Mosquito Traps: An Innovative, Environmentally Friendly Technique to Control Mosquitoes
Abstract
:1. Introduction
2. Materials and Methods
3. Results
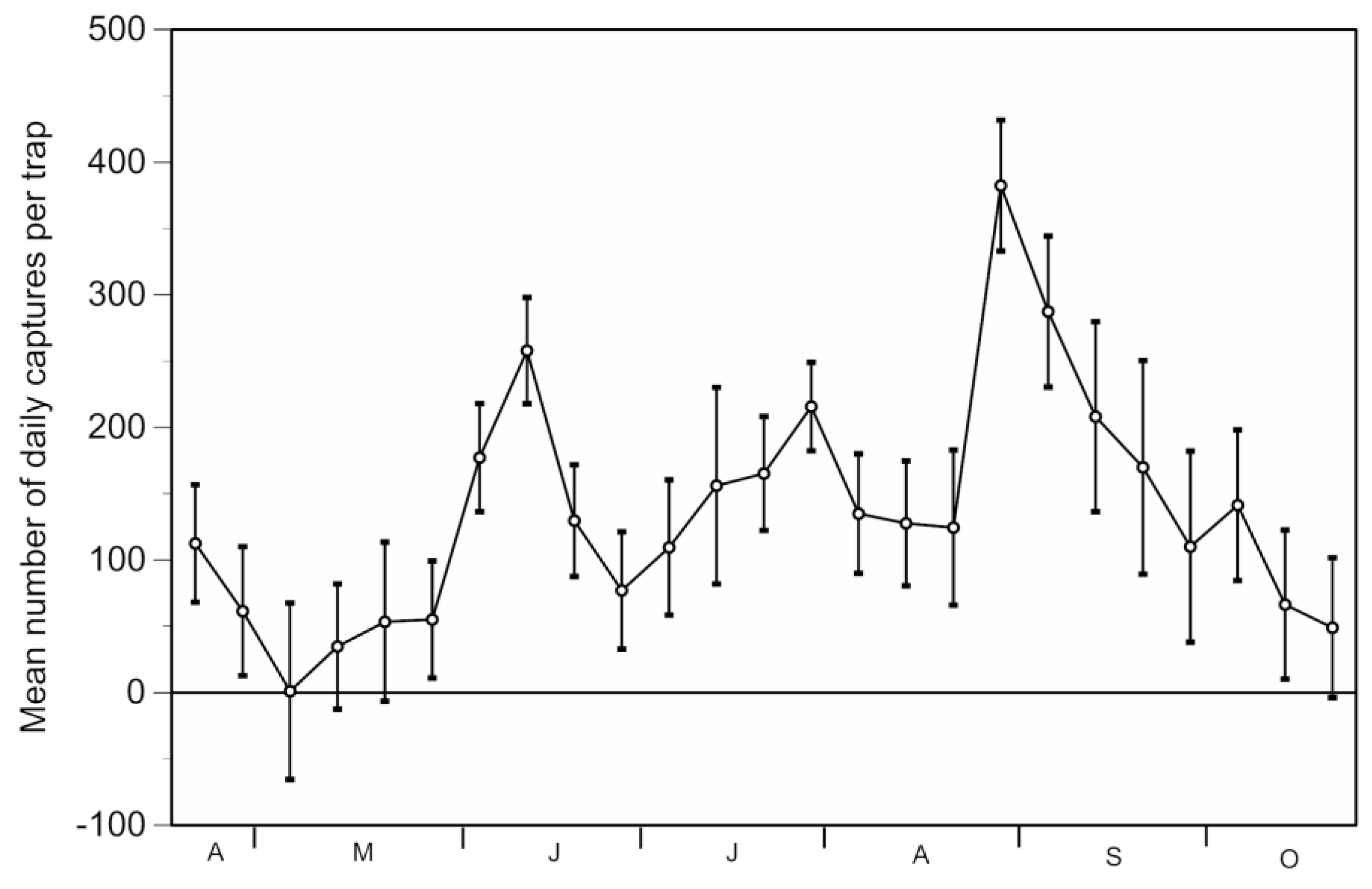
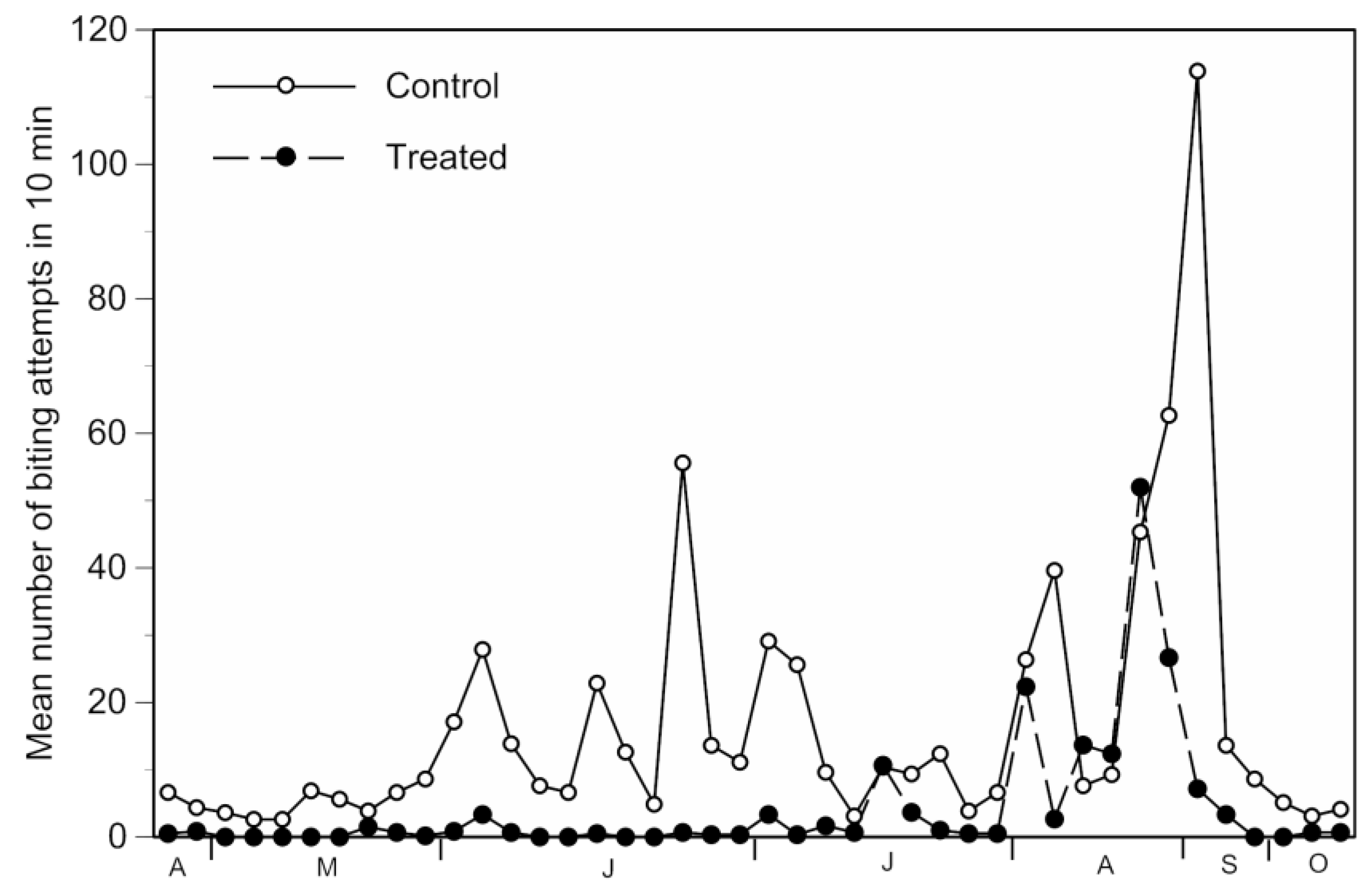
3.1. Trap Performance
3.2. Environmental Impacts of Techno Bam Traps
3.2.1. Direct Effects
3.2.2. Indirect Effects
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Boyce, R.; Lenhart, A.; Kroeger, A.; Velayudhan, R.; Roberts, B.; Horstick, O. Bacillus thuringiensis israelensis (Bti) for the control of dengue vectors: systematic literature review. Trop. Med. Int. Health 2013, 18, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Poulin, B. Indirect effects of bioinsecticides on the nontarget fauna: The Camargue experiment calls for future research. Acta Oecologica. 2012, 44, 28–32. [Google Scholar] [CrossRef]
- Poulin, B.; Lefebvre, G.; Paz, L. Red flag for green spray: adverse trophic effects of Bti on breeding birds. J. Appl. Ecol. 2010, 47, 884–889. [Google Scholar] [CrossRef]
- Jakob, C.; Poulin, B. Indirect effects of mosquito control using Bti on dragonflies and damselflies (Odonata) in the Camargue. Insect Conserv. Divers. 2016, 9, 161–169. [Google Scholar] [CrossRef]
- Poulin, B.; Lefebvre, G. Perturbation and delayed recovery of the reed invertebrate assemblage in Camargue marshes sprayed with Bacillus thuringiensis israelensis. Insect Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Duchet, C.; Tetreau, G.; Albane, M.; Rey, D.; Besnard, G.; Perrin, Y.; Paris, M.; David, J.-P.; Lagneau, C.; Després, L. Persistence and recycling of bioinsecticidal Bacillus thuringiensis subsp. israelensis spores in contrasting environments: Evidence from field monitoring and laboratory experiments. Microb. Ecol. 2014, 67, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Poulin, B.; Lefebvre, G.; Després, L.; Université de Grenoble, LECA-UMR 5553, Grenoble, France. Unpublished data. 2016.
- Lühken, R.; Pfitzner, W.P.; Börstler, J.; Garms, R.; Huber, K.; Schork, N.; Steinke, S.; Kiel, E.; Becker, N.; Tannich, E.; et al. Field evaluation of four widely used mosquito traps in Central Europe. Parasites Vectors 2014, 7, 268. [Google Scholar] [CrossRef] [PubMed]
- Drago, A.; Marini, F.; Caputo, B.; Coluzzi, M.; della Torre, A.; Pombi, M. Looking for the gold standard: assessment of the effectiveness of four traps for monitoring mosquitoes in Italy. J. Vector Ecol. 2012, 37, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Bryant, D.M. The factors influencing the selection of food by the house martin Delichon urbica (L.). J. Appl. Ecol. 1973, 42, 539–564. [Google Scholar] [CrossRef]
- Bryant, D.M.; Turner, A.K. Central place foraging by swallows (Hirundinidae): the question of load size. Anim. Behav. 1982, 30, 845–856. [Google Scholar] [CrossRef]
- Krockel, U.; Rose, A.; Eiras, L.E.; Geier, M. New tools for surveillance of adult yellow fever mosquitoes: Comparison of trap catches with human landing rates in an urban environment. J. Am. Mosq. Contr. Assoc. 2006, 22, 229–238. [Google Scholar] [CrossRef]
- Farajollahi, A.; Kesavaraju, B.; Price, D.C.; Williams, G.M.; Healy, S.P.; Gaugler, R.; Nelder, M.P. Field efficacy of BG-Sentinel and industry-standard traps for Aedes albopictus (Diptera: Culicidae) and West Nile virus surveillance. J. Med. Entomol. 2009, 46, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Englbrecht, C.; Gordon, S.; Venturelli, C.; Rose, A.; Geier, M. Evaluation of BG-Sentinel trap as a management tool to reduce Aedes albopictus nuisance in an urban environment in Italy. J. Am. Mosq. Contr. Assoc. 2015, 31, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Marina, C.F.; Bond, J.G.; Muñoz, J.; Valle, J.; Novelo-Gutiérrez, R.; Williams, T. Efficacy and non-target impact of spinosad, Bti and temephos larvicides for control of Anopheles spp. in an endemic malaria region of southern Mexico. Parasites Vectors 2014, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Okumu, F.O.; Killeen, G.F.; Ogoma, S.; Biswaro, L.; Renate, C.; Smallegange, R.C.; Mbeyela, E.; Titus, E.; Munk, C.; Ngonyani, H.; et al. Development and field evaluation of a synthetic mosquito lure that is more attractive than humans. PLoS ONE 2010, 5, e8951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crepeau, T.N.; Healy, S.P.; Bartlett-Healy, K.; Unlu, I.; Farajollahi, A.; Fonseca, D.M. Effects of Biogents sentinel trap field placement on capture rates of adult Asian tiger mosquitoes, Aedes albopictus. PLoS ONE 2013, 8, e60524. [Google Scholar] [CrossRef] [PubMed]
- Faraji, A.; Unlu, I. The eye of the tiger, the thrill of the fight: effective larval and adult control measures against the Asian tiger mosquito, Aedes albopictus (Diptera: Culicidae), in North America. J. Med. Entomol. 2016, 53, 1029–1047. [Google Scholar] [CrossRef] [PubMed]
- Salazar, F.V.; Achee, N.L.; Grieco, J.P.; Prabaripai, A.; Eisen, L.; Shah, P.; Chareonviriyaphap, T. Evaluation of a peridomestic mosquito trap for integration into an Aedes aegypti (Diptera: Culicidae) push-pull control strategy. J. Vector Ecol. 2012, 37, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Barrera, R.; Amador, M.; Acevedo, V.; Hemme, R.R.; Felix, G. Sustained, area-wide control of Aedes aegypti using CDC autocidal gravid ovitraps. Am. J. Trop. Med. Hyg. 2014, 91, 1269–1276. [Google Scholar] [CrossRef] [PubMed]



| Traps | Human bait (calf tests) | ||||||
|---|---|---|---|---|---|---|---|
| % | % biting | Mean (SE) biting rate/10 min | Reduction | ANOVA Statistics | |||
| Mosquito species | captures | attempts | Control | Treated | Rate (%) | F(1,294) | p value |
| Ochlerotatus caspius | 82.76 | 51.39 | 7.68 (0.92) | 1.97 (0.54) | 74 | 28.7 | <0.00001 |
| Anopheles hyrcanus | 8.73 | 35.27 | 3.44 (1.54) | 1.87 (0.89) | 46 | 0.4 | 0.37 |
| Aedes vexans | 4.76 | 2.05 | 0.57 (0.07) | 0.03 (0.04) | 94 | 38.7 | <0.00001 |
| Culex pipiens | 1.99 | 0.23 | 0.03 (0.01) | 0.01 (0.01) | 67 | 1.57 | 0.21 |
| Oc. detritus/coluzzii | 1.40 | 6.77 | 1.86 (0.19) | 0.03 (0.11) | 98 | 70.8 | <0.00001 |
| Culex modestus | 0.14 | 4.16 | 0.44 (0.41) | 0.22 (0.24) | 50 | 0.22 | 0.64 |
| Culiseta annulata | 0.06 | 0.03 | 0.017 (0.01) | 0 | 100 | 6.05 | 0.014 |
| Aedes albopictus | 0.01 | 0.07 | 0 | 0.007 (0.01) | 0.5 | 0.48 | |
| Anopheles maculipennis | 0.14 | ||||||
| Coquillettidia richiardii | 0.03 | 0.017 (0.01) | 0 | 100 | 6.06 | 0.014 | |
| Total | 299,408 | 3051 | 14.06 (1.16) | 4.15 (1.99) | 70.5% | 18.46 | 0.00002 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poulin, B.; Lefebvre, G.; Muranyi-Kovacs, C.; Hilaire, S. Mosquito Traps: An Innovative, Environmentally Friendly Technique to Control Mosquitoes. Int. J. Environ. Res. Public Health 2017, 14, 313. https://doi.org/10.3390/ijerph14030313
Poulin B, Lefebvre G, Muranyi-Kovacs C, Hilaire S. Mosquito Traps: An Innovative, Environmentally Friendly Technique to Control Mosquitoes. International Journal of Environmental Research and Public Health. 2017; 14(3):313. https://doi.org/10.3390/ijerph14030313
Chicago/Turabian StylePoulin, Brigitte, Gaëtan Lefebvre, Camille Muranyi-Kovacs, and Samuel Hilaire. 2017. "Mosquito Traps: An Innovative, Environmentally Friendly Technique to Control Mosquitoes" International Journal of Environmental Research and Public Health 14, no. 3: 313. https://doi.org/10.3390/ijerph14030313





