The Evaluation on the Cadmium Net Concentration for Soil Ecosystems
Abstract
:1. Introduction
2. Experimental
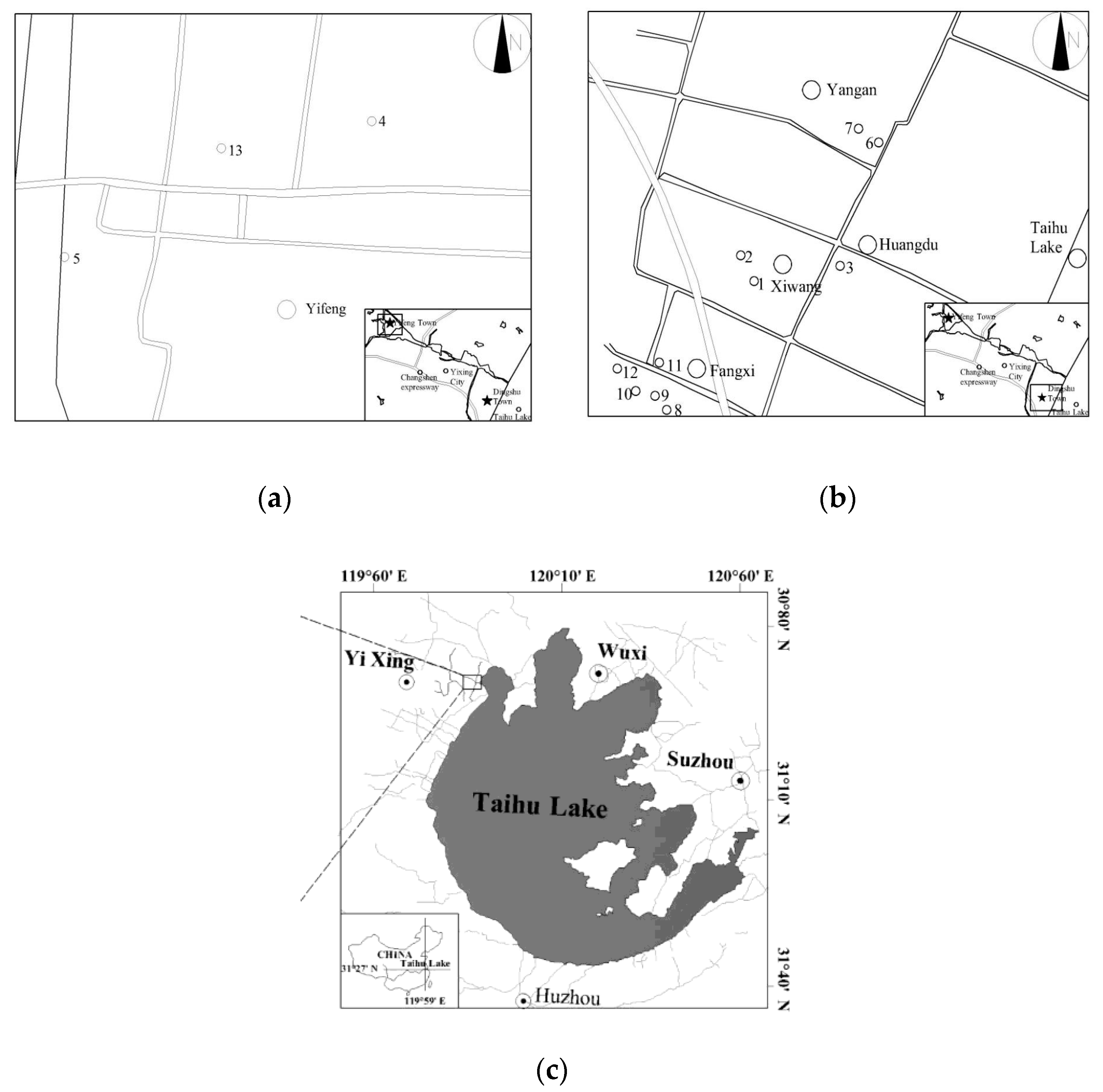
2.1. Soil Sites
2.2. Greenhouse Pot Experiment
2.3. Analytical Methods for Cd Evaluation
2.3.1. Ex Situ Measurement
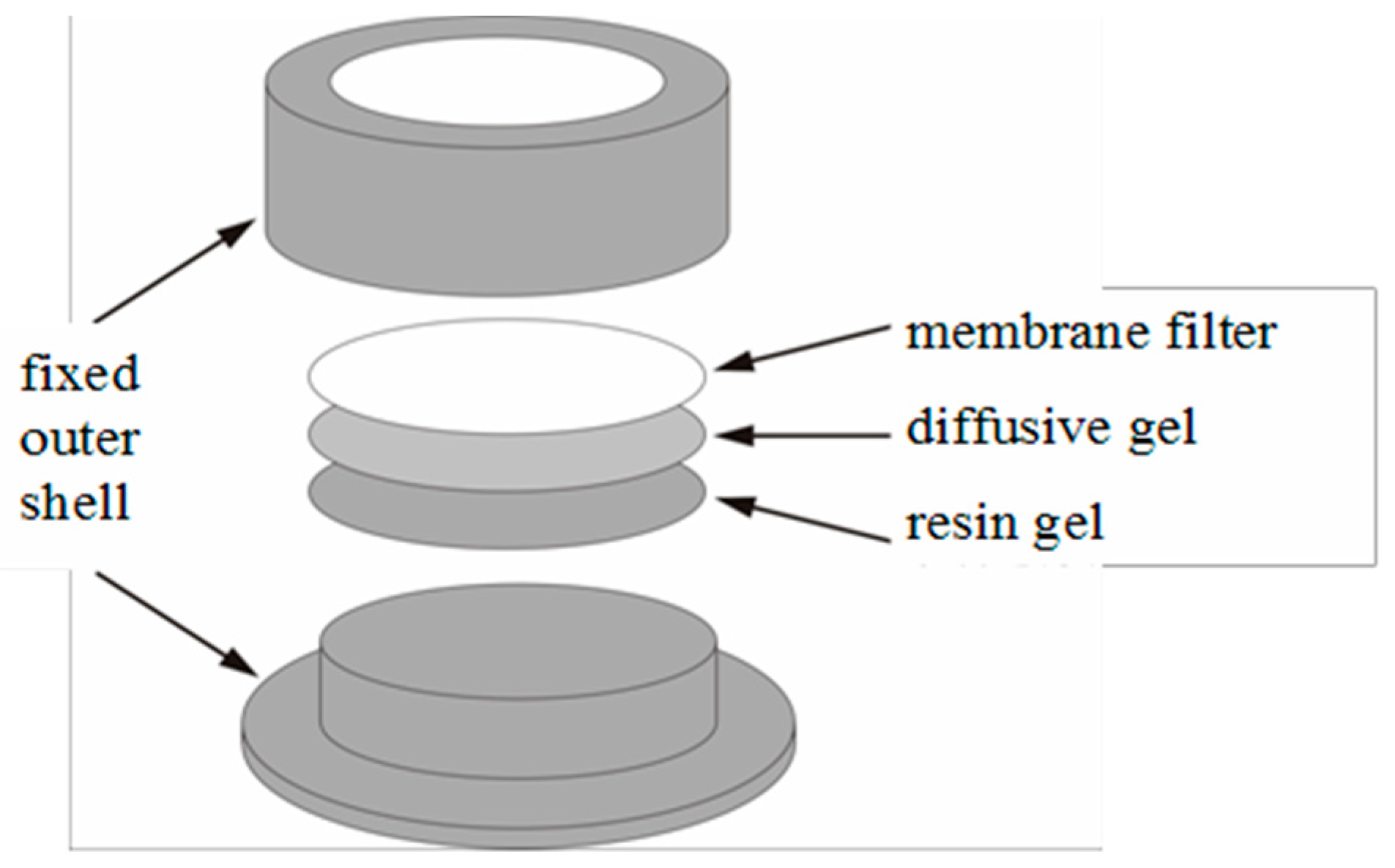
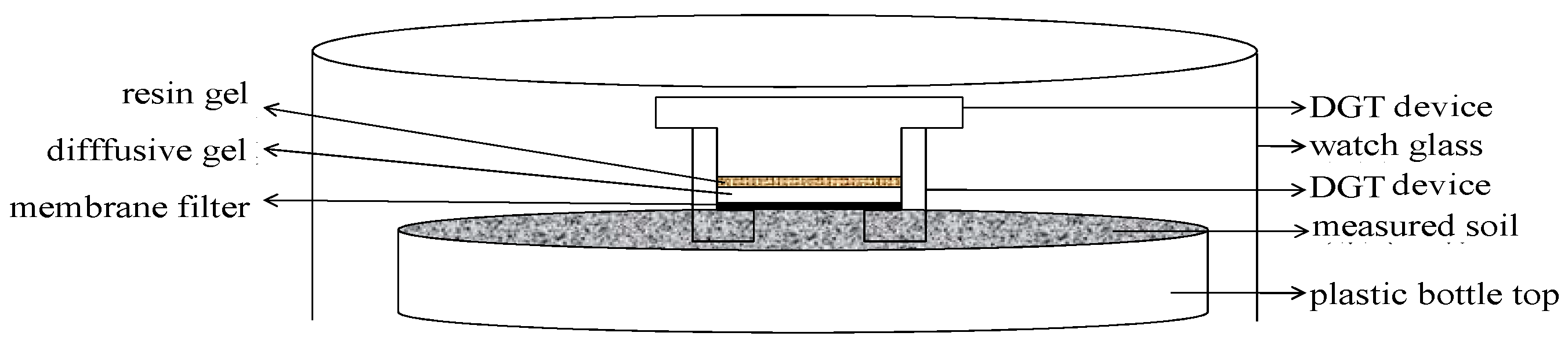
2.3.2. In Situ Measurement
2.4. Data Analyses
3. Results and Discussion
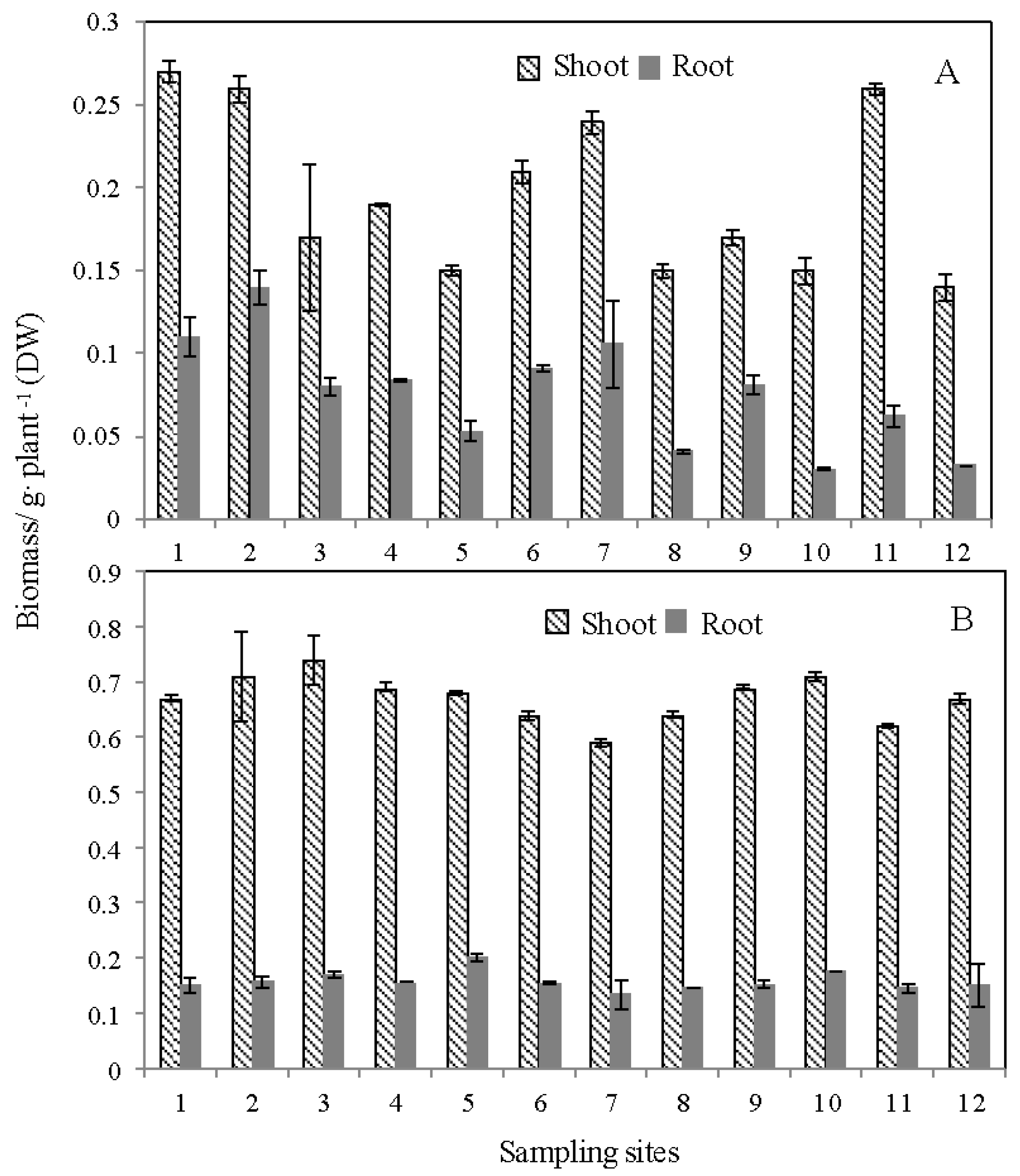
3.1. Plant Growth
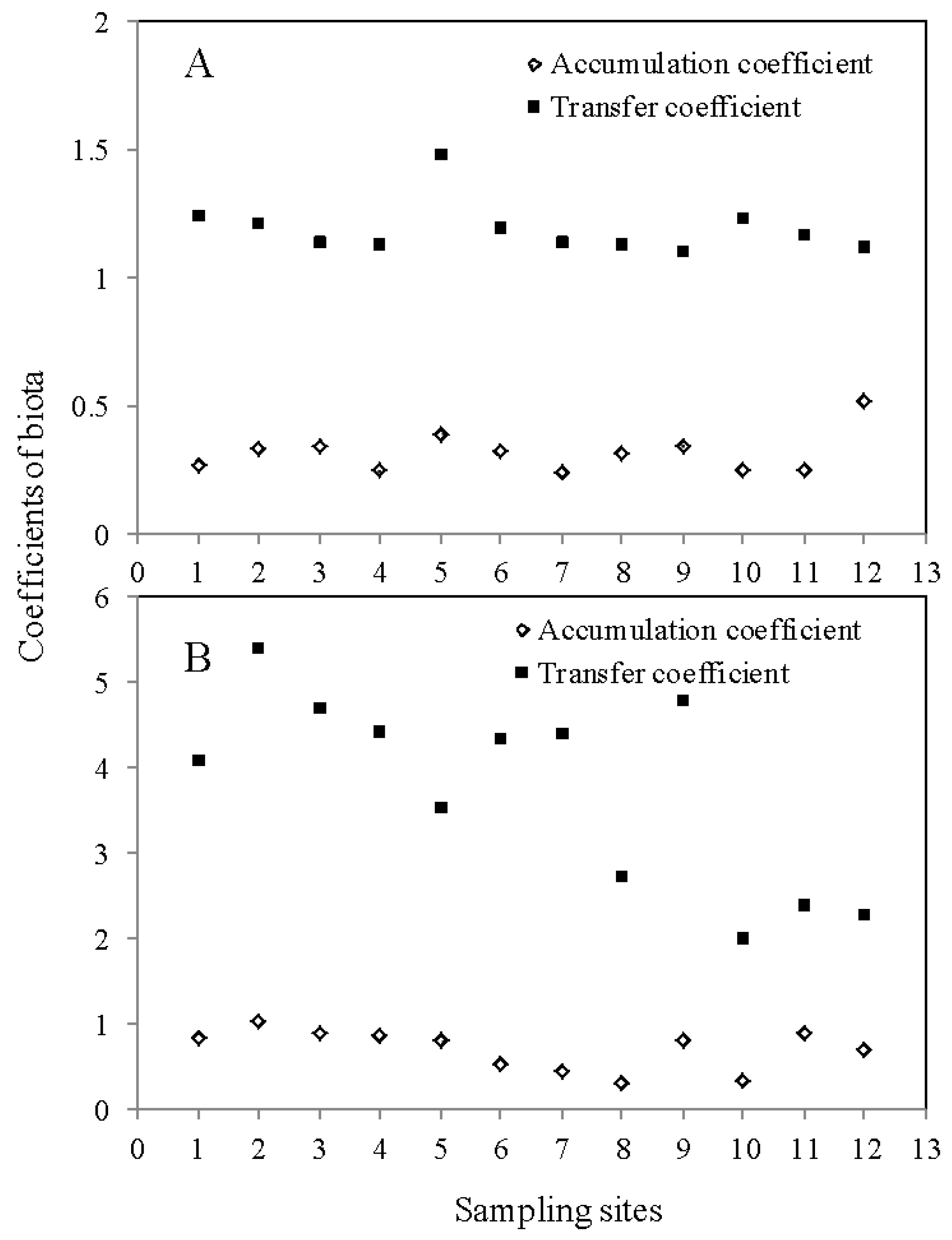
3.2. Different Indictors for Cd Bioavailability Evaluation
3.3. Multivariate Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Egan, S.K.; Bolger, P.M.; Carrington, C.D. Update of U.S. FDA’s Total Diet Study food list and diets. Expo. Sci. Environ. Epidemiol. 2007, 17, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Jarup, L.; Akesson, A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.F.; Wang, T.; Yao, Y.; Wang, C.; Liu, C.; Yuan, Y. A Diffusive Gradient-in-Thin-Film Technique for Evaluation of the Bioavailability of Cd in Soil Contaminated with Cd and Pb. Int. J. Environ. Res. Public Health 2016, 13. [Google Scholar] [CrossRef] [PubMed]
- Kippler, M.; Ekström, E.C.; Lönnerdal, B.; Goessler, W.; Åkesson, A.; El Arifeen, S.; Persson, L.; Vahter, M. Influence of iron and zinc status on cadmium accumulation in Bangladeshi women. Toxicol. Appl. Pharmacol. 2007, 222, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Link, B.; Gabrio, T.; Piechotowsk, I.; Zöllner, I.; Schwenk, M. Baden-Wuerttemberg Environmental Health Survey (BW-EHS) from 1996 to 2003: toxic metals in blood and urine of children. Environ. Health 2007, 210, 357–371. [Google Scholar]
- Shan, X.Q.; Wang, Z.W.; Wang, W.S.; Zhang, S.; Wen, B. Labile rhizosphere soil solution fraction for prediction of bioavailability of heavy metals and rare earth elements to plants. Anal. Bioanal. Chem. 2003, 375, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.H.; Shan, X.Q.; Zhang, S.Z.; Wen, B. Comparison of a rhizosphere-based method with other one-step extraction methods for assessing the bioavailability of soil metals to wheat. Chemosphere 2005, 59, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Menzies, N.W.; Donn, M.J.; Kopittke, P.M. Evaluation of extractants for estimation of the phytoavailable trace metals in soils. Environ. Pollut. 2007, 145, 121–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Y.; Sun, Q.; Wang, C.; Wang, P.F.; Miao, L.Z.; Ding, S.M. The Combination of DGT Technique and Traditional Chemical Methods for Evaluation of Cadmium Bioavailability in Contaminated Soils with Organic Amendment. Int. J. Environ. Res. Public Health 2016, 13. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.M.; Yan, W.D.; Christie, P. Soil solution dynamics of Cu and Zn in a Cu- and Zn-polluted soil as influenced by γ-irradiation and Cu-Zn interaction. Chemosphere 2001, 42, 179–184. [Google Scholar] [CrossRef]
- Nolan, A.L.; Zhang, H.; McLaughlin, M.J. Prediction of zinc, cadmium, lead, and copper availability to wheat in contaminated soils using chemical speciation, diffusive gradients in thin films, extraction, and isotopic dilution techniques. J. Environ. Qual. 2005, 34, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Kamewada, K.; Nakayama, M. Cadmium uptake by garland chrysanthemum can be predicted from the cadmium in the soil solution, independent of soil type. Soil Sci. Plant Nutr. 2009, 55, 441–451. [Google Scholar] [CrossRef]
- Tye, A.M.; Young, S.D.; Crout, N.M.J.; Zhang, H.; Preston, S.; Barbosa-Jefferson, V.L.; Davison, W.; McGrath, S.P.; Paton, G.I.; Kilham, K.; et al. Predicting the activity of Cd2+ and Zn2+ in soil pore water from the radio-labile metal fraction. Geochim. Cosmochim. Acta 2003, 67, 375–385. [Google Scholar] [CrossRef]
- Zhang, H.; Davison, W. Performance characteristics of diffusion gradients in thin films for the in situ measurement of trace metals in aqueous solution. Anal. Chem. 1995, 67, 3391–3400. [Google Scholar] [CrossRef]
- Sabry, M.S.; Jörg, R.; Tina, F.; John, R.W.; Ron, D.D. Redox effects on release kinetics of arsenic, cadmium, cobalt, and vanadium in Wax Lake Deltaic freshwater marsh soils. Chemosphere 2016, 150, 740–748. [Google Scholar]
- Collins, R.N.; Merrington, G.; McLaughlin, M.J.; Morel, J.L. Organic ligand and pH effects on isotopically exchangeable cadmium in polluted soils. Soil Sci. Soc. Am. J. 2003, 67, 112–121. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, F.J.; Sun, B.; Davison, W.; McGrath, S.P. A new method to measure effective soil solution concentration predicts copper availability to plants. Environ. Sci. Technol. 2001, 35, 2602–2607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lombi, E.; Smolders, E.; McGrath, S. Kinetic of Zn release in soils and prediction of Zn concentration in plants using diffusive gradients in thin films. Environ. Sci. Technol. 2004, 38, 3608–3613. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.Y.; Davison, W.; Zhang, H.; Kelly, M. Performance characteristics for the measurement of Cs and Sr by diffusive gradients in thin films (DGT). Anal. Chim. Acta 1998, 368, 243–253. [Google Scholar] [CrossRef]
- Teasdale, P.R.; Hayward, S.; Davison, W. In situ, high-resolution measurement of dissolved sulfide using diffusive gradients in thin films with computer imaging densitometry. Anal. Chem. 1999, 71, 2186–2191. [Google Scholar] [CrossRef] [PubMed]
- Docekalova, H.; Divis, P. Application of diffusive gradient in thin filmstechnique (DGT) to measurement of mercury in aquatic systems. Talanta 2005, 65, 1174–1178. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yao, Y.; Wang, P.F.; Hou, J.; Qian, J.; Yuan, Y.; Fan, X. In situ high-resolution evaluation of labile arsenic and mercury in sediment of a large shallow lake. Sci. Total Environ. 2016, 541, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Divis, P.; Szkandera, R.; Brulik, L.; Docekalová, H.; Matús, P.; Bujdos, M. Application of New Resin Gels for Measuring Mercury by Diffusive Gradients ina Thin-films Technique. Anal. Sci. 2009, 25, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Cheng, J.; Ding, S.M.; Yao, Y.; Chen, Y. Comparison of diffusive gradients in thin film technique with traditional methods for evaluation of zinc bioavailability in soil. Environ. Monit. Assess. 2014, 186, 6553–6564. [Google Scholar] [CrossRef] [PubMed]
- Black, A.; McLaren, R.G.; Reichman, S.M.; Speir, T.W.; Condron, L.M. Evaluation of soil metal bioavailablity estimates using two plant species (L. perenne and T. aestivum) grown in a range of agricultural soils treated with biosolids and metal salts. Environ. Pollut. 2011, 159, 1523–1535. [Google Scholar] [CrossRef] [PubMed]
- Mundus, S.; Lombi, E.; Holm, P.E.; Zhang, H.; Huste, S. Assessing the plant availability of manganese in soils using diffusive gradients in thin films (DGT). Geoderma 2012, 183–184, 92–99. [Google Scholar] [CrossRef]
- Soriano-Disla, J.M.; Speir, T.W.; Gómez, I.; Clucas, L.M.; McLaren, R.G.; Navarro-Pedreño, J. Evaluation of different extraction methods for the assessment of heavy metal bioavailability in various soils. Water. Air Soil Pollut. 2010, 213, 471–483. [Google Scholar] [CrossRef]
- Cornu, J.Y.; Denaix, L. Prediction of zinc and cadmium phytoavailability within a contaminated agricultural site using DGT. Environ. Chem. 2006, 3, 61–64. [Google Scholar] [CrossRef]
- AlmAs, A.R.; Lombns, P.; Sogn, T.A.; Mulder, J. Speciation of Cd and Zn in contaminated soils assessed by DGT-DIFS, and WHAM/Model VI in relation to uptake by spinach and ryegrass. Chemosphere 2006, 62, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Ure, A.M.; Quevauviller, P.H.; Muntau, H.; Griepink, B. Speciation of heavy metals in soils and sediments. An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the commission of the European communities. Int. J. Environ. Anal. Chem. 1993, 51, 135–151. [Google Scholar] [CrossRef]
- Houba, V.J.G.; Lexmond, T.M.; Novozamsky, I.; van der Lee, J.J. State of the art and future developments in soil analysis for bioavailability assessment. Sci. Total Environ. 1996, 178, 21–28. [Google Scholar] [CrossRef]
- Wear, J.I.; Evans, C.E. Relationship of zinc uptake by corn and sorghum to soil zinc measured by three extractants. Soil Sci. Soc. Am. J. 1968, 32, 543–546. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Novozamsky, I.; Lexmond, T.H.M.; Houba, V.J.G. A single extraction procedure of soil for evaluation of uptake of some heavy metals by plants. Int. J. Environ. Anal. Chem. 1993, 51, 47–58. [Google Scholar] [CrossRef]
- Tandy, S.; Mundus, S.; Yngvesson, J.; de Bang, T.C.; Lombi, E.; Schjoerring, J.K.; Husted, S. The use of DGT for prediction of plant available copper, zinc and phosphorus in agricultural soils. Plant Soil 2011, 346, 167–180. [Google Scholar] [CrossRef]
- Qiu, H.; Gu, H.H.; He, E.K.; Wang, S.Z.; Qiu, R.L. Attenuation of metal bioavailability in acidic multi-metal contaminated soil treated with fly ash and steel slag. Pedosphere 2012, 22, 544–553. [Google Scholar] [CrossRef]
- Yao, Y.; Sun, Q.; Wang, C.; Wang, P.; Miao, L.; Ding, S. The combination of DGT technique and traditional chemical methods for evaluation of cadmium bioavailability in contaminated soils with organic amendment. Int. J Environ. Res. Public Health 2016, 13, 595. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Sun, Q.; Cheng, J.; Ding, S.M.; Liu, H.; Wang, C.; Wang, P.F. Evaluation of Cadmium Bioavailability in Soils Using Diffusive Gradients in Thin Film Technique and Traditional Methods. J. Donghua Univ. 2015, 32, 426–433. [Google Scholar]
- Song, J.; Zhao, F.J.; Luo, Y.M.; McGrath, S.P.; Zhang, H. Copper uptake by Elsholtzia splendens and Silene vulgaris and assessment of copper phytoavailability in contaminated soils. Environ. Pollut. 2004, 128, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Desorption of cadmium from goethite: Effects of pH, temperature and aging. Chemosphere 2006, 65, 856–865.
- Seuntient, P.; Tirez, K.; Simunek, J.; van Genuchten, M.T.; Cornelis, C.; Geuzens, P. Aging Effect on Cadmium Transport in Undisturbed Contaminated Sandy soil columns. J. Environ. Qual. 2001, 30, 1040–1050. [Google Scholar]
- Martinez, C.E.; McBride, M.B. Solubility of Cd2+, Cu2+, Pb2+, and Zn2+ in aged coprecipitates with amorphous iron hydroxides. Environ. Sci. Technol. 1998, 32, 743–748. [Google Scholar] [CrossRef]
- Barrow, N.J.; Gerth, J.; Brummer, G.W. Reaction kinetics of the adsorption and desorption of nickel, zinc and cadmium by goethite. II. Modelling the extent and rate of reaction. Soil Sci. 1989, 40, 437–450. [Google Scholar] [CrossRef]
- Wang, K.; Xing, B. Adsorption and desorption of cadmium by goethite pretreated with phosphate. Chemosphere 2002, 48, 665–670. [Google Scholar] [CrossRef]
- Randall, S.; Sherman, D.M.; Ragnersdottir, K.V.; Collinsa, C.R. The mechanism of cadmium surface complexation on iron oxyhydroxide minerals. Geochim. Cosmochim. Acta 1999, 63, 2971–2987. [Google Scholar] [CrossRef]
- Souza-Talarico, J.N.; Marcourakis, T.; Barbosa, F., Jr.; Moraes Barros, S.B.; Rivelli, D.P.; Pompéia, S.; Caramelli, P.; Plusquellec, P.; Lupien, S.J.; Catucci, R.F.; et al. Association between heavy metal exposure and poor working memory and possible mediation effect of antioxidant defenses during aging. Sci. Total Environ. 2017, 575, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhang, H.; Zhao, F.J.; Davison, W. Distinguishing diffusional and plant control of Cd and Ni uptake by hyperaccumulator and nonhyperaccumulator plants. Environ. Sci. Technol. 2010, 44, 6636–6641. [Google Scholar] [CrossRef] [PubMed]
- Scally, S.; Davison, W.; Zhang, H. Diffusion coefficients of metals and metal complexes in hydrogels used in diffusive gradients in thin films. Anal. Chim. Acta 2006, 558, 222–229. [Google Scholar] [CrossRef]
- Wang, D.; Gong, M.D.; Li, Y.Y.; Xu, L.; Wang, Y.; Jing, R.; Ding, S.; Zhang, C. In Situ, High-Resolution Profiles of Labile Metals in Sediments of Lake Taihu. Int. J. Environ. Res. Public Health 2016, 13, 884. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wang, X.R.; Luo, J.; Yu, H.; Zhang, H. Evaluation of holistic approaches to predicting the concentrations of metals in field-cultivated rice. Environ. Sci. Technol. 2008, 42, 7649–7654. [Google Scholar] [CrossRef] [PubMed]


| Site | pH | MC (%) | OM (%) | Soil Mechanical Composition | CEC | Zn Centration (mg·kg−1) | Pb Centration (mg·kg−1) | ||
|---|---|---|---|---|---|---|---|---|---|
| Clay | Silt | Sand | |||||||
| 1 | 5.93 | 35.6 | 4.31 | 8.51 | 47.19 | 14.42 | 14.3 | 103.5 | 53.98 |
| 2 | 6.18 | 38.1 | 4.19 | 13.62 | 35.27 | 19.46 | 16.6 | 132.4 | 67.37 |
| 3 | 6.75 | 39.8 | 5.35 | 16.47 | 47.98 | 14.12 | 18.7 | 178.9 | 94.76 |
| 4 | 6.73 | 41.1 | 2.49 | 13.11 | 49.07 | 15.01 | 16.1 | 141.7 | 65.07 |
| 5 | 6.54 | 43.4 | 5.17 | 12.48 | 49.94 | 11.13 | 15.8 | 176.9 | 59.98 |
| 6 | 5.78 | 29.8 | 4.59 | 9.85 | 50.19 | 14.34 | 15.3 | 153.8 | 71.79 |
| 7 | 5.43 | 31.9 | 3.39 | 14.28 | 44.97 | 12.92 | 16.5 | 201.2 | 65.34 |
| 8 | 5.76 | 35.7 | 5.41 | 9.89 | 56.18 | 10.59 | 16.2 | 191.5 | 61.31 |
| 9 | 5.94 | 40.8 | 2.77 | 13.78 | 51.03 | 13.09 | 18.8 | 163.2 | 71.59 |
| 10 | 6.32 | 41.6 | 3.19 | 14.78 | 52.76 | 12.91 | 19.1 | 167.3 | 81.35 |
| 11 | 7.45 | 43.2 | 2.99 | 17.61 | 53.15 | 11.48 | 21.2 | 178.9 | 77.08 |
| 12 | 7.27 | 42.7 | 4.79 | 17.11 | 46.14 | 16.41 | 15.7 | 213.2 | 61.34 |
| Extractants | Procedure | References |
|---|---|---|
| HAc | 0.5 g of soil was extracted with 20 ml of 0.11 mol·L−1 HAc and shaken for at least 16 h (overnight) | Houba et al. [30,33] |
| EDTA | 2.0 g of soil was extracted with 20 mL of 0.05 mol·L−1 EDTA adjusted using an ammonia solution to pH = 7.0 and shaken for 2 h | Wear and Evans [31,32] |
| CaCl2 | 2.0 g of soil was extracted with 20 mL of 0.01 mol·L−1 CaCl2 and shaken for 3 h | Novozamsky et al. [31,33] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.; Wang, P.-F.; Wang, C.; Hou, J.; Miao, L.-Z. The Evaluation on the Cadmium Net Concentration for Soil Ecosystems. Int. J. Environ. Res. Public Health 2017, 14, 297. https://doi.org/10.3390/ijerph14030297
Yao Y, Wang P-F, Wang C, Hou J, Miao L-Z. The Evaluation on the Cadmium Net Concentration for Soil Ecosystems. International Journal of Environmental Research and Public Health. 2017; 14(3):297. https://doi.org/10.3390/ijerph14030297
Chicago/Turabian StyleYao, Yu, Pei-Fang Wang, Chao Wang, Jun Hou, and Ling-Zhan Miao. 2017. "The Evaluation on the Cadmium Net Concentration for Soil Ecosystems" International Journal of Environmental Research and Public Health 14, no. 3: 297. https://doi.org/10.3390/ijerph14030297





