Sustainable Methods for Decontamination of Microcystin in Water Using Cold Plasma and UV with Reusable TiO2 Nanoparticle Coating
Abstract
:1. Introduction
2. Materials and Methods
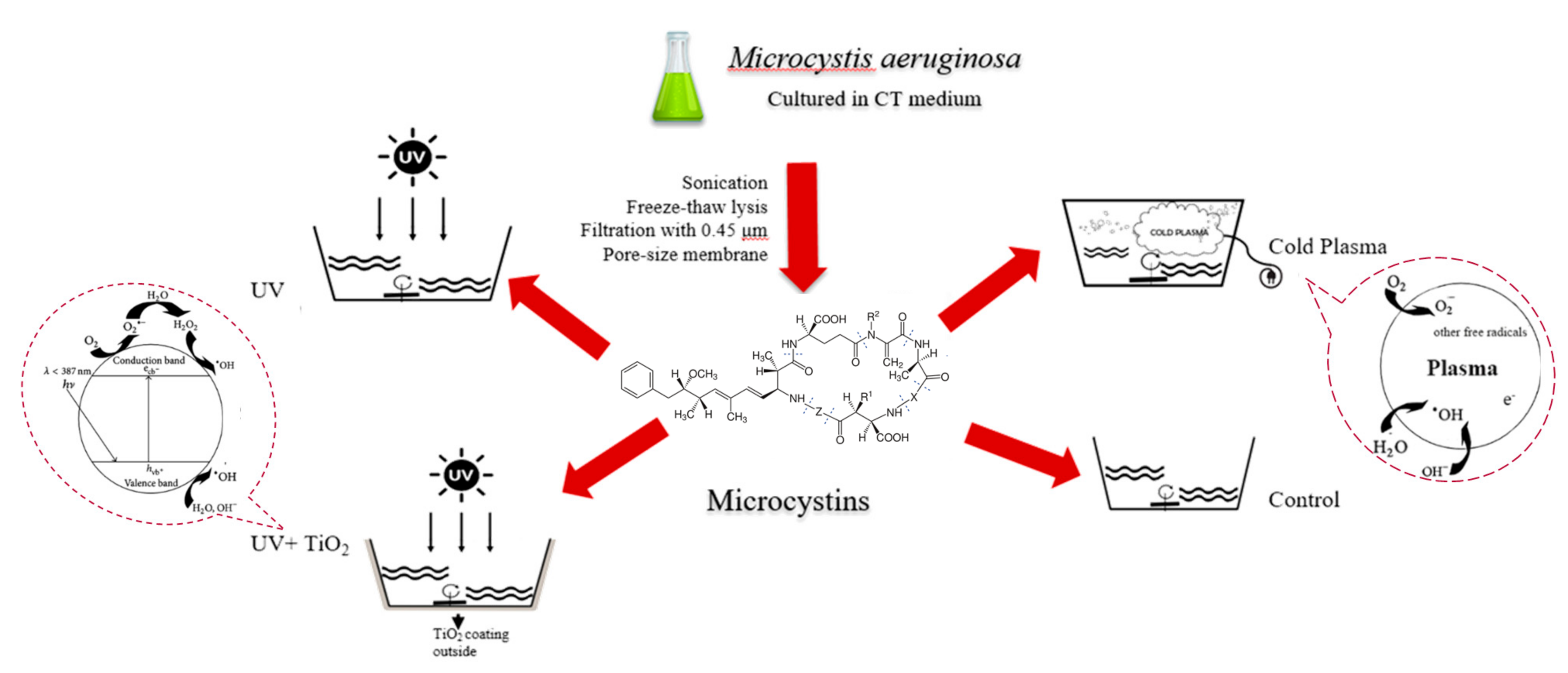
2.1. MC Preparation
2.2. MC Treatments and Measurement
2.3. Statistical Analysis
2.4. Energy per Order (EEO) of Each Treatment
3. Results
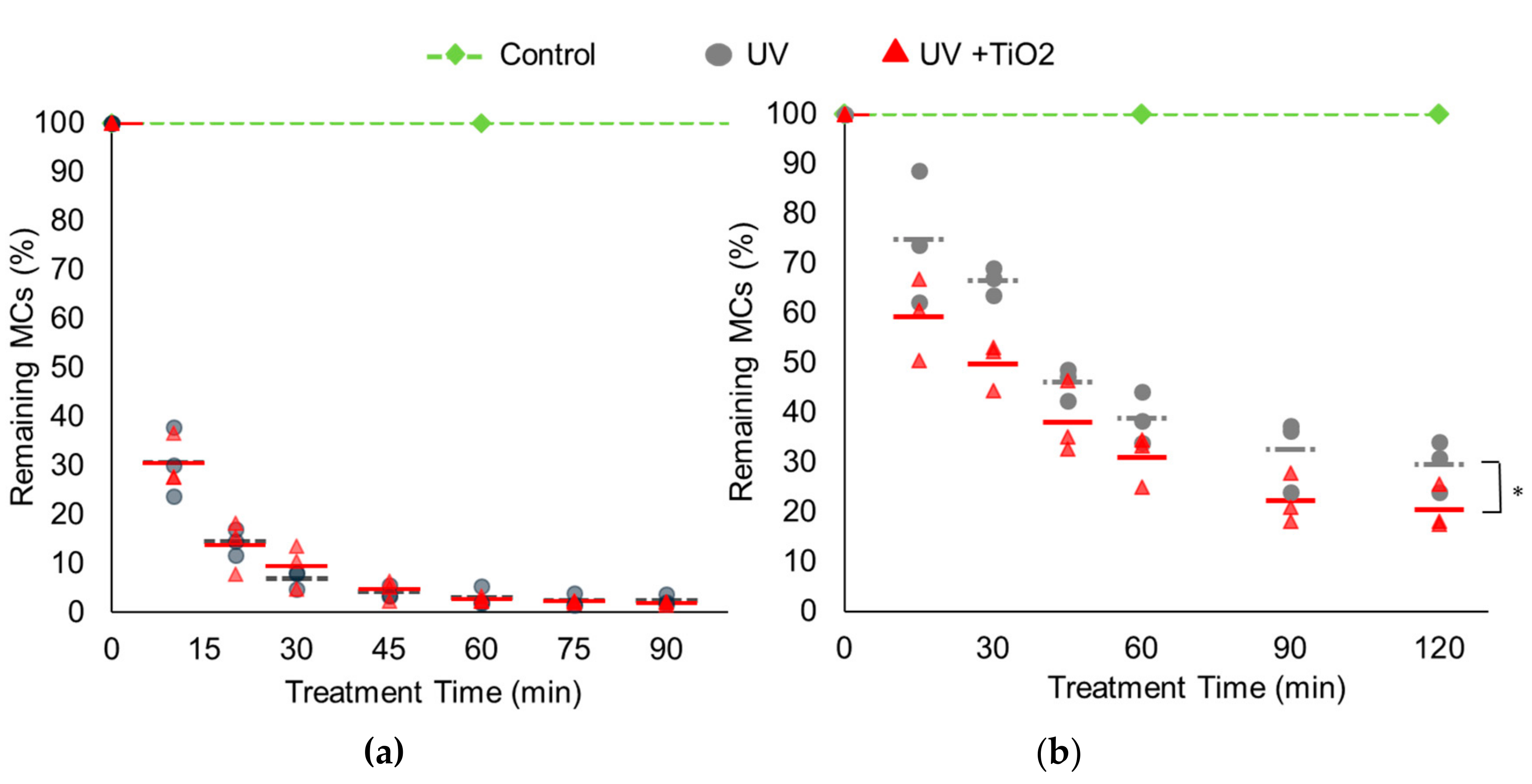
3.1. Synergistic Effects of TiO2 with UV on MC Degradation
3.2. Effectiveness of Cold Plasma on MC Degradation
3.3. Kinetic Analysis
3.4. Energy per Order (EEO)
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
Appendix A
| Time (min) | 0 | 30 | 90 | 120 | ||
|---|---|---|---|---|---|---|
| Concentrstion (ppb) | ||||||
| Treatment | ||||||
| UV (Irradiance: 1470 μW/cm2) | 6.76 ± 1.50 | 0.48 ± 0.15 | 0.16 ± 0.03 | N/A | ||
| UV with TiO2 (Irradiance: 1470 μW/cm2) | 6.95 ± 0.92 | 0.66 ± 0.34 | 0.13 ± 0.016 | |||
| UV (Irradiance: 180 μW/cm2) | 8.63 ± 0.0 | 5.74 ± 0.24 | 2.81 ± 0.64 | 2.55 ± 0.45 | ||
| UV with TiO2 (Irradiance: 180 μW/cm2) | 12.72 ± 0.0 | 6.33 ± 0.61 | 2.83 ± 0.63 | 2.59 ± 0.58 | ||
| Cold plasma | 6.13 ± 0.78 | 2.20 ± 0.45 | 0.85 ± 0.12 | 0.50 ± 0.11 | ||
References
- Cheung, M.Y.; Liang, S.; Lee, J. Toxin-producing cyanobacteria in freshwater: A review of the problems, impact on drinking water safety, and efforts for protecting public health. J. Microbiol. 2013, 51, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Harke, M.J.; Steffen, M.M.; Gobler, C.J.; Otten, T.G.; Wilhelm, S.W.; Wood, S.A.; Paerl, H.W. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 2016, 54, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Funari, E.; Testai, E. Human health risk assessment related to cyanotoxins exposure. Crit. Rev. Toxicol. 2008, 38, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, B.; Gervais, M.; Chevalier, P.; Gauvin, D. Prospective study of acute health effects in relation to exposure to cyanobacteria. Sci. Total 2014, 466, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Rudolph-Böhner, S.; Mierke, D.; Moroder, L. Molecular structure of the cyanobacterial tumor-promoting microcystins. FEBS Lett. 1994, 349, 319–323. [Google Scholar] [CrossRef]
- Dawson, R.M. The toxicology of microcystins. Toxicon 1998, 36, 953–962. [Google Scholar] [CrossRef]
- Ferrão-Filho, A.D.S.; Kozlowsky-Suzuki, B. Cyanotoxins: Bioaccumulation and Effects on Aquatic Animals. Mar. Drugs 2011, 9, 2729–2772. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Guidelines for Drinking-Water Quality, Fourth Edition. Available online: http://www.who.int/water_sanitation_health/publications/2011/dwq_guidelines/en/ (accessed on 23 April 2017).
- EPA, U.S.E.P.A. Toxicological Review of Cyanobacterial Toxins: Microcystins LR, RR, YR and LA (External Review Draft). Available online: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=160548&CFID=79569862&CFTOKEN=96333965 (accessed on 11 April 2017).
- EPA, U.S.E.P.A. Drinking Water Health Advisory for the Cyanobacterial Toxin Cylindrospermopsin. Available online: https://www.google.ch/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=0ahUKEwi4j-_yg7_TAhWrA8AKHQgBCzoQFggnMAA&url=https%3A%2F%2Fwww.epa.gov%2Fsites%2Fproduction%2Ffiles%2F2015-06%2Fdocuments%2Fcylindrospermopsin-report-2015.pdf&usg=AFQjCNE0OOTCg5ehEXmN0gZrMNcOUnfYeg&cad=rja (accessed on 11 April 2017).
- EPA Office of Water, U.S. Human Health Recreational Ambient Water Quality Criteria or Swimming Advisories for Microcystins and Cylindrospermopsin—Draft. Available online: https://www.epa.gov/sites/production/files/2016-12/documents/draft-hh-rec-ambient-water-swimming-document.pdf (accessed on 23 April 2017).
- Hitzfeld, B.; Höger, S.; Dietrich, D. Cyanobacterial toxins: Removal during drinking water treatment, and human risk assessment. Environ. Health Perspect. 2000, 108, 113. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Chen, Z.; Wang, Z.; Kang, J.; Chen, Q. Oxidation of microcystin-LR in water by ozone combined with UV radiation: The removal and degradation pathway. Chem. Eng. J. 2015, 276, 97–105. [Google Scholar] [CrossRef]
- He, X.; Armah, A.; Hiskia, A.; Kaloudis, T.; O’Shea, K. Destruction of microcystins (cyanotoxins) by UV-254 nm-based direct photolysis and advanced oxidation processes (AOPs): Influence of variable amino acids on the degradation kinetics and reaction mechanisms. Water Res. 2015, 74, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Pinho, L.; Azevedo, J.; Brito, Â.; Santos, A. Effect of TiO2 photocatalysis on the destruction of Microcystis aeruginosa cells and degradation of cyanotoxins microcystin-LR and cylindrospermopsin. Chem. Eng. J. 2015, 268, 144–152. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, Y.; Gao, N.; Chu, W.; Sun, Z. Removal of microcystin-LR by free chlorine: Identify of transformation products and disinfection by-products formation. Chem. Eng. J. 2016, 287, 189–195. [Google Scholar] [CrossRef]
- Liu, S.; Hu, X.; Jiang, W.; Ma, L.; Cai, M.; Xu, H.; Wu, M.; Ma, F. Degradation of Microcystins from Microcystis aeruginosa by 185-nm UV Irradiation. Water Air Soil Pollut. 2016, 227, 129. [Google Scholar] [CrossRef]
- Chang, J.; Chen, Z.; Wang, Z.; Shen, J.; Chen, Q.; Kang, J. Ozonation degradation of microcystin-LR in aqueous solution: intermediates, byproducts and pathways. Water Res. 2014, 63, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Phujomjai, Y.; Somdee, A.; Somdee, T. Biodegradation of microcystin [Dha7]MC-LR by a novel microcystin-degrading bacterium in an internal airlift loop bioreactor. Water Sci. Technol. 2016, 73, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Pestana, C.; Edwards, C.; Prabhu, R.; Robertson, P. Photocatalytic degradation of eleven microcystin analogues and nodularin by TiO2 coated glass microspheres. J. Hazard. Mater. 2015, 300, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Michalak, A. Challenges in tracking harmful algal blooms: A synthesis of evidence from Lake Erie. J. Great Lakes Res. 2015, 41, 317–325. [Google Scholar] [CrossRef]
- De Rijcke, M.; Vandegehuchte, M.; Bussche, J. Common European Harmful Algal Blooms affect the viability and innate immune responses of Mytilus edulis larvae. Fish Shellfish Immunol. 2015, 47, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Selcuk, M.; Oksuz, L.; Basaran, P. Decontamination of grains and legumes infected with Aspergillus spp. and Penicillum spp. by cold plasma treatment. Bioresour. Technol. 2008, 99, 5104–5109. [Google Scholar] [CrossRef] [PubMed]
- Dhayal, M.; Lee, S.; Park, S. Using low-pressure plasma for Carthamus tinctorium L. seed surface modification. Vacuum 2006, 80, 499–506. [Google Scholar] [CrossRef]
- Hickling, A. Electrochemical Processes in Glow Discharge at the Gas-Solution Interface. In Modern Aspects of Electrochemistry No. 6; Springer: Boston, MA, USA, 1971; pp. 329–373. [Google Scholar]
- Perni, S.; Shama, G.; Kong, M.G. Cold Atmospheric Plasma Disinfection of Cut Fruit Surfaces Contaminated with Migrating Microorganisms. J. Food Prot. 2008, 71, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Montie, T.; Kelly-Wintenberg, K. An overview of research using the one atmosphere uniform glow discharge plasma (OAUGDP) for sterilization of surfaces and materials. IEEE Trans. Plasma Sci. 2000, 28, 41–50. [Google Scholar] [CrossRef]
- Sarangapani, C.; Misra, N.; Milosavljevic, V. Pesticide degradation in water using atmospheric air cold plasma. J. Water Process Eng. 2016, 9, 225–232. [Google Scholar] [CrossRef]
- Wang, H.; Chen, X. Kinetic analysis and energy efficiency of phenol degradation in a plasma-photocatalysis system. J. Hazard. Mater. 2011, 186, 1888–1892. [Google Scholar] [CrossRef] [PubMed]
- Thirumdas, R.; Sarangapani, C.; Annapure, U.S. Cold Plasma: A novel Non-Thermal Technology for Food Processing. Food Biophys. 2014, 10, 1–11. [Google Scholar] [CrossRef]
- Misra, N.; Pankaj, S.; Walsh, T.; O’Regan, F. In-package nonthermal plasma degradation of pesticides on fresh produce. J. Hazard. Mater. 2014, 271, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Min, Z.W.; Hong, S.-M.; Mok, C.-K.; Im, G.-J. Degradation of the Selected Pesticides by Gas Discharge Plasma. Korean J. Pestic. Sci. 2012, 16, 11–20. [Google Scholar] [CrossRef]
- Barillas, L. Design of a Prototype of Water Purification by Plasma Technology as the Foundation for an Industrial Wastewater Plant. J. Phys. Conf. Ser. 2015, 591, 12057. [Google Scholar] [CrossRef]
- Lawton, L.; Marsalek, B.; Padisák, J.; Chorus, I. Determination of Cyanobacteria in the Laboratory. In Toxic Cyanobacteria Water: A Guid to Their Public Health Consequences, Monitoring and Management; St Edmundsbury Press: England, UK, 1999. [Google Scholar]
- Fagan, R.; McCormack, D.E.; Dionysiou, D.D.; Pillai, S.C. A review of solar and visible light active TiO2 photocatalysis for treating bacteria, cyanotoxins and contaminants of emerging concern. Mater. Sci. Semicond. Process. 2016, 42, 2–14. [Google Scholar] [CrossRef]
- Liu, I.; Lawton, L.A.; Robertson, P.K.J. Mechanistic studies of the photocatalytic oxidation of microcystin-LR: An investigation of byproducts of the decomposition process. Environ. Sci. Technol. 2003, 37, 3214–3219. [Google Scholar] [CrossRef] [PubMed]
- Buthiyappan, A.; Abdul Aziz, A.R.; Wan Daud, W.M.A. Degradation performance and cost implication of UV-integrated advanced oxidation processes for wastewater treatments. Rev. Chem. Eng. 2015, 31, 263–302. [Google Scholar] [CrossRef]
- Hu, C.; Rea, C.; Yu, Z.; Lee, J. Relative importance of Microcystis abundance and diversity in determining microcystin dynamics in Lake Erie coastal wetland and downstream beach water. J. Appl. Microbiol. 2016, 120, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.M.; Kawachi, M.; Hiroki, M.; Kasai, F. Microalgae and Protozoa. Microbial Culture Collections. In NIES Collection List of Strains, 6th ed.; National Institute for Environmental Studies: Tsukuba, Japan, 2000. [Google Scholar]
- Rapala, J.; Erkomaa, K.; Kukkonen, J.; Sivonen, K.; Lahti, K. Detection of microcystins with protein phosphatase inhibition assay, high-performance liquid chromatography–UV detection and enzyme-linked immunosorbent assay: Comparison of methods. Anal. Chim. Acta 2002, 466, 213–231. [Google Scholar] [CrossRef]
- Choi, H.; Antoniou, M.G.; de la Cruz, A.A.; Stathatos, E.; Dionysiou, D.D. Photocatalytic TiO2 films and membranes for the development of efficient wastewater treatment and reuse systems. Desalination 2007, 202, 199–206. [Google Scholar] [CrossRef]
- Ohio EPA Total (Extracellular and Intracellular) Microcystins—ADDA by ELISA Analytical Methodology. Available online: https://www.google.ch/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=0ahUKEwiB4N6-hL_TAhVHIcAKHSnTBvkQFggiMAA&url=http%3A%2F%2Fepa.ohio.gov%2FPortals%2F28%2Fdocuments%2Fhabs%2FHAB_Analytical_Methodology.pdf&usg=AFQjCNFNYAXrJr2Q-1M_vlw_jL_pU2sOqg&cad=rja (accessed on 11 April 2017).
- Bolton, J.R.; Bircher, K.G.; Tumas, W.; Tolman, C.A. Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric- and solar-driven systems (IUPAC Technical Report). Pure Appl. Chem. 2001, 73, 627–637. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Q.; Ke, Z.; Yang, L.; Wang, X.; Yu, Z. Degradation of microcystin-LR in water by glow discharge plasma oxidation at the gas-solution interface and its safety evaluation. Water Res. 2012, 46, 6554–6562. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, M.; Silva, C.; Silva, A. Carbon-based TiO2 materials for the degradation of Microcystin-LA. Appl. Catal. B 2015, 170, 74–82. [Google Scholar] [CrossRef]
- Xagoraraki, I.; Harrington, G.W.; Zulliger, K.; Zeier, B.; Krick, W.; Karner, D.A.; Standridge, J.H.; Westrick, J. Inactivation kinetics of the cyanobacterial toxin microcystin-LR by free chlorine. J. Environ. Eng. 2006, 132, 818–823. [Google Scholar] [CrossRef]
- Qiao, R.P.; Li, N.; Qi, X.H.; Wang, Q.S.; Zhuang, Y.Y. Degradation of microcystin-RR by UV radiation in the presence of hydrogen peroxide. Toxicon 2005, 45, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Xin, Q.; Zhang, Y.; Wu, K. Degradation of Microcystin-LR by Gas-Liquid Interfacial Discharge Plasma. Plasma Sci. Technol. 2013, 15, 1221–1225. [Google Scholar] [CrossRef]
- Niedermeyer, T. Microcystin Congeners Described in the Literature. Available online: https://figshare.com/articles/_Microcystin_congeners_described_in_the_literature/880756 (accessed on 8 March 2017).
- EPA, U.S.E.P.A. Method 546: Determination of Total Microcystins and Nodularins in Drinking Water and Ambient Water by Adda Enzyme-Linked Immunosorbent Assay. Available online: https://www.google.ch/url?sa=t&rct=j&q=&esrc=s&source=web&cd=2&ved=0ahUKEwjQnJD5hL_TAhUsK8AKHdJDD2wQFggnMAE&url=https%3A%2F%2Fwww.epa.gov%2Fsites%2Fproduction%2Ffiles%2F2016-09%2Fdocuments%2Fmethod-546-determination-total-microcystins-nodularins-drinking-water-ambient-water-adda-enzyme-linked-immunosorbent-assay.pdf&usg=AFQjCNHUb2XiT27dC0x3YawD5PgF0MRQlw&cad=rja (accessed on 11 April 2017).
- Tsuji, K.; Watanuki, T.; Kondo, F.; Watanabe, M.F.; Suzuki, S.; Nakazawa, H.; Suzuki, M.; Uchida, H.; Harada, K.I. Stability of microcystins from cyanobacteria-II. Effect of UV light on decomposition and isomerization. Toxicon 1995, 33, 1619–1631. [Google Scholar] [CrossRef]
- Schnabel, U.; Sydow, D.; Schlüter, O.; Andrasch, M.; Ehlbeck, J. Decontamination of Fresh-Cut Iceberg Lettuce and Fresh Mung Bean Sprouts by Non-Thermal Atmospheric Pressure Plasma Processed Water (PPW). Modern Agric. Sci. Technol. 2015, 1, 23–39. [Google Scholar]
- Pignata, C.; D’Angelo, D.; Fea, E.; Gilli, G. A review on microbiological decontamination of fresh produce with nonthermal plasma. J. Appl. Microbiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.X.; Nian, W.F.; Wu, H.Y.; Feng, H.Q.; Zhang, K.; Zhang, J.; Zhu, W.D.; Becker, K.H.; Fang, J. Atmospheric-pressure cold plasma treatment of contaminated fresh fruit and vegetable slices: inactivation and physiochemical properties evaluation. Eur. Phys. J. D 2012, 66, 276. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Jiang, X. Cyanobacterial Toxins in Freshwater and Food: Important Sources of Exposure to Humans. Annu. Rev. Food Sci. 2017, 8, 281–304. [Google Scholar] [CrossRef] [PubMed]

| Treatment | First-order (Ln(C0/C) = −kt) | Reference | ||
|---|---|---|---|---|
| k (min−1) | t½ (min) | R2 | ||
| UV (Irradiance: 1470 μW/cm2) | 0.0394 | 17.59 | 0.89 | This study |
| UV with TiO2 (Irradiance: 1470 μW/cm2) | 0.0412 | 16.82 | 0.91 | This study |
| UV (Irradiance: 180 μW/cm2) | 0.0104 | 66.64 | 0.92 | This study |
| UV with TiO2 (Irradiance: 180 μW/cm2) | 0.0129 | 53.73 | 0.93 | This study |
| UV with TiO2 added (~182 ppb MC-LA; 47,100 μW/cm2) | 0.0277 | 25.02 | 0.99 | [29] |
| Treatment | Second-order (1/C − 1/C0 = kt) | Reference | ||
| k (ppb × min)−1 | t½ (min) | R2 | ||
| UV (Irradiance: 1470 μW/cm2) | 0.0848 | 1.74 | 0.92 | This study |
| UV with TiO2 (Irradiance: 1470 μW/cm2) | 0.0913 | 1.58 | 0.95 | This study |
| UV (Irradiance: 180 μW/cm2) | 0.0025 | 46.34 | 0.91 | This study |
| UV with TiO2 (Irradiance: 180 μW/cm2) | 0.0028 | 28.09 | 0.93 | This study |
| Treatment | First-order (Ln(C0/C) = −kt) | Reference | ||
|---|---|---|---|---|
| k (min−1) | t½ (min) | R2 | ||
| Cold plasma | 0.0192 | 36.10 | 0.95 | This study |
| Treatment | Second-order (1/C − 1/C0 = k t) | Reference | ||
| k (ppb × min)−1 | t½ (min) | R2 | ||
| Cold plasma | 0.0150 | 10.88 | 0.93 | This study |
| Glow discharge plasma (4025.5 ppb MC-LR) | 38.81 | 0.018 | Not mentioned | [44] |
| Treatment | EEO (kWh/m3/order) | Reference |
|---|---|---|
| UV (Irradiance: 1470 μW/cm2) | 27.96 | This study |
| UV with TiO2 (Irradiance: 1470 μW/cm2) | 29.85 | This study |
| UV (Irradiance: 180 μW/cm2) | 110.73 | This study |
| UV with TiO2 (Irradiance: 180 μW/cm2) | 89.26 | This study |
| UV with TiO2 added | 415.71 | [45] |
| Cold plasma | 59.97 | This study |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Lee, S.; Mok, C.; Lee, J. Sustainable Methods for Decontamination of Microcystin in Water Using Cold Plasma and UV with Reusable TiO2 Nanoparticle Coating. Int. J. Environ. Res. Public Health 2017, 14, 480. https://doi.org/10.3390/ijerph14050480
Jiang X, Lee S, Mok C, Lee J. Sustainable Methods for Decontamination of Microcystin in Water Using Cold Plasma and UV with Reusable TiO2 Nanoparticle Coating. International Journal of Environmental Research and Public Health. 2017; 14(5):480. https://doi.org/10.3390/ijerph14050480
Chicago/Turabian StyleJiang, Xuewen, Seungjun Lee, Chulkyoon Mok, and Jiyoung Lee. 2017. "Sustainable Methods for Decontamination of Microcystin in Water Using Cold Plasma and UV with Reusable TiO2 Nanoparticle Coating" International Journal of Environmental Research and Public Health 14, no. 5: 480. https://doi.org/10.3390/ijerph14050480





