Enteric Viruses and Fecal Bacteria Indicators to Assess Groundwater Quality and Suitability for Irrigation
Abstract
:1. Introduction
2. Materials and Methods
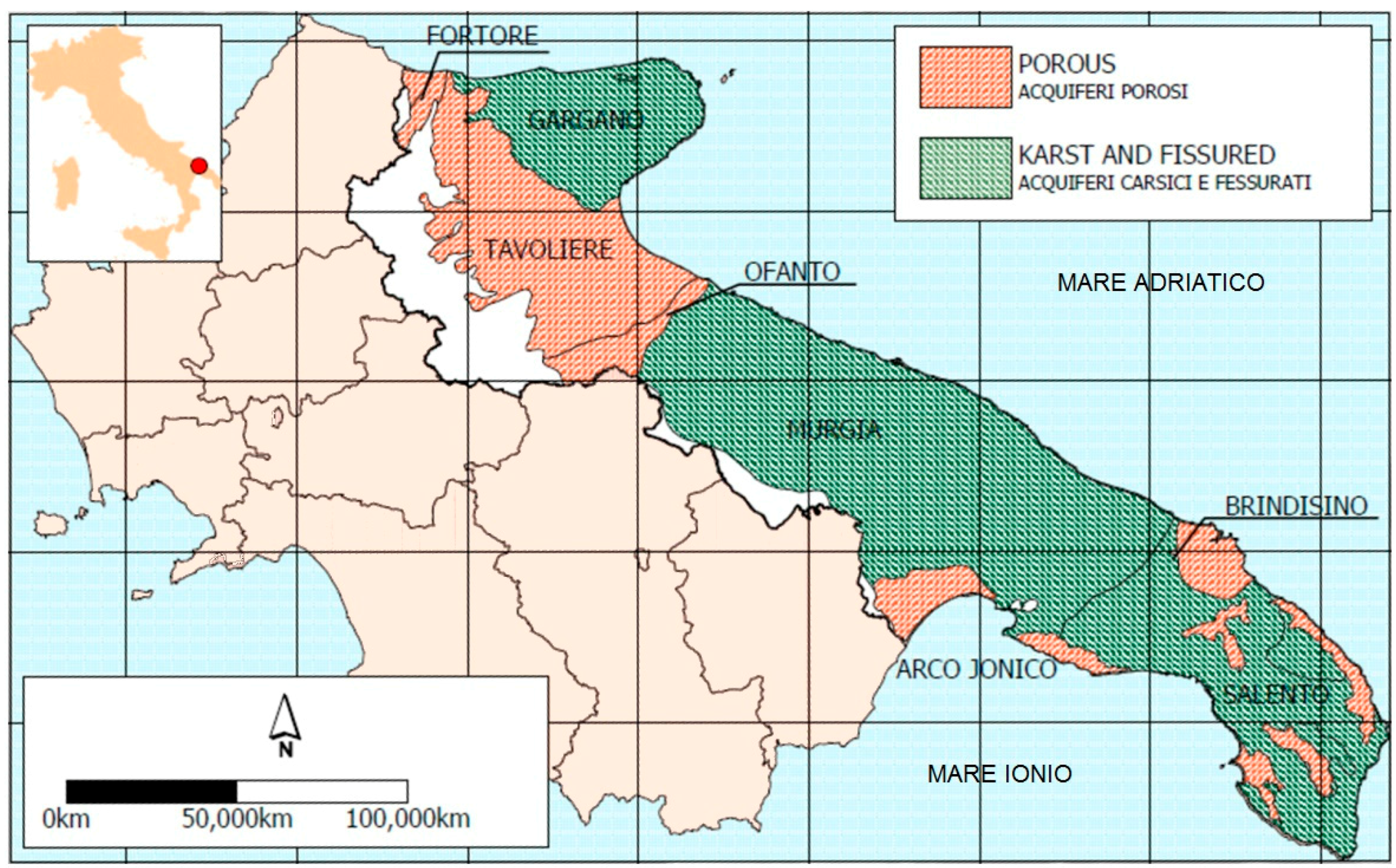
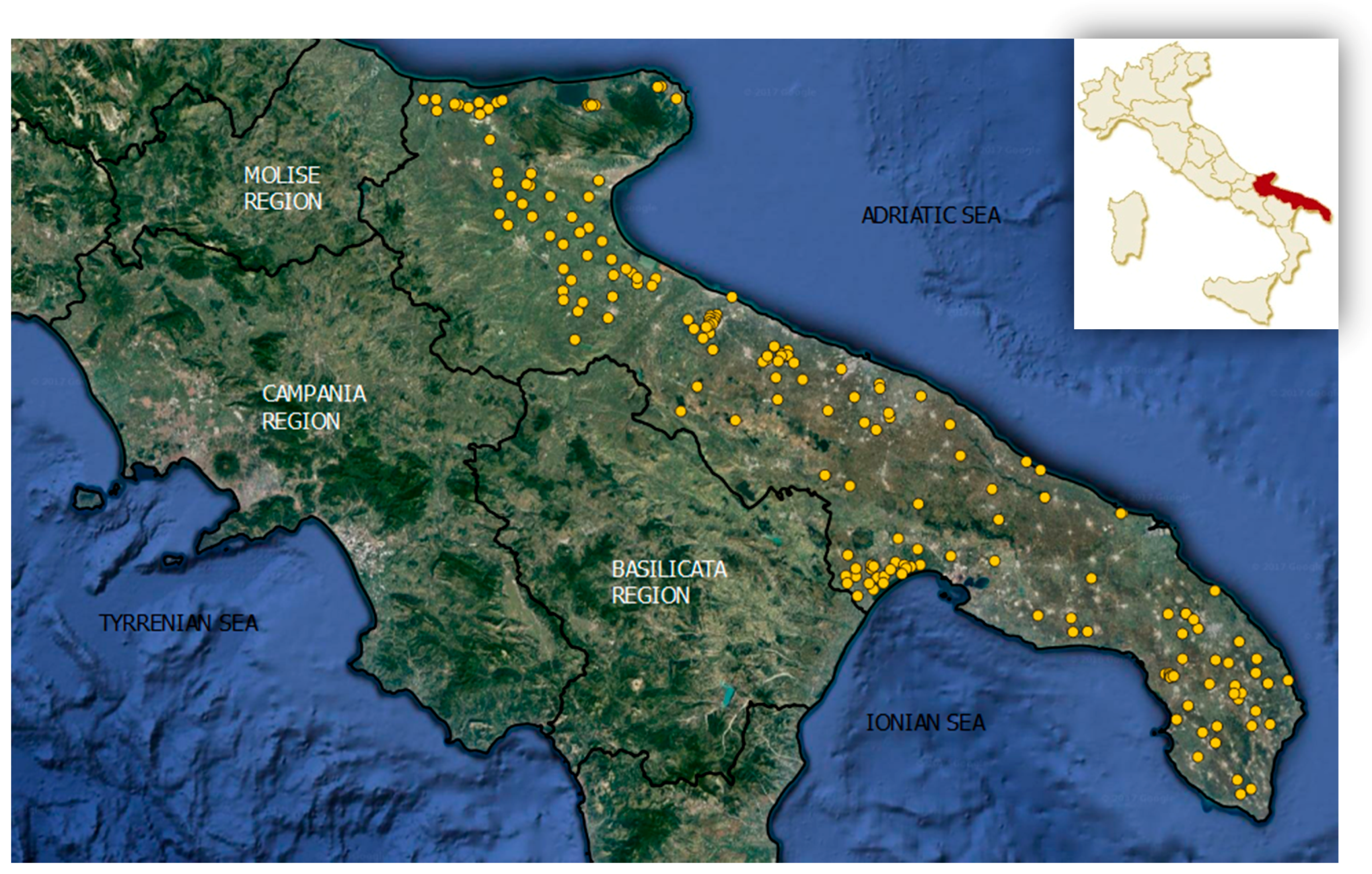
2.1. Study Area
2.2. Water Sampling
2.3. Bacteria Detection
2.4. Enteric Virus Detection
2.4.1. Concentration Process
2.4.2. Viral Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Danon-Schaffer, M.N. Walkerton’s contaminated water supply system: A forensic approach to identifying the source. Environ. Forensics 2001, 2, 197–200. [Google Scholar] [CrossRef]
- Wu, J.; van Geen, A.; Ahmed, K.M.; Alam, Y.A.J.; Culligan, P.J.; Escamilla, V.; Feighery, J.; Ferguson, A.S.; Knappett, P.; Mailloux, B.J.; et al. Increase in diarrheal disease associated with arsenic mitigation in Bangladesh. PLoS ONE 2011, 6, e29593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kelly, W.R.; Panno, S.V.; Liu, W.T. Tracing fecal pollution sources in karst groundwater by Bacteroidales genetic biomarkers, bacterial indicators, and environmental variables. Sci. Total Environ. 2014, 490, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, D.L.; Barry, D.A.J.; Goss, M.J. Contamination in Ontario farmstead domestic wells and its association with agriculture: 2. Results from multilevel monitoring well installations. J. Contam. Hydrol. 1998, 32, 295–311. [Google Scholar] [CrossRef]
- Pang, L.P.; Nokes, C.; Simunek, J.; Kikkert, H.; Hector, R. Modeling the impact of clustered septic tank systems on groundwater quality. Vadose Zone J. 2006, 5, 599–609. [Google Scholar] [CrossRef]
- Embrey, S.S.; Runkle, D.L. Microbial Quality of the Nation’s Ground-Water Resources, 1993–2004, USGS Scientific Investigations Report 2006-5290. In National Water-Quality Assessment Program Principal Aquifers; United States Geological Survey: Reston, VA, USA, 2006. [Google Scholar]
- Luby, S.P.; Gupta, S.K.; Sheikh, M.A.; Johnston, R.B.; Ram, P.K.; Islam, M.S. Tube well water quality and predictors of contamination in three flood-prone areas in Bangladesh. J. Appl. Microbiol. 2008, 105, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Lugoli, F.; Leopizzi, I.; Bagordo, F.; Grassi, T.; Guido, M.; De Donno, A. Widespread microbiological groundwater contamination in the South-eastern Salento (Puglia-Italy). J. Environ. Monit. 2011, 13, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Van Geen, A.; Ahmed, K.M.; Akita, Y.; Alam, M.J.; Culligan, P.J.; Emch, M.; Escamilla, V.; Feighery, J.; Ferguson, A.S.; Knappett, P.; et al. Fecal contamination of shallow tube wells in Bangladesh inversely related to arsenic. Environ. Sci. Technol. 2011, 45, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Knappett, P.S.K.; McKay, L.D.; Layton, A.; Williams, D.E.; Alam, M.J.; Huq, M.R.; Mey, J.; Feighery, J.E.; Culligan, P.J.; Mailloux, B.J.; et al. Implications of fecal bacteria input from latrine-polluted ponds for wells in sandy aquifers. Environ. Sci. Technol. 2012, 46, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, A.S.; Layton, A.C.; Mailloux, B.J.; Culligan, P.J.; Williams, D.E.; Smartt, A.E.; Sayler, G.S.; Feighery, J.; McKay, L.; Knappett, P.S.K.; et al. Comparison of fecal indicators with pathogenic Rotavirus in groundwater. Sci. Total Environ. 2012, 431, 314–322. [Google Scholar] [CrossRef] [PubMed]
- De Giglio, O.; Quaranta, A.; Barbuti, G.; Napoli, C.; Caggiano, G.; Montagna, M.T. Factors influencing groundwater quality: Towards an integrated management approach. Ann. Ig. 2015, 27, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Alamgir, A.; Ali Khan, M.; Schilling, J.; Shaukat, S.S.; Shahab, S. Assessment of groundwater quality in the coastal area of Sindh province, Pakistan. Environ. Monit. Assess. 2016, 188, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bagordo, F.; Migoni, D.; Grassi, T.; Serio, F.; Idolo, A.; Guido, M.; Zaccarelli, N.; Fanizzi, F.P.; De Donno, A. Using the DPSIR framework to identify factors influencing the quality of groundwater in GrecìaSalentina (Puglia, Italy). Rend. Lincei 2016, 27, 113–125. [Google Scholar] [CrossRef]
- Heaton, J.C.; Jones, K. Microbial contamination of fruit and vegetables and the behaviour of enteropathogens in the phyllosphere: A review. J. Appl. Microbiol. 2008, 104, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Cooley, M.; Carychao, D.; Crawford-Miksza, L.; Jay, M.T.; Myers, C.; Rose, C.; Keys, C.; Farrar, J.; Mandrell, R.E. Incidence and tracking of Escherichia coli O157:H7 in a major produce production region in California. PLoS ONE 2007, 2, e1159. [Google Scholar] [CrossRef] [PubMed]
- Uyttendaele, M.; Jaykus, L.-A.; Amoah, P.; Chiodini, A.; Cunliffe, D.; Jacxsens, L.; Holvoet, K.; Korsten, L.; Lau, M.; McClure, P.; et al. Microbial hazards in irrigation water: Standards, norms, and testing to manage use of water in fresh produce primary production. Compr. Rev. Food Sci. Food Saf. 2015, 14, 336–356. [Google Scholar] [CrossRef]
- Callejón, R.M.; Rodríguez-Naranjo, M.I.; Ubeda, C.; Hornedo-Ortega, R.; Garcia-Parrilla, M.C.; Troncoso, A.M. Reported foodborne outbreaks due to fresh produce in the United States and European Union: Trends and causes. Foodborne Pathog Dis. 2015, 12, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Grassi, T.; Bagordo, F.; Idolo, A.; Lugoli, F.; Gabutti, G.; De Donno, A. Rotavirus detection in environmental water samples by tangential flow ultrafiltration and RT-nested PCR. Environ. Monit. Assess. 2010, 164, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Ogorzaly, L.; Tissier, A.; Bertrand, I.; Maul, A.; Gantzer, C. Relationship between F-specific RNA phage genogroups, faecal pollution indicators and human adenoviruses in river water. Water Res. 2009, 43, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, G.; Muscillo, M. Molecular Detection of Viruses in Water and Sewage. In Viruses in Food and Water: Risks, Surveillance and Control; Cook, N., Ed.; Wood Head Publishing: Cambridge, UK, 2013; pp. 97–125. [Google Scholar]
- European Food Safety Authority (EFSA), Parma, Italy. Scientific Opinion on the Risk Posed by Pathogens in Food of Non-Animal Origin. Part 2 (Salmonella, Yersinia, Shigella and Norovirus in Bulb and Stem Vegetables, and Carrots). EFSA Panel on Biological Hazards (BIOHAZ). Available online: https://www.efsa.europa.eu/en/efsajournal/pub/3937 (accessed on 24 March 2017).
- Regulation (EC) No 852/2004 of the European Parliament and of the Council of 29 April 2004 on the Hygiene of Foodstuffs. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32004R0852 (accessed on 22 May 2017).
- Ministry of Environment Territory. Ministerial Decree of 12 June 2003, No 185, Regulations on Technical Standards for Reuse Wastewater, OJ No. 169 of 23/07/2003; Ministry of Environment Territory: Roma, Italy, 2003. [Google Scholar]
- De Giglio, O.; Barbuti, G.; Trerotoli, P.; Brigida, S.; Calabrese, A.; Di Vittorio, G.; Lovero, G.; Caggiano, G.; Uricchio, V.F.; Montagna, M.T. Microbiological and hydrogeological assessment of groundwater in Southern Italy. Environ. Monit. Assess. 2016, 188, 638. [Google Scholar] [CrossRef] [PubMed]
- Piano di Tutela delle Acque della Regione Puglia–Relazione Generale 2009. Available online: http://www.regione.puglia.it/index.php?page=documenti&id=29&opz=getdoc (accessed on 12 May 2017).
- Tema Ambientale Agenti Fisici Attività Specifica—Servizio Meteo. Available online: http://www.arpa.puglia.it/web/guest/serviziometeo (accessed on 12 May 2017).
- International Organization for Standardization (ISO). EN ISO 9308-1:2000 Water Quality—Detection and Enumeration of Escherichia coli and Coliform Bacteria—Part 1. Membrane Filtration Method; ISO: Geneva, Switzerland, 2000. [Google Scholar]
- Standard Method 9260D: Quantitative Salmonella Procedures. Available online: http://www.standardmethods.org/store/ProductView.cfm?ProductID=115 (accessed on 22 May 2017).
- International Organization for Standardization (ISO). EN ISO 7899-2 Water Quality—Detection and Enumeration of Intestinal Enterococci—Part 2: Membrane Filtration Method; ISO: Geneva, Switzerland, 2003. [Google Scholar]
- International Organization for Standardization Technical Specification (ISO/TS). ISO/TS 15216-2 Microbiology of Food and Animal Feed—Horizontal Method for Determination of Hepatitis A Virus and Norovirus in Food Using Real-Time RT-PCR—Part 2: Method for Qualitative Detection; ISO: Geneva, Switzerland, 2013. [Google Scholar]
- Steele, M.; Odumeru, J. Irrigation water as source of foodborne pathogens on fruit and vegetables. J. Food Prot. 2004, 67, 2839–2849. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, A.C.; Arias, C.F.; Sánchez-Colón, S.; Mazari-Hiriart, M. Comparative study of enteric viruses, coliphages and indicator bacteria for evaluating water quality in a tropical high-altitude system. Environ. Health 2009, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Gin, K.Y.; Ngo, H.H. Fecal pollution source tracking toolbox for identification, evaluation and characterization of fecal contamination in receiving urban surface waters and groundwater. Sci. Total Environ. 2015, 538, 38–57. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, G.; Fratini, M.; della Libera, S.; Iaconelli, M.; Muscillo, M. Emerging and potentially emerging viruses in water environments. Annali Istituto Superiore Sanità 2012, 48, 397–406. [Google Scholar] [CrossRef]
- Masciopinto, C.; La Mantia, R.; Pollice, A.; Laera, G. Manages Aquifer Recharge of a karstic aquifer in Nardò, Italy. In Water Reclamation Technologies for Safe Managed Aquifer Recharge; Kazner, C., Wintgens, T., Dillon, P., Eds.; IWA Publishing: London, UK, 2012; ISBN 9781843393443. [Google Scholar]
- European Food Safety Authority. Tracing of food items in connection to the multinational hepatitis A virus outbreak in Europe. EFSA J. 2014, 12, 186. [Google Scholar]
- Suffredini, E.; La Rosa, G. Norovirus in Italy. In Noroviruses: Outbreaks, Control and Prevention Strategies; Romalde, J.L., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2017; in press. [Google Scholar]
- Gabrieli, R.; Maccari, F.; Ruta, A.; Panà, A.; Divizia, M. Norovirus detection in groundwater. Food Environ. Virol. 2009, 1, 92–96. [Google Scholar] [CrossRef]
- Fongaro, G.; Padilha, J.; Schissi, C.D.; Nascimento, M.A.; Bampi, G.B.; Viancelli, A.; Barardi, C.R. Human and animal enteric virus in groundwater from deep wells, and recreational and network water. Environ. Sci. Pollut. Res. Int. 2015, 22, 20060–20066. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, D.; Prato, R.; Chironna, M.; Sallustio, A.; Caputi, G.; Conversano, M.; Ciofi Degli Atti, M.; D’Ancona, F.P.; Germinario, C.A.; Quarto, M. Large outbreak of viral gastroenteritis caused by contaminated drinking water in Apulia, Italy, May–October 2006. Euro Surveill. 2007, 12, E070419.1. [Google Scholar] [PubMed]
- Ruggeri, F.M.; Bonomo, P.; Ianiro, G.; Battistone, A.; Delogu, R.; Germinario, C.; Chironna, M.; Triassi, M.; Campagnuolo, R.; Cicala, A.; et al. Rotavirus genotypes in sewage treatment plants and in children hospitalized with acute diarrhea in Italy in 2010 and 2011. Appl. Environ. Microbiol. 2015, 81, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Gallone, M.F.; Desiante, F.; Gallone, M.S.; Barbuti, G.; Tafuri, S.; Germinario, C. Serosurveillance of hepatitis A in a region which adopted the universal mass vaccination. Medicine 2017, 96, e5884. [Google Scholar] [CrossRef] [PubMed]
- Ajelli, M.; Iannelli, M.; Manfredi, P.; Ciofi degli Atti, M.L. Basic mathematical model for the temporal dynamics of HAV in medium-endemicity Italian areas. Vaccine 2008, 26, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, K.R.; Borchardt, M.A.; Gotkowitz, M.; Spencer, S.K.; Zhu, J.; Hunt, R.J. Source and transport of human enteric viruses in deep municipal water supply wells. Environ. Sci. Technol. 2013, 47, 4096–4103. [Google Scholar] [CrossRef] [PubMed]
- Parshionikar, S.; Laseke, I.; Fout, G.S. Use of propidium monoazide in reverse transcriptase PCR to distinguish between infectious and noninfectious enteric viruses in water samples. Appl. Environ. Microbiol. 2010, 76, 4318–4326. [Google Scholar] [CrossRef] [PubMed]
- Goeppert, N.; Goldscheider, N. Transport and variability of fecal bacteria carbonate conglomerate aquifers. Ground Water 2011, 49, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Bitton, G.; Harvey, R.W. Transport of Pathogens through Soils and Aquifers. In Environmental Microbiology; Mitchell, R., Ed.; Wiley-Liss: New York, NY, USA, 1992; pp. 103–124. [Google Scholar]
- Borchardt, M.A.; Bradbury, K.R.; Gotkowitz, M.B.; Cherry, J.A.; Parker, B.L. Human enteric viruses in groundwater from a confined bedrock aquifer. Environ. Sci. Technol. 2007, 41, 6606–6612. [Google Scholar] [CrossRef] [PubMed]
- Fong, T.T.; Lipp, E.K. Enteric viruses of humans and animals in aquatic environments: Health risks, detection, and potential water quality assessment tools. Microbiol. Mol. Biol. Rev. 2005, 69, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Krauss, S.; Griebler, C. Pathogenic Microorganisms and Viruses in Groundwater. Available online: http://www.acatech.de/fileadmin/user_upload/Baumstruktur_nach_Website/Acatech/root/de/Publikationen/Materialienbaende/acatech_Materialband_Nr6_WEB.pdf (accessed on 21 March 2017).
- Pronk, M.; Goldscheider, N.; Zopfi, J. Particle-size distribution as indicator for fecal bacteria contamination of drinking water from karst springs. Environ. Sci. Technol. 2007, 41, 8400–8405. [Google Scholar] [CrossRef] [PubMed]
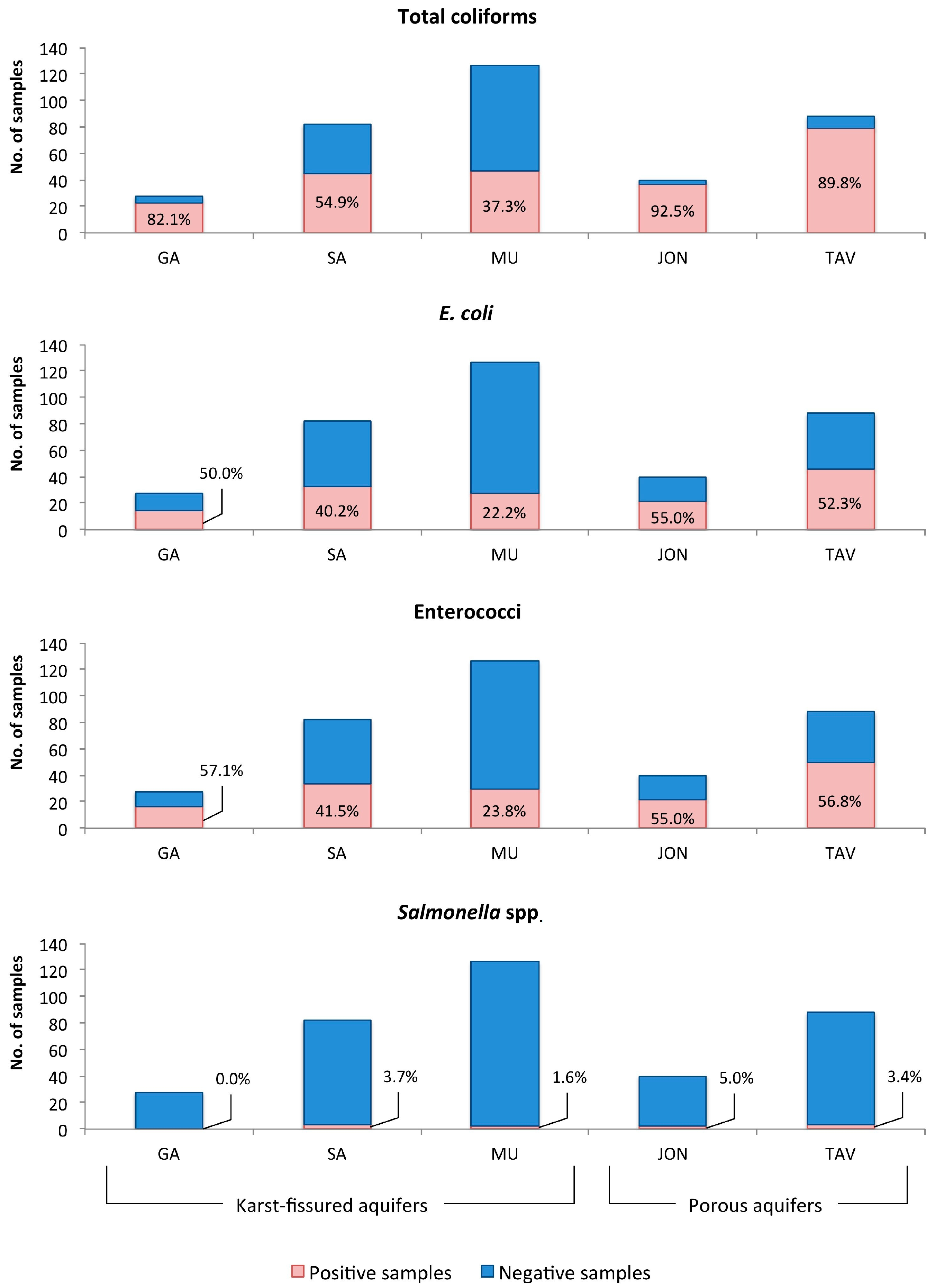

| Bacteria | Karst-Fissured Aquifers | Porous Aquifers | Total | p-Value | |||
|---|---|---|---|---|---|---|---|
| GA | SA | MU | JON | TAV | |||
| E. coli a | 0.5 (0–120) | 0 (0–3000) | 0 (0–1700) | 1 (0–1600) | 1 (0–900) | 0 (0–3000) | <0.001 |
| Salmonella spp. b | 0 (0–0) | 0 (0–120) | 0 (0–20) | 0 (0–8) | 0 (0–7) | 0 (0–120) | 0.638 |
| Total coliforms a | 30 (0–900) | 2 (0–6000) | 0 (0–2000) | 24 (0–6000) | 35 (0–2000) | 10 (0–6000) | <0.001 |
| Enterococci a | 2.5 (0–50) | 0 (0–900) | 0 (0–500) | 3 (0–300) | 1 (0–500) | 0 (0–900) | <0.001 |
| Virus | Karst-Fissured Aquifers | Porous Aquifers | Total (364) No. (%) | p-Value | |||
|---|---|---|---|---|---|---|---|
| GA (28) No. (%) | SA (82) No. (%) | MU (126) No. (%) | JON (40) No. (%) | TAV (88) No. (%) | |||
| Enterovirus | 0 (-) | 2 (2.4) | 0 (-) | 1 (2.5) | 1 (1.1) | 4 (1.1) | 0.435 |
| Norovirus | 1 (3.6) | 3 (3.7) | 10 (7.9) | 3 (7.5) | 1 (1.1) | 18 (4.9) | 0.194 |
| Rotavirus | 1 (3.6) | 2 (2.4) | 0 (-) | 0 (-) | 2 (2.3) | 5 (1.4) | 0.340 |
| Hepatitis A virus | 0 (-) | 0 (-) | 0 (-) | 0 (-) | 0 (-) | 0 (-) | - |
| TOTAL | 2 (7.1) | 7 (8.5) | 10 (7.9) | 4 (10.0) | 4 (4.5) | 27 (7.4) | 0.800 |
| Aquifers | Unsuitable Wells */Total Wells No. (%) | Presence of Virus/Suitable Wells * No. (%) | Total Wells with Potential Infectious Risk No. (%) |
|---|---|---|---|
| Karst-Fissured | 20/118 (16.9) | 17/98 (17.3) | 37/118 (31.3) |
| GA | 2/14 (14.3) | 2/12 (16.7) | 4/14 (28.6) |
| SA | 9/41 (22.0) | 6/32 (18.7) | 15/41 (36.6) |
| MU | 9/63 (14.3) | 9/54 (16.7) | 18/63 (28.6) |
| Porous | 15/64 (23.4) | 6/49 (12.2) | 21/64 (32.8) |
| JON | 3/20 (15.0) | 4/17 (23.5) | 7/20 (35.0) |
| TAV | 12/44 (27.3) | 2/32 (6.3) | 14/44 (31.8) |
| Total | 35/182 (19.2) | 23/147 (15.6) | 58/182 (31.9) |
| Microbiological Parameters | Winter | Summer | p-Value | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Total coliforms | 113 | 62.1 | 118 | 64.8 | 0.586 |
| E. coli | 69 | 37.9 | 74 | 40.7 | 0.592 |
| Enterococci | 73 | 40.1 | 79 | 43.4 | 0.524 |
| Salmonella spp. | 3 | 1.6 | 7 | 3.8 | 0.200 |
| Enteric viruses | 15 | 8.2 | 12 | 6.6 | 0.549 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Giglio, O.; Caggiano, G.; Bagordo, F.; Barbuti, G.; Brigida, S.; Lugoli, F.; Grassi, T.; La Rosa, G.; Lucentini, L.; Uricchio, V.F.; et al. Enteric Viruses and Fecal Bacteria Indicators to Assess Groundwater Quality and Suitability for Irrigation. Int. J. Environ. Res. Public Health 2017, 14, 558. https://doi.org/10.3390/ijerph14060558
De Giglio O, Caggiano G, Bagordo F, Barbuti G, Brigida S, Lugoli F, Grassi T, La Rosa G, Lucentini L, Uricchio VF, et al. Enteric Viruses and Fecal Bacteria Indicators to Assess Groundwater Quality and Suitability for Irrigation. International Journal of Environmental Research and Public Health. 2017; 14(6):558. https://doi.org/10.3390/ijerph14060558
Chicago/Turabian StyleDe Giglio, Osvalda, Giuseppina Caggiano, Francesco Bagordo, Giovanna Barbuti, Silvia Brigida, Federica Lugoli, Tiziana Grassi, Giuseppina La Rosa, Luca Lucentini, Vito Felice Uricchio, and et al. 2017. "Enteric Viruses and Fecal Bacteria Indicators to Assess Groundwater Quality and Suitability for Irrigation" International Journal of Environmental Research and Public Health 14, no. 6: 558. https://doi.org/10.3390/ijerph14060558







