactivity to Acetylcholine and Reduced pO2
2.4. Measurement of Se Content in Whole Blood and in Thoracic Aorta Tissue
2.5. Measurement of Oxidative Stress and Antioxidant Capacity
2.6. Measurement of Glutathione Peroxidase 1 Serum Concentration
2.7. mRNA Expression of Antioxidative Enzymes in Rat Aortas
2.8. Reagents
2.9. Statistical Analysis
3. Results
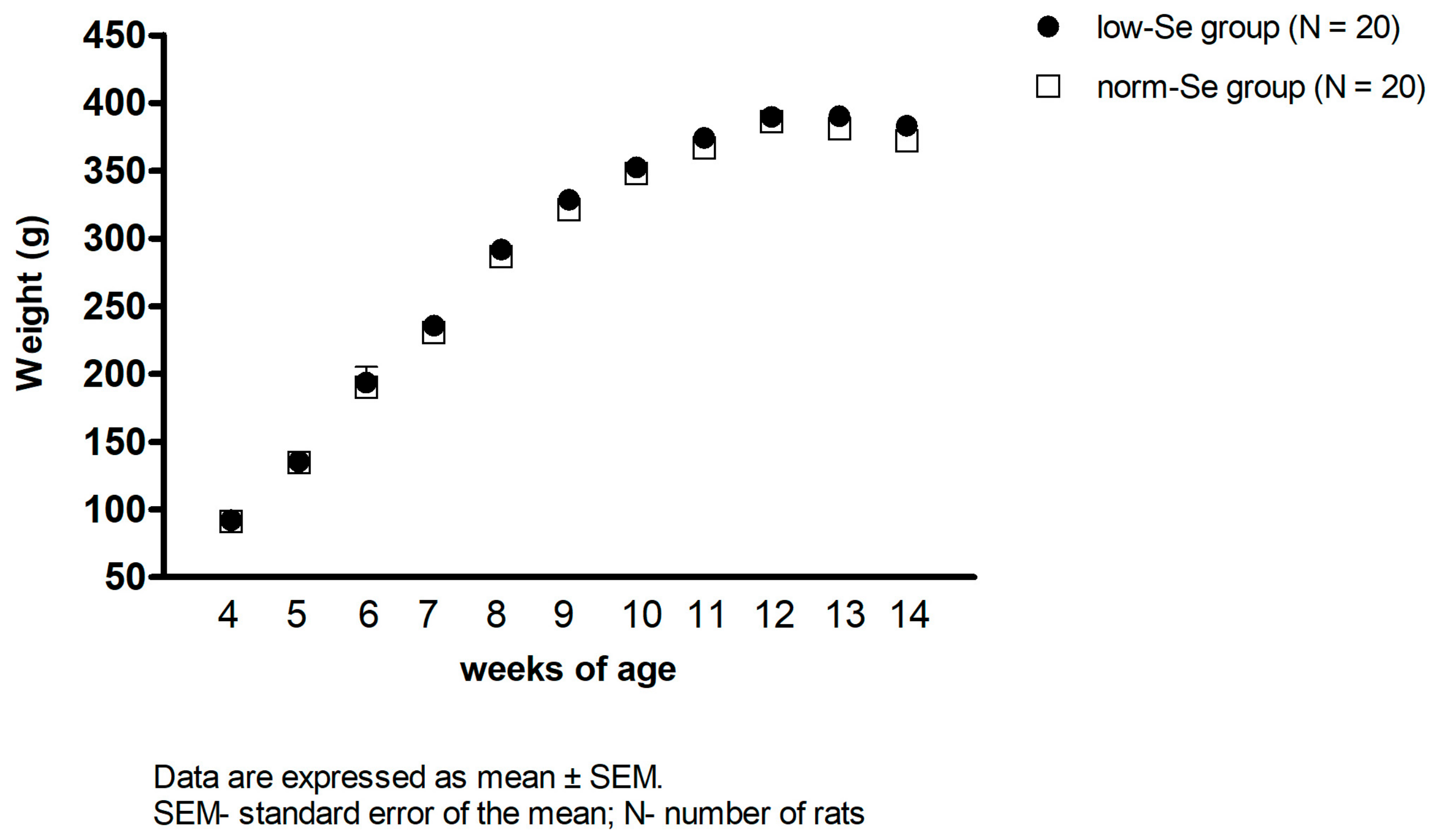
3.1. Body Weight of Experimental Animals
3.2. Acetylcholine Induced Relaxation of Isolated Rat Aortic Rings
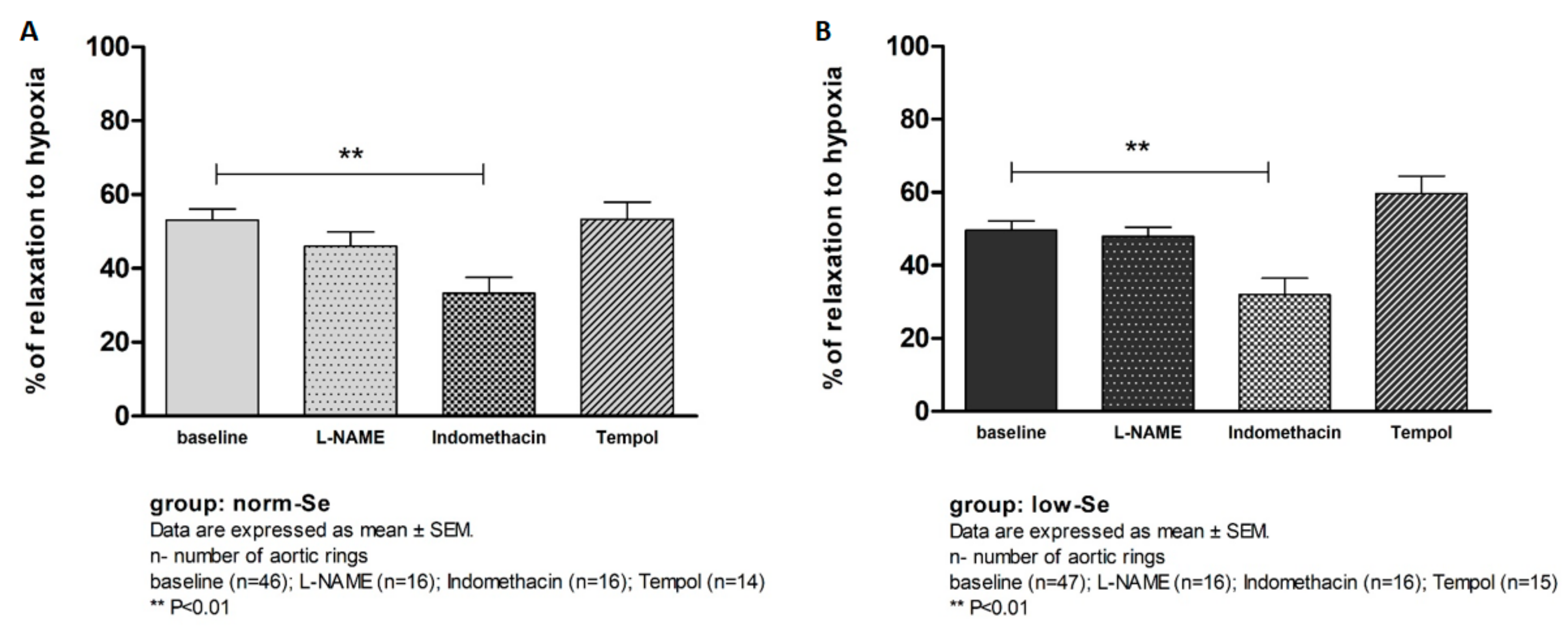
3.3. Hypoxia Induced Relaxation of Isolated Rat Aortic Rings
3.4. Se Content in Whole Blood and in Thoracic Aorta Tissue
3.5. Plasma Oxidative Stress (TBARS) and Antioxidant Capacity (FRAP)
3.6. Glutathione Peroxidase 1 Serum Concentration
3.7. mRNA Expression of Antioxidative Enzymes in Rat Aorta
4. Discussion
4.1. Se Dietary Content and Supplementation
4.2. Influence of Se Dietary Content on Vascular Function
4.3. Influence of Se Dietary Content on Oxidative Stress
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Le Brocq, M.; Leslie, S.J.; Milliken, P.; Megson, I.L. Endothelial dysfunction: From molecular mechanisms to measurement, clinical implications, and therapeutic opportunities. Antioxid. Redox Signal 2008, 10, 1631–1674. [Google Scholar] [CrossRef] [PubMed]
- Goszcz, K.; Deakin, S.J.; Duthie, G.G.; Stewart, D.; Leslie, S.J.; Megson, I.L. Antioxidants in Cardiovascular Therapy: Panacea or False Hope? Front. Cardiovasc. Med. 2015, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Harrison, D.G. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.G. Endothelial function and oxidant stress. Clin. Cardiol. 1997, 20, II-11–II-17. [Google Scholar] [PubMed]
- Zureik, M.; Galan, P.; Bertrais, S.; Mennen, L.; Czernichow, S.; Blacher, J.; Ducimetière, P.; Hercberg, S. Effects of long-term daily low-dose supplementation with antioxidant vitamins and minerals on structure and function of large arteries. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Jialal, I.; Devaraj, S. Antioxidants and atherosclerosis: Don’t throw out the baby with the bath water. Circulation 2003, 164, 926–928. [Google Scholar] [CrossRef]
- Salonen, R.M.; Nyyssonen, K.; Kaikkonen, J.; Porkkala-Sarataho, E.; Voutilainen, S.; Rissanen, T.H.; Tuomainen, T.P.; Valkonen, V.P.; Ristonmaa, U.; Lakka, H.M.; et al. Antioxidant Supplementation in Atherosclerosis Prevention Study. Six year effect of combined vitamin C and E supplementation on atherosclerotic progression: ASAP study. Circulation 2003, 107, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Stephens, N.G.; Parsons, A.; Schofield, P.; Kelly, F.; Cheeseman, K.; Mitchinson, M.J. Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS). Lancet 1996, 347, 781–786. [Google Scholar] [CrossRef]
- Fang, J.; Kinlay, S.; Beltrame, J.; Hikiti, H.; Wainstein, M.; Behrendt, D.; Suh, J.; Frei, B.; Mudge, G.H.; Selwyn, A.P.; et al. Effect of vitamins C and E on progression of transplant-associated arteriosclerosis: A randomized trial. Lancet 2002, 359, 1108–1113. [Google Scholar] [CrossRef]
- Hercberg, S.; Galan, P.; Preziosi, P.; Bertrais, S.; Mennen, L.; Malvy, D.; Roussel, A.M.; Favier, A.; Briançon, S. The SU.VI.MAX Study: A randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch. Intern. Med. 2004, 164, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Collaborative Group of the Primary Prevention Project. Low-dose aspirin and vitamin E in people at cardiovascular risk: A randomised trial in general practice. Lancet 2001, 357, 89–95. [Google Scholar]
- Yusuf, S.; Dagenais, G.; Pogue, J.; Bosch, J.; Sleight, P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N. Engl. J. Med. 2000, 342, 154–160. [Google Scholar] [PubMed]
- Schwarz, K.; Foltz, C.M. Selenium as an integral part of factor 3 against dietary necrotic liver degeneration. J. Am. Chem. Soc. 1957, 79, 3292–3293. [Google Scholar] [CrossRef]
- Berry, M.J.; Bannu, L.; Harney, J.W.; Larsen, P.R. Functional characterization of the eukaryotic SECIS elements which direct selenocysteine insertion at UGA codons. EMBO J. 1993, 12, 3315–3322. [Google Scholar] [PubMed]
- Combs, G.F., Jr. Selenium in global food systems. Br. J. Nutr. 2001, 85, 517–547. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Banning, A.; Schnurr, K. Selenium-dependent enzymes in endothelial cell function. Antioxid. Redox Signal. 2003, 5, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Ruseva, B.; Atanasova, M.; Georgieva, M.; Shumkov, N.; Laleva, P. Effects of selenium on the vessel walls and anti-elastin antibodies in spontaneously hypertensive rats. Exp. Biol. Med. 2012, 237, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Ruseva, B.; Atanasova, M.; Tsvetkova, R.; Betova, T.; Mollova, M.; Alexandrova, M.; Laleva, P.; Dimitrova, A. Effect of Selenium Supplementation on Redox Status of the Aortic Wall in Young Spontaneously Hypertensive Rats. Oxid. Med. Cell. Longev. 2015, 609053. [Google Scholar] [CrossRef] [PubMed]
- Blankenberg, S.; Rupprecht, H.J.; Bickel, C.; Torzewski, M.; Hafner, G.; Tiret, L.; Smieja, M.; Cambien, F.; Meyer, J.; Lackner, K.J.; et al. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N. Engl. J. Med. 2003, 349, 1605–1613. [Google Scholar] [CrossRef] [PubMed]
- Moghadaszadeh, B.; Beggs, A.H. Selenoproteins and their impact on human health through diverse physiological pathways. Physiology 2006, 21, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ji, W.J.; Zhu, Y.; He, B.; Li, H.; Huang, T.G.; Li, Y.M. Enhancement of endogenous defenses against ROS by supra-nutritional level of selenium is more safe and effective than antioxidant supplementation in reducing hypertensive target organ damage. Med. Hypotheses 2007, 68, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, R.; Lubos, E.; Messow, C.M.; Sinning, C.R.; Zeller, T.; Wild, P.S.; Peetz, D.; Handy, D.E.; Munzel, T.; Loscalzo, J.; et al. Selenium supplementation improves antioxidant capacity in vitro and in vivo in patients with coronary artery disease: The SElenium Therapy in Coronary Artery disease Patients (SETCAP) Study. Am. Heart J. 2008, 156, 1201.e1–1201.e11. [Google Scholar] [CrossRef] [PubMed]
- Baliga, M.S.; Wang, H.; Zhuo, P.; Schwartz, J.L.; Diamond, A.M. Selenium and GPx-1 overexpression protect mammalian cells against UV-induced DNA damage. Biol. Trace Elem. Res. 2007, 115, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Bermingham, E.N.; Hesketh, J.E.; Sinclair, B.R.; Koolaard, J.P.; Roy, N.C. Selenium-enriched foods are more effective at increasing glutathione peroxidase (GPx) activity compared with selenomethionine: A meta-analysis. Nutrients 2014, 6, 4002–4031. [Google Scholar] [CrossRef] [PubMed]
- Lewin, M.H.; Arthur, J.R.; Riemersma, R.A.; Nicol, F.; Walker, S.W.; Millar, E.M.; Howie, A.F.; Beckett, G.J. Selenium supplementation acting through the induction of thioredoxin reductase and glutathione peroxidase protects the human endothelial cell line EAhy926 from damage by lipid hydroperoxides. Biochim. Biophys. Acta 2002, 1593, 85–92. [Google Scholar] [CrossRef]
- Wu, Q.; Huang, K.; Xu, H. Effects of long term selenium deficiency on glutathione peroxidase and thioredoxin reductase activities and expressions in rat aorta. J. Inorg. Biochem. 2003, 94, 301–306. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Trace Elements in Human Nutrition and Health; Selenium; WHO: Geneva, Switzerland, 1996; pp. 105–122. [Google Scholar]
- Reilly, C. Selenium in Food and Health; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006; p. 9. [Google Scholar]
- Reeves, P. Components of the AIN-93 Diets as Improvements in the AIN-76A Diet. J. Nutr. 1997, 127, 838S–841S. [Google Scholar] [PubMed]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Lyons, G.H.; Lewis, J.; Lorimer, M.F.; Holloway, R.E.; Brace, D.M.; Stangoulis, J.C.R.; Graham, R.D. High-selenium wheat: Agronomic biofortification strategies to improve human nutrition. Food Agric. Environ. 2004, 2, 171–178. [Google Scholar]
- Kibel, A.; Novak, S.; Cosic, A.; Mihaljevic, Z.; Falck, J.R.; Drenjancevic, I. Hyperbaric oxygenation modulates vascular reactivity to angiotensin-(1-7) in diabetic rats: Potential role of epoxyeicosatrienoic acids. Diab Vasc. Dis. Res. 2015, 12, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Kunert, M.P.; Dwinell, M.R.; Lombard, J.H. Vascular responses in aortic rings of a consomic rat panel derived from the Fawn Hooded Hypertensive strain. Physiol. Genomics 2010, 42A, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Prasad, A.S.; Beck, F.W.; Fitzgerald, J.T.; Snell, D.; Bao, G.W.; Singh, T.; Cardozo, L.J. Zinc decreases C-reactive protein, lipid peroxidation, and inflammatory cytokines in elderly subjects: A potential implication of zinc as an atheroprotective agent. Am. J. Clin. Nutr. 2010, 91, 1634–1641. [Google Scholar] [CrossRef] [PubMed]
- Cosic, A.; Jukic, I.; Stupin, A.; Mihalj, M.; Mihaljevic, Z.; Novak, S.; Vukovic, R.; Drenjancevic, I. Attenuated flow-induced dilatation of middle cerebral arteries is related to increased vascular oxidative stress in rats on a short-term high salt diet. J. Physiol. 2016, 594, 4917–4931. [Google Scholar] [CrossRef] [PubMed]
- Oakes, K.D.; Van Der Kraak, G.J. Utility of the TBARS assay in detecting oxidative stress in white sucker (Catostomus commersoni) populations exposed to pulp mill effluent. Aquat. Toxicol. 2003, 63, 447. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidiniumthiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Buss, C.; Marinho, C.; Maranhão, P.A.; Bouskela, E.; Kraemer-Aguiar, L.G. Long-term dietary intake of selenium, calcium, and dairy products is associated with improved capillary recruitment in healthy young men. Eur. J. Nutr. 2013, 52, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.H.; Siu, C.W.; Yiu, K.H.; Chan, H.T.; Li, S.W.; Tam, S.; Cheung, B.M.; Lau, C.P.; Lam, T.H.; Tse, H.F. Adverse systemic arterial function in patients with selenium deficiency. J. Nutr. Health Aging 2012, 16, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.D.; Boubez, W.; Powell, W.S. Effects of selenium-deficient diets on the production of prostaglandins and other oxygenated metabolites of arachidonic acid and linoleic acid by rat and rabbit aortae. Biochim. Biophys. Acta 1987, 921, 213–220. [Google Scholar] [CrossRef]
- Hampel, G.; Watanabe, K.; Weksler, B.B.; Jaffe, E.A. Selenium deficiency inhibits prostacyclin release and enhances production of platelet activating factor by human endothelial cells. Biochim. Biophys. Acta 1989, 1006, 151–158. [Google Scholar] [CrossRef]
- Oztürk, Z.; Gurpinar, T.; Vural, K.; Boyacıoglu, S.; Korkmaz, M.; Var, A. Effects of selenium on endothelial dysfunction and metabolic profile in low dose streptozotocin induced diabetic rats fed a high fat diet. Biotech. Histochem. 2015, 90, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Raij, L.; Nagy, J.; Coffee, K.; DeMaster, E.G. Hypercholesterolemia promotes endothelial dysfunction in vitamin E- and selenium-deficient rats. Hypertension 1993, 22, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, S.Y.; Man, R.Y. Enhancement of endothelium dependent relaxation in the rat aortic ring by selenium supplement. Cardiovasc. Res. 1994, 28, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Michiels, C.; Raes, M.; Toussaint, O.; Remacle, J. Importance of Se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress. Free Radic. Biol. Med. 1994, 17, 235–348. [Google Scholar] [CrossRef]
- Glass, C.K.; Witztum, J.L. Atherosclerosis: The road ahead. Cell 2001, 104, 503–516. [Google Scholar] [CrossRef]
- Forgione, M.A.; Cap, A.; Liao, R.; Moldovan, N.I.; Eberhardt, R.T.; Lim, C.C.; Jones, J.; Goldschmidt-Clermont, P.J.; Loscalzo, J. Heterozygouscellular glutathione peroxidase deficiency in the mouse: abnormalities in vascular and cardiac function and structure. Circulation 2002, 106, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Forgione, M.A.; Weiss, N.; Heydrick, S.; Cap, A.; Klings, E.S.; Bierl, C.; Eberhardt, R.T.; Farber, H.W.; Loscalzo, J. Cellular glutathione peroxidase deficiency and endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1255–H1261. [Google Scholar] [CrossRef] [PubMed]
- Bermano, G.; Nicol, F.; Dyer, J.A.; Sunde, R.A.; Beckett, G.J.; Arthur, J.R.; Hesketh, J.E. Selenoprotein gene expression during selenium-repletion of selenium-deficient rats. Biol. Trace Elem. Res. 1996, 51, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Sunde, R.A. Molecular biomarker panels for assessment of selenium status in rats. Exp. Biol. Med. 2010, 235, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Barnes, K.M.; Evenson, J.K.; Raines, A.M.; Sunde, R.A. Transcript analysis of the selenoproteome indicates that dietary selenium requirements of rats based on selenium-regulated selenoprotein mRNA levels are uniformly less than those based on glutathione peroxidase activity. J. Nutr. 2009, 139, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Goldson, A.J.; Fairweather-Tait, S.J.; Armah, C.N.; Bao, Y.; Broadley, M.R.; Dainty, J.R.; Furniss, C.; Hart, D.J.; Teucher, B.; Hurst, R. Effects of selenium supplementation on selenoprotein gene expression and response to influenza vaccine challenge: A randomised controlled trial. PLoS ONE 2011, 6, e14771. [Google Scholar] [CrossRef] [PubMed]
- Sunde, R.A.; Thompson, K.M.; Evenson, J.K.; Thompson, B.M. Blood glutathione peroxidase-1 mRNA levels can be used as molecular biomarkers to determine dietary selenium requirements in rats. Exp. Biol. Med. Maywood 2009, 234, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Zhao, X.; Fan, R.; Zhao, J.; Luan, Y.; Zhang, Z.; Xu, S. Dietary selenium increases the antioxidant levels and ATPase activity in the arteries and veins of poultry. Biol. Trace Elem. Res. 2016, 172, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Huang, K.; Gao, J.; Huang, D.; Cai, T.; Pan, C. Comparison of glutathione peroxidase 1 and iodothyronine deiodinase 1 mRNA expression in murine liver after feeding selenite or selenized yeast. J. Trace Elem. Med. Biol. 2009, 23, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Yan, L.; Cheng, W.H.; Uthus, E.O. Dietary selenomethionine increases exon-specific DNA methylation of the p53 gene in rat liver and colon mucosa. J. Nutr. 2011, 141, 1464–1468. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; McIntosh, G.H.; Le Leu, R.K.; Young, G.P. Selenium-enriched milk proteins and selenium yeast affect selenoprotein activity and expression differently in mouse colon. Br. J. Nutr. 2010, 104, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, U.; Chiu, J.; Köhrle, J. Peroxides and peroxide-degrading enzymes in the thyroid. Antioxid. Redox Signal. 2008, 10, 1577–1592. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ha, P.C.; Butler, J.A.; Ou, B.R.; Yeh, J.Y.; Whanger, P. Effect of dietary selenium on selenoprotein W and glutathione peroxidase in 28 tissues of the rat. J. Nutr. Biochem. 1998, 9, 23–27. [Google Scholar] [CrossRef]
- Wilcox, C.S. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol. Ther. 2010, 126, 119–1145. [Google Scholar] [CrossRef] [PubMed]


| Experimental Group | Low-Se Group | Norm-Se Group | |
|---|---|---|---|
| Whole blood | Se (μg/mL) | 0.45 ± 0.01 | 0.54 ± 0.02 *** |
| Thoracic aorta tissue | Se (μg/mg) | 0.12 ± 0.01 | 0.20 ± 0.01 *** |
| Experimental Group | GPx1 | CAT | Cu/Zn SOD |
|---|---|---|---|
| low-Se group | 1.70 ± 0.37 * | 8.90 ± 0.84 | 1.91 ± 0.24 |
| norm-Se group | 3.52 ± 0.37 | 15.64 ± 3.19 | 2.13 ± 0.37 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stupin, A.; Cosic, A.; Novak, S.; Vesel, M.; Jukic, I.; Popovic, B.; Karalic, K.; Loncaric, Z.; Drenjancevic, I. Reduced Dietary Selenium Impairs Vascular Function by Increasing Oxidative Stress in Sprague-Dawley Rat Aortas. Int. J. Environ. Res. Public Health 2017, 14, 591. https://doi.org/10.3390/ijerph14060591
Stupin A, Cosic A, Novak S, Vesel M, Jukic I, Popovic B, Karalic K, Loncaric Z, Drenjancevic I. Reduced Dietary Selenium Impairs Vascular Function by Increasing Oxidative Stress in Sprague-Dawley Rat Aortas. International Journal of Environmental Research and Public Health. 2017; 14(6):591. https://doi.org/10.3390/ijerph14060591
Chicago/Turabian StyleStupin, Ana, Anita Cosic, Sanja Novak, Monika Vesel, Ivana Jukic, Brigita Popovic, Krunoslav Karalic, Zdenko Loncaric, and Ines Drenjancevic. 2017. "Reduced Dietary Selenium Impairs Vascular Function by Increasing Oxidative Stress in Sprague-Dawley Rat Aortas" International Journal of Environmental Research and Public Health 14, no. 6: 591. https://doi.org/10.3390/ijerph14060591





