Evaluation of the Effects of Airborne Particulate Matter on Bone Marrow-Mesenchymal Stem Cells (BM-MSCs): Cellular, Molecular and Systems Biological Approaches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Particle Sample Collection
2.2. Element Metal Analysis
2.3. Particle Extraction
2.4. Culture of Bone Marrow Mesenchymal Stem Cells (BM-MSCs)
2.5. Cell Morphology
2.6. Cell Proliferation
2.7. Ingenuity Pathway Analyses (IPA) of Heavy Metals in the Dust Particles
2.8. Quantitative Real-Time Gene Expression Analysis (qRT-PCR)
2.9. Statistical Analysis
3. Results
3.1. Cell Morphology
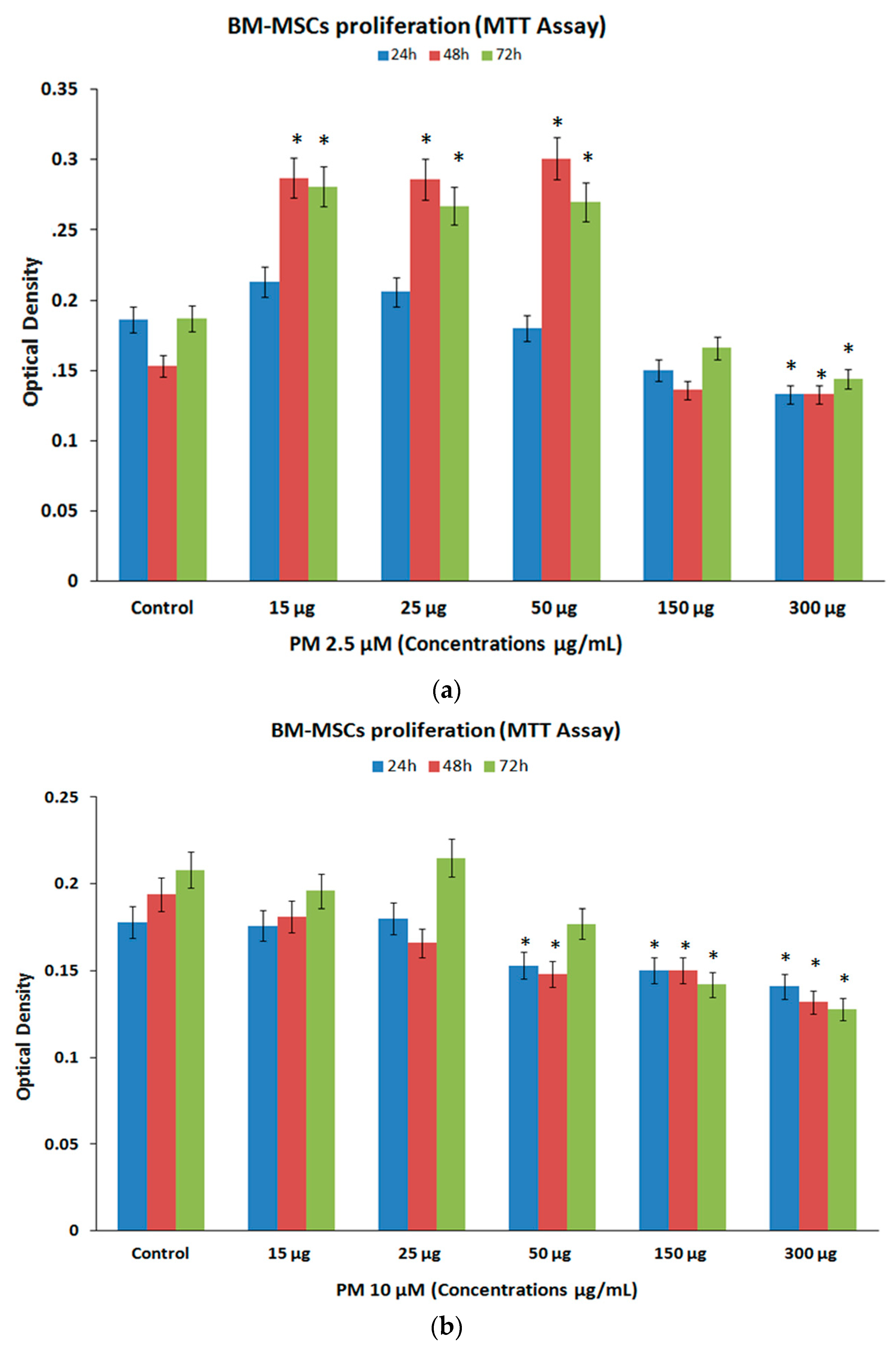
3.2. Cell Proliferation (MTT Assay)
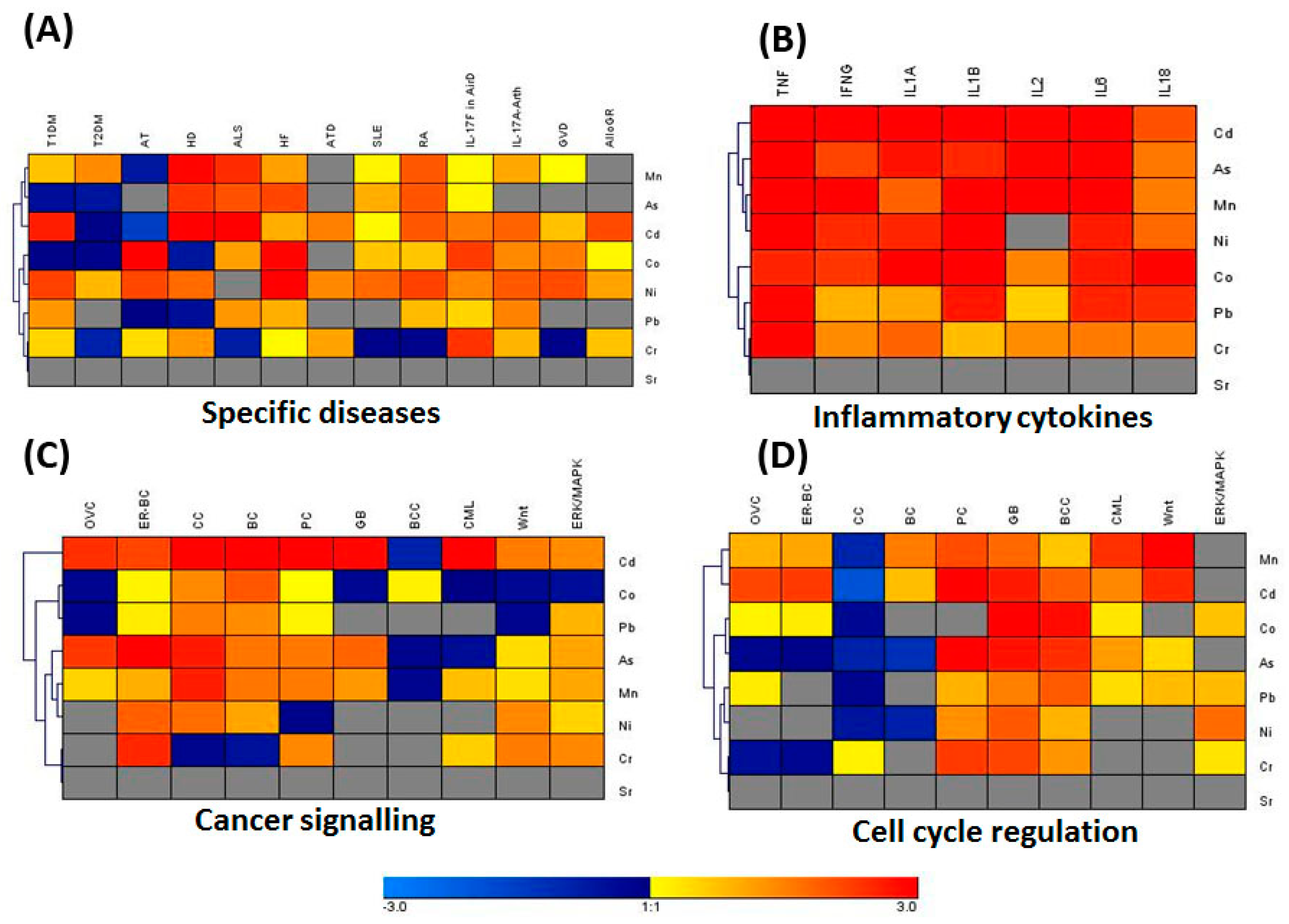
3.3. IPA Analysis for Associated Genes and Networks
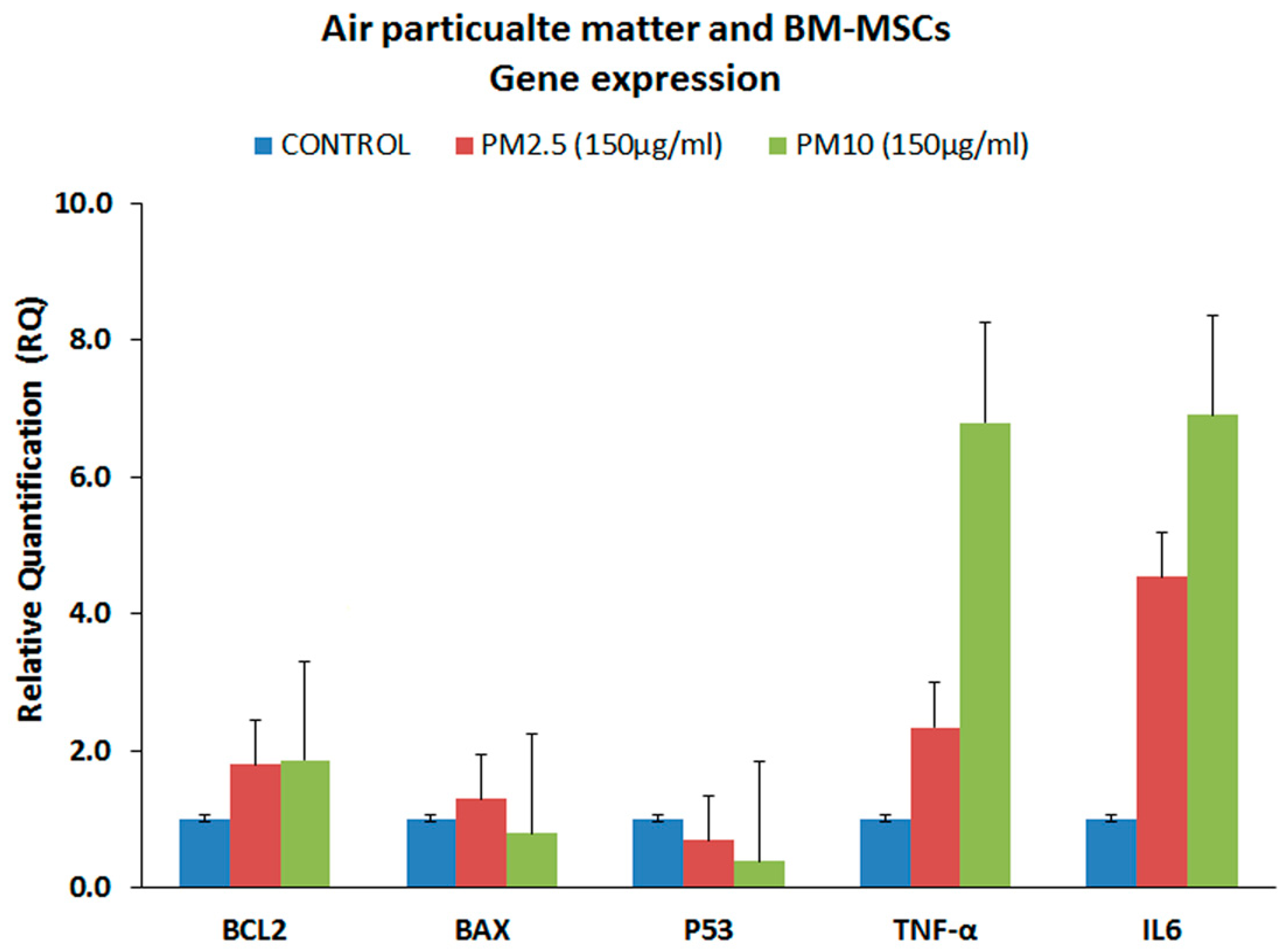
3.4. Gene Expression Analysis of the Proinflammatory Markers (qRT-PCR)
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ALS | Amyotrophic Lateral Sclerosis |
| As | Arsenic |
| bFGF | Basic fibroblast growth factor |
| BM-MSC | Bone-marrow mesenchymal stem cells |
| CC | Colorectal cancer |
| Cd | Cadmium |
| cDNA | Complementary DNA |
| CML | Chronic myeloid leukemia |
| Co | Cobalt |
| CO2 | Carbon Dioxide |
| Cr | Chromium |
| ER-BC | Estrogen-dependent breast cancer |
| FADD | Fas Associated protein with Death Domain |
| GB | Glioblastoma |
| HCL | Heatmap and complete hierarchical clustering |
| HD | Huntington’s Disease |
| IPA | Ingenuity Pathway Analysis |
| Mn | Manganese |
| Mo | Molybdenum |
| MTT | 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazoliumbromide assay |
| Ni | Nickel |
| NSCLC | Non-small-cell lung cancer cells |
| OVC | Ovarian cancer |
| ORS | oxygen reactive species |
| Pb | Lead |
| PC | Prostate cancer |
| PM | Particulate matter |
| qRT-PCR | Quantitative real-time gene expression analysis |
| RIP | Receptor interacting protein |
| ROS | Reactive oxygen species |
| RA | Rheumatoid Arthritis |
| SLE | Systemic lupus erythematosus |
| SPSS | Statistical package for social sciences |
| Sr | Strontium |
| TIDM | Type I Diabetes Mellitus |
| TIIDM | Type II Diabetes Mellitus |
| WHO | World Health Organization |
| XRF | X-ray Fluorescence |
References
- Kelly, F.J.; Fussell, J.C. Air pollution and public health: Emerging hazards and improved understanding of risk. Environ. Geochem. Health 2015, 37, 631–649. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Fiotakis, K.; Vlachogianni, T. Airborne particulate matter and human health: Toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2008, 26, 339–362. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Azarmi, F.; Kumar, P.; Mulheron, M. The exposure to coarse, fine and ultrafine particle emissions from concrete mixing, drilling and cutting activities. J. Hazard. Mater. 2014, 279, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.F.; Xu, Y.H.; Shi, M.H.; Lian, Y.X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, E69–E74. [Google Scholar] [PubMed]
- Ghio, A.J.; Carraway, M.S.; Madden, M.C. Composition of air pollution particles and oxidative stress in cells, tissues, and living systems. J. Toxicol. Environ. Health Part B Crit. Rev. 2012, 15, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Mancebo, S.E.; Wang, S.Q. Recognizing the impact of ambient air pollution on skin health. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2326–2332. [Google Scholar] [CrossRef] [PubMed]
- Lensen, G.; Jungbauer, F.; Goncalo, M.; Coenraads, P.J. Airborne irritant contact dermatitis and conjunctivitis after occupational exposure to chlorothalonil in textiles. Contact Dermat. 2007, 57, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Strak, M.; Janssen, N.A.; Godri, K.J.; Gosens, I.; Mudway, I.S.; Cassee, F.R.; Lebret, E.; Kelly, F.J.; Harrison, R.M.; Brunekreef, B.; et al. Respiratory health effects of airborne particulate matter: The role of particle size, composition, and oxidative potential-the raptes project. Environ. Health Perspect. 2012, 120, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Zemp, E.; Elsasser, S.; Schindler, C.; Kunzli, N.; Perruchoud, A.P.; Domenighetti, G.; Medici, T.; Ackermann-Liebrich, U.; Leuenberger, P.; Monn, C.; et al. Long-term ambient air pollution and respiratory symptoms in adults (sapaldia study). The sapaldia team. Am. J. Respir. Crit. Care Med. 1999, 159, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.M.; Forti, M.C.; de Freitas, C.U.; Nascimento, F.P.; Junger, W.L.; Gouveia, N. Effects of particulate matter and its chemical constituents on elderly hospital admissions due to circulatory and respiratory diseases. Int. J. Environ. Res. Public Health 2016, 13, 947. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Kuo, C.C.; Liou, S.H.; Yang, C.Y. Fine particulate air pollution and hospital admissions for myocardial infarction in a subtropical city: Taipei, Taiwan. J. Toxicol. Environ. Health Part A 2013, 76, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Yeatts, K.; Svendsen, E.; Creason, J.; Alexis, N.; Herbst, M.; Scott, J.; Kupper, L.; Williams, R.; Neas, L.; Cascio, W.; et al. Coarse particulate matter (PM2.5–10) affects heart rate variability, blood lipids, and circulating eosinophils in adults with asthma. Environ. Health Perspect. 2007, 115, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A. Neurological disorders from ambient (urban) air pollution emphasizing ufpm and PM2.5. Curr. Pollut. Rep. 2016, 2, 203–211. [Google Scholar] [CrossRef]
- Genc, S.; Zadeoglulari, Z.; Fuss, S.H.; Genc, K. The adverse effects of air pollution on the nervous system. J. Toxicol. 2012, 2012, 782462. [Google Scholar] [CrossRef] [PubMed]
- Heusinkveld, H.J.; Wahle, T.; Campbell, A.; Westerink, R.H.; Tran, L.; Johnston, H.; Stone, V.; Cassee, F.R.; Schins, R.P. Neurodegenerative and neurological disorders by small inhaled particles. Neurotoxicology 2016, 56, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Maher, B.A.; Ahmed, I.A. Magnetite pollution nanoparticles in the human brain. Proc. Natl. Acad. Sci. USA 2016, 113, 10797–10801. [Google Scholar] [CrossRef] [PubMed]
- Sagai, M.; Win-Shwe, T.T. Oxidative stress derived from airborne fine and ultrafine particles and the effects on brain-nervous system: Part 1. Nihon eiseigaku zasshi. Jpn. J. Hyg. 2015, 70, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Vacher, G.; Niculita-Hirzel, H.; Roger, T. Immune responses to airborne fungi and non-invasive airway diseases. Semin. Immunopathol. 2015, 37, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Holian, A.; Hamilton, R.F., Jr.; Morandi, M.T.; Brown, S.D.; Li, L. Urban particle-induced apoptosis and phenotype shifts in human alveolar macrophages. Environ. Health Perspect. 1998, 106, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Balti, E.V.; Echouffo-Tcheugui, J.B.; Yako, Y.Y.; Kengne, A.P. Air pollution and risk of type 2 diabetes mellitus: A systematic review and meta-analysis. Diabet. Res. Clin. Pract. 2014, 106, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Brocato, J.; Sun, H.; Shamy, M.; Kluz, T.; Alghamdi, M.A.; Khoder, M.I.; Chen, L.C.; Costa, M. Particulate matter from saudi arabia induces genes involved in inflammation, metabolic syndrome and atherosclerosis. J. Toxicol. Environ. Health Part A 2014, 77, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Raaschou-Nielsen, O.; Beelen, R.; Wang, M.; Hoek, G.; Andersen, Z.J.; Hoffmann, B.; Stafoggia, M.; Samoli, E.; Weinmayr, G.; Dimakopoulou, K.; et al. Particulate matter air pollution components and risk for lung cancer. Environ. Int. 2016, 87, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, R.; Mastrantonio, M.; Altavista, P.; Caiaffa, E.; Cattani, G.; Belli, S.; Comba, P. Female lung cancer mortality and long-term exposure to particulate matter in Italy. Eur. J. Public Health 2017, 27, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Shamy, M.; Kluz, T.; Munoz, A.B.; Zhong, M.; Laulicht, F.; Alghamdi, M.A.; Khoder, M.I.; Chen, L.C.; Costa, M. Gene expression profiling and pathway analysis of human bronchial epithelial cells exposed to airborne particulate matter collected from Saudi Arabia. Toxicol. Appl. Pharmacol. 2012, 265, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Hougaard, K.S.; Campagnolo, L.; Chavatte-Palmer, P.; Tarrade, A.; Rousseau-Ralliard, D.; Valentino, S.; Park, M.V.; de Jong, W.H.; Wolterink, G.; Piersma, A.H.; et al. A perspective on the developmental toxicity of inhaled nanoparticles. Reprod. Toxicol. 2015, 56, 118–140. [Google Scholar] [CrossRef] [PubMed]
- Tosh, D.; Slack, J.M.W. How cells change their phenotype. Nat. Rev. Mol. Cell. Biol. 2002, 3, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Maciejczyk, P.; Chen, L.C. Effects of subchronic exposures to concentrated ambient particles (caps) in mice. VIII. Source-related daily variations in in vitro responses to caps. Inhal. Toxicol. 2005, 17, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Duvall, R.M.; Norris, G.A.; Dailey, L.A.; Burke, J.M.; McGee, J.K.; Gilmour, M.I.; Gordon, T.; Devlin, R.B. Source apportionment of particulate matter in the U.S. and associations with lung inflammatory markers. Inhal. Toxicol. 2008, 20, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Kelloff, G.J.; Lippman, S.M.; Dannenberg, A.J.; Sigman, C.C.; Pearce, H.L.; Reid, B.J.; Szabo, E.; Jordan, V.C.; Spitz, M.R.; Mills, G.B.; et al. Progress in chemoprevention drug development: The promise of molecular biomarkers for prevention of intraepithelial neoplasia and cancer—A plan to move forward. Clin. Cancer Res. 2006, 12, 3661–3697. [Google Scholar] [CrossRef] [PubMed]
- Kanaji, A.; Caicedo, M.S.; Virdi, A.S.; Sumner, D.R.; Hallab, N.J.; Sena, K. Co-cr-mo alloy particles induce tumor necrosis factor alpha production in mlo-y4 osteocytes: A role for osteocytes in particle-induced inflammation. Bone 2009, 45, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Buggiano, V.; Petrillo, E.; Alló, M.; Lafaille, C.; Redal, M.A.; Alghamdi, M.A.; Khoder, M.I.; Shamy, M.; Muñoz, M.J.; Kornblihtt, A.R. Effects of airborne particulate matter on alternative pre-mrna splicing in colon cancer cells. Environ. Res. 2015, 140, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Sturn, A.; Quackenbush, J.; Trajanoski, Z. Genesis: Cluster analysis of microarray data. Bioinformatics 2002, 18, 207–208. [Google Scholar] [CrossRef] [PubMed]
- Zytoon, M.A.; Aburas, H.M.; Abdulsalam, M.I. Determination of 40k, 232th and 238u activity concentrations in ambient PM2.5 aerosols and the associated inhalation effective dose to the public in Jeddah city, Saudi Arabia. J. Environ. Radioact. 2014, 129, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Balkhyour, M.A.; Goknil, M.K. Total fume and metal concentrations during welding in selected factories in Jeddah, Saudi Arabia. Int. J. Environ. Res. Public Health 2010, 7, 2978–2987. [Google Scholar] [CrossRef] [PubMed]
- Elassouli, S.M.; Alqahtani, M.H.; Milaat, W. Genotoxicity of air borne particulates assessed by comet and the salmonella mutagenicity test in Jeddah, Saudi Arabia. Int. J. Environ. Res. Public Health 2007, 4, 216–233. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, A.; Moretta, L.; Pistoia, V. Immunoregulatory function of mesenchymal stem cells. Eur. J. Immunol. 2006, 36, 2566–2573. [Google Scholar] [CrossRef] [PubMed]
- Denburg, J.A.; Inman, M.D.; Wood, L.; Ellis, R.; Sehmi, R.; Dahlback, M.; O′Byrne, P. Bone marrow progenitors in allergic airways diseases: Studies in canine and human models. Int. Arch. Allergy Immunol. 1997, 113, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Denburg, J.A.; Inman, M.D.; Sehmi, R.; Uno, M.; O′Byrne, P.M. Hemopoietic mechanisms in allergic airway inflammation. Int. Arch. Allergy Immunol. 1998, 117, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.W.; Moon, C.; Kim, H.Y.; Oh, S.I.; Park, J.; Lee, J.H.; Chang, I.Y.; Kim, K.S.; Kim, S.H. Phase I trial of repeated intrathecal autologous bone marrow-derived mesenchymal stromal cells in amyotrophic lateral sclerosis. Stem Cells Transl. Med. 2015, 4, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chiu, S.M.; Motan, D.A.; Zhang, Z.; Chen, L.; Ji, H.L.; Tse, H.F.; Fu, Q.L.; Lian, Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, e2062. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Stanke, J.; Lahti, J.M. The connections between neural crest development and neuroblastoma. Curr. Top. Dev. Biol. 2011, 94, 77–127. [Google Scholar] [PubMed]
- Howlader, N.; Krapcho, M.; Garshell, J.; Neyman, N.; Altekruse, S.F.; Kosary, C.L.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Cho, H.; et al. (Eds.) Seer Cancer Statistics Review, 1975–2010; SEER Web Site, April 2013 ed.; National Cancer Institute: Bethesda, MD, USA, 2012; SEER Data Submission. [Google Scholar]
- Humphries, F.; Yang, S.; Wang, B.; Moynagh, P.N. Rip kinases: Key decision makers in cell death and innate immunity. Cell Death Diff. 2015, 22, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Hunter, P. The inflammation theory of disease. The growing realization that chronic inflammation is crucial in many diseases opens new avenues for treatment. EMBO Rep. 2012, 13, 968–970. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Xie, X.; Jia, F.; He, J.; Li, Z.; Fu, M.; Hao, H.; Liu, Y.; Liu, J.Z.; Cowan, P.J.; et al. Ambient fine particulate matter induces apoptosis of endothelial progenitor cells through reactive oxygen species formation. Cell. Physiol. Biochem. 2015, 35, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Monn, C.; Becker, S. Cytotoxicity and induction of proinflammatory cytokines from human monocytes exposed to fine (PM2.5) and coarse particles (PM10–2.5) in outdoor and indoor air. Toxicol. Appl. Pharmacol. 1999, 155, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.H.; Fiel, M.I.; Sun, Q.; Guo, J.; Gordon, R.E.; Chen, L.C.; Friedman, S.L.; Odin, J.A.; Allina, J. Kupffer cell activation by ambient air particulate matter exposure may exacerbate non-alcoholic fatty liver disease. J. Immunotoxicol. 2009, 6, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Michael, S.; Montag, M.; Dott, W. Pro-inflammatory effects and oxidative stress in lung macrophages and epithelial cells induced by ambient particulate matter. Environ. Pollut. (Barking Essex: 1987) 2013, 183, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Zhang, F.; Qu, F.; Ding, W. Water-insoluble fraction of airborne particulate matter (PM10) induces oxidative stress in human lung epithelial a549 cells. Environ. Toxicol. 2014, 29, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.G.; Wang, M.; Li, N.; Loo, J.A.; Nel, A.E. Use of proteomics to demonstrate a hierarchical oxidative stress response to diesel exhaust particle chemicals in a macrophage cell line. J. Biol. Chem. 2003, 278, 50781–50790. [Google Scholar] [CrossRef] [PubMed]
- Teeguarden, J.G.; Hinderliter, P.M.; Orr, G.; Thrall, B.D.; Pounds, J.G. Particokinetics in vitro: Dosimetry considerations for in vitro nanoparticle toxicity assessments. Toxicol. Sci. 2007, 95, 300–312. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer Sequence |
|---|---|
| GAPDH | F: 5′-ACCACAGTCCATGCCATCAC-3′ R: 5′-TCCACCACCCTGTTGCTGTA-3′ |
| BCL2 | F: 5’-GGCTGGGATGCCTTTGTG-3’ R: 5’-CAGCCAGGAGAAATCAAACAGA-3’ |
| BAX | F: 5’-TGGAGCTGCAGAGGATGATTG-3’ R: 5’-GCTGCCACTCGGAAAAAGAC-3’ |
| p53 | F: 5’-GCGCACAGAGGAAGAGAATC-3’ R: 5’-CTCTCGGAACATCTCGAAGC-3’ |
| TNF-α | F: 5′-GGT-GCTTGT-TCC-TCA-GCC-TC-3′ R: 5′-CAG-GCA-GAAGAG-CGT-GGT-G-3′ |
| IL-6 | F: 5′-CCACTCACCTCTTCAGAA-3′ R: 5’-GCGCAAAATGAGATGAGT-3’ |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Elmagd, M.; Alghamdi, M.A.; Shamy, M.; Khoder, M.I.; Costa, M.; Assidi, M.; Kadam, R.; Alsehli, H.; Gari, M.; Pushparaj, P.N.; et al. Evaluation of the Effects of Airborne Particulate Matter on Bone Marrow-Mesenchymal Stem Cells (BM-MSCs): Cellular, Molecular and Systems Biological Approaches. Int. J. Environ. Res. Public Health 2017, 14, 440. https://doi.org/10.3390/ijerph14040440
Abu-Elmagd M, Alghamdi MA, Shamy M, Khoder MI, Costa M, Assidi M, Kadam R, Alsehli H, Gari M, Pushparaj PN, et al. Evaluation of the Effects of Airborne Particulate Matter on Bone Marrow-Mesenchymal Stem Cells (BM-MSCs): Cellular, Molecular and Systems Biological Approaches. International Journal of Environmental Research and Public Health. 2017; 14(4):440. https://doi.org/10.3390/ijerph14040440
Chicago/Turabian StyleAbu-Elmagd, Muhammad, Mansour A. Alghamdi, Magdy Shamy, Mamdouh I. Khoder, Max Costa, Mourad Assidi, Roaa Kadam, Haneen Alsehli, Mamdooh Gari, Peter Natesan Pushparaj, and et al. 2017. "Evaluation of the Effects of Airborne Particulate Matter on Bone Marrow-Mesenchymal Stem Cells (BM-MSCs): Cellular, Molecular and Systems Biological Approaches" International Journal of Environmental Research and Public Health 14, no. 4: 440. https://doi.org/10.3390/ijerph14040440






