Use of the QuantiFERON-TB Gold In-Tube Test in the Diagnosis and Monitoring of Treatment Efficacy in Active Pulmonary Tuberculosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Study Population
2.3. Study Procedures
2.4. Laboratory Tests
2.5. Statistical Analysis
2.6. Definition
3. Results
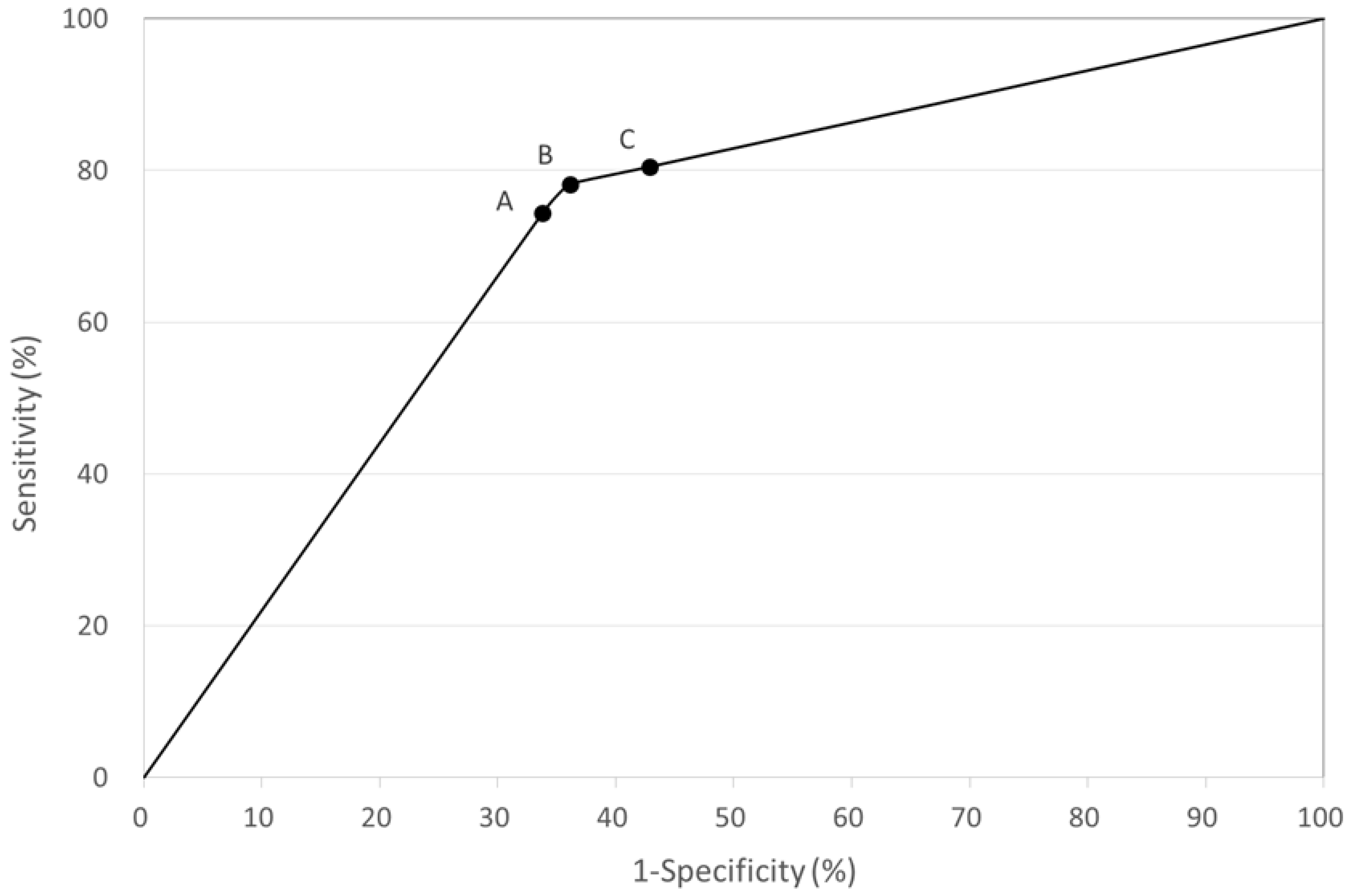
Accuracy of the QFT-GIT Test
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- WHO. Global Tuberculosis Report 2016. Available online: http://www.who.int/tb/publications/global_report/en/ (accessed on 2 January 2017).
- Houben, R.M.; Dodd, P.J. The Global Burden of Latent Tuberculosis Infection: A Re-Estimation Using Mathematical Modelling. PLoS Med. 2016, 25, e1002152. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Chen, K.L.; Chen, K.H.; Chien, S.T.; Chen, K.T. Updated diagnosis and treatment of childhood tuberculosis. World J. Pediatr. 2013, 9, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Detjen, A.K.; Keil, T.; Roll, S.; Hauer, B.; Mauch, H.; Wahn, U.; Magdorf, K. Interferon-gamma release assays improve the diagnosis of tuberculosis and nontuberculous mycobacterial disease in children in a country with a low incidence of tuberculosis. Clin. Infect. Dis. 2007, 45, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.D.O.; Gordon, S.B.; Davies, G. Clinical Tuberculosis, 5th ed.; Taylor & Francis Group, LLC: Baca Raton, FL, USA, 2014; pp. 96–110. [Google Scholar]
- Lu, P.; Chen, X.; Zhu, L.M.; Yang, H.T. Interferon-Gamma Release Assays for the Diagnosis of Tuberculosis: A Systematic Review and Meta-analysis. Lung 2016, 194, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Diel, R.; Loddenkemper, R.; Nienhaus, A. Evidence-based comparison of commercial interferon-gamma release assays for detecting active TB: A metaanalysis. Chest 2010, 137, 952–968. [Google Scholar] [CrossRef] [PubMed]
- Dilektasli, A.G.; Erdem, E.; Durukan, E.; Eyüboğlu, F.Ö. Is the T-cell-based interferon-gamma releasing assay feasible for diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country? Jpn. J. Infect. Dis. 2010, 63, 433–436. [Google Scholar] [PubMed]
- Carvalho, A.C.; Pezzoli, M.C.; El-Hamad, I.; Arce, P.; Bigoni, S.; Scarcella, C.; Indelicato, A.M.; Scolari, C.; Carosi, G.; Matteelli, A. QuantiFERONTB Gold test in the identification of latent tuberculosis infection in immigrants. J. Infect. 2007, 55, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Dheda, K.; Pooran, A.; Pai, M.; Miller, R.F.; Lesley, K.; Booth, H.L.; Scott, G.M.; Akbar, A.N.; Zumla, A.; Rook, G.A. Interpretation of Mycobacterium tuberculosis antigen-specific IFN-γ release assays (T-SPOT.TB) and factors that may modulate test results. J. Infect. 2007, 55, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Kobashi, Y.; Obase, Y.; Fukuda, M.; Yoshida, K.; Miyashita, N.; Oka, M. Clinical reevaluation of the QuantiFERON TB-2G test as a diagnostic method for differentiating active tuberculosis from nontuberculous mycobacteriosis. Clin. Infect. Dis. 2006, 43, 1540–1546. [Google Scholar] [CrossRef] [PubMed]
- Lalvani, A. Diagnosing tuberculosis infection in the 21st century: New tools to tackle an old enemy. Chest 2007, 131, 1898–1906. [Google Scholar] [CrossRef] [PubMed]
- Menzies, D.; Pai, M.; Comstock, G. Meta-analysis: New tests for the diagnosis of latent tuberculosis infection: Areas of uncertainty and recommendations for research. Ann. Intern. Med. 2007, 146, 340–354. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, K.A.; Kon, O.M.; Newton, S.M.; Meintjes, G.; Davidson, R.N.; Pasvol, G.; Wilkinson, R.J. Effect of treatment of latent tuberculosis infection on the T-cell response to Mycobacterium tuberculosis antigens. J. Infect. Dis. 2006, 193, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.; Joshi, R.; Dogra, S.; Mendiratta, D.K.; Narang, P.; Dheda, K.; Kalantri, S. Persistently elevated T-cell interferon-gamma responses after treatment for latent tuberculosis infection among health care workers in India: A preliminary report. J. Occup. Med. Toxicol. 2006, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, K.; Harada, N.; Mori, T. Interferon-gamma responses after isoniazid chemotherapy for latent tuberculosis. Respirology 2008, 13, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Chee, C.B.; KhinMar, K.W.; Gan, S.H.; Barkham, T.M.; Pushparani, M.; Wang, Y.T. Latent tuberculosis infection treatment and T-cell responses to Mycobacterium tuberculosis-specific antigens. Am. J. Respir. Crit. Care Med. 2007, 175, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Goletti, D.; Parracino, M.P.; Butera, O.; Bizzoni, F.; Casetti, R.; Dainotto, D.; Anzidei, G.; Nisii, C.; Ippolito, G.; Poccia, F.; et al. Isoniazid prophylaxis differently modulates T-cell responses to RD1-epitopes in contacts recently exposed to Mycobacterium tuberculosis: A pilot study. Respir. Res. 2007, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Lalvani, A.; Nagvenkar, P.; Udwadia, Z.; Pathan, A.A.; Wilkinson, K.A.; Shastri, J.S.; Ewer, K.; Hill, A.V.; Mehta, A.; Rodrigues, C. Enumeration of T-cells specific for RD1-encoded antigens suggests a high prevalence of latent Mycobacterium tuberculosis infection in healthy urban Indians. J. Infect. Dis. 2001, 183, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Carrara, S.; Vincenti, D.; Petrosillo, N.; Amicosante, M.; Girardi, E.; Goletti, D. Use of a T cell-based assay for monitoring efficacy of antituberculosis therapy. Clin. Infect. Dis. 2004, 38, 754–756. [Google Scholar] [CrossRef] [PubMed]
- Aiken, A.M.; Hill, P.C.; Fox, A.; McAdam, K.P.; Jackson-Sillah, D.; Lugos, M.D.; Donkor, S.A.; Adegbola, R.A.; Brookes, R.H. Reversion of the ELISPOT test after treatment in Gambian tuberculosis cases. BMC Infect. Dis. 2006, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Ulrichs, T.; Anding, R.; Kaufmann, S.H.; Munk, M.E. Numbers of IFN-γ-producing cells against ESAT-6 increase in tuberculosis patients during chemotherapy. Int. J. Tuberc. Lung Dis. 2000, 4, 1181–1183. [Google Scholar] [PubMed]
- Wu-Hsieh, B.A.; Chen, C.K.; Chang, J.H.; Lai, S.Y.; Wu, C.H.; Cheng, W.C.; Andersen, P.; Doherty, T.M. Long-lived immune response to early secretory antigenic target 6 in individuals who had recovered from tuberculosis. Clin. Infect. Dis. 2001, 33, 1336–1340. [Google Scholar] [CrossRef] [PubMed]
- Toulza, F.; Tsang, L.; Ottenhoff, T.H.; Brown, M.; Dockrell, H.M. Mycobacterium tuberculosis-specific CD4+ T-cell response is increased, and Treg cells decreased, in anthelmintic-treated patients with latent TB. Eur. J. Immunol. 2016, 46, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.; Joshi, R.; Bandyopadhyay, M.; Narang, P.; Dogra, S.; Taksande, B.; Kalantri, S. Sensitivity of a wholeblood interferon-gamma assay among patients with pulmonary tuberculosis and variations in T-cell responses during antituberculosis treatment. Infection 2007, 35, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Tsou, P.H.; Huang, W.C.; Huang, C.C.; Lin, C.F.; Wu, K.M.; Hsu, J.Y.; Shen, G.H. Quantiferon TB-Gold conversion can predict active tuberculosis development in elderly nursing home residents. Geriatr. Gerontol. Int. 2015, 15, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Chen, H.C.; Cheng, H.Y.; Chian, C.F.; Chang, F.Y.; Chen, H.I.; Ku, C.H.; Lin, J.C. Role of QuantiFERON-TB-Gold In Tube assay for active and latent tuberculosis infection in investigation of tuberculosis outbreak in a university. J. Microbiol. Immunol. Infect. 2015, 48, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.T.; Lee, S.S.; Sy, C.L.; Wu, K.S.; Chen, J.K.; Tsai, H.C.; Chen, Y.S. Prevalence of latent tuberculosis infection in BCG-vaccinated healthcare workers by using an interferon-gamma release assay and the tuberculin skin test in an intermediate tuberculosis burden country. J. Microbiol. Immunol. Infect. 2015, 48, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Tan, C.K.; Lin, S.H.; Liao, C.H.; Huang, Y.T.; Hsueh, P.R. Diagnostic performance of whole-blood interferon-γ assay and enzyme-linked immunospot assay for active tuberculosis. Diagn. Microbiol. Infect. Dis. 2011, 71, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.T.; Wang, P.H.; Chien, S.T.; Chen, S.C. Performance of the QuantiFERON-TB Gold In-Tube test to Monitor Treatment of Active Pulmonary Tuberculosis in Taiwan. J. Community Med. Public Health Care 2016, 3, 022. [Google Scholar]
- Bjerrum, S.; Oliver-Commey, J.; Kenu, E.; Lartey, M.; Newman, M.J.; Addo, K.K.; Hilleman, D.; Andersen, A.B.; Johansen, I.S. Tuberculosis and nontuberculous mycobacteria among HIV-infected individuals in Ghana. Trop. Med. Int. Health 2016, 21, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Metz, M.Z. Basic principles of ROC analysis. Semin. Nucl. Med. 1978, 8, 283–298. [Google Scholar] [CrossRef]
- Kang, Y.A.; Lee, H.W.; Hwang, S.S.; Um, S.W.; Han, S.K.; Shim, Y.S.; Yim, J.J. Usefulness of whole-blood interferon-gamma assay and interferon-gamma enzyme-linked immunospot assay in the diagnosis of active pulmonary tuberculosis. Chest 2007, 132, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Sampath, A.; Bihari, S.; Mamtani, M.; Kulkarni, H. Use of the QuantiFERON-TB Gold In-Tube test to monitor treatment efficacy in active pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 2008, 12, 1146–1152. [Google Scholar] [PubMed]
- Dewan, P.K.; Grinsdale, J.; Kawamura, L.M. Low sensitivity of a whole-blood interferon-gamma release assay for detection of active tuberculosis. Clin. Infect. Dis. 2007, 44, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Goletti, D.; Carrara, S.; Vincenti, D.; Saltini, C.; Rizzi, E.B.; Schininà, V.; Ippolito, G.; Amicosante, M.; Girardi, E. Accuracy of an immune diagnostic assay based on RD1 selected epitopes for active tuberculosis in a clinical setting: A pilot study. Clin. Microbiol. Infect. 2006, 12, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Sester, M.; Sotgiu, G.; Lange, C.; Giehl, C.; Girardi, E.; Migliori, G.B.; Bossink, A.; Dheda, K.; Diel, R.; Dominguez, J.; et al. Interferon-γ release assays for the diagnosis of active tuberculosis: A systematic review and meta-analysis. Eur. Respir. J. 2011, 37, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Hasegawa, N.; Mori, M.; Takebayashi, T.; Harada, N.; Higuchi, K.; Tasaka, S.; Ishizaka, A. Accuracy of an interferon-gamma release assay to detect active pulmonary and extra-pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 2008, 12, 269–274. [Google Scholar] [PubMed]
- Vincenti, D.; Carrara, S.; Butera, O.; Bizzoni, F.; Casetti, R.; Girardi, E.; Goletti, D. Response to region of difference 1 (RD1) epitopes in human immunodeficiency virus(HIV)-infected individuals enrolled with suspected active tuberculosis: A pilot study. Clin. Exp. Immunol. 2007, 150, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Tian, J.L.; Yin, Q.Q.; Xiao, J.; Li, J.Q.; Guo, Y.J.; Feng, G.S.; Peng, X.X.; Qi, H.; Xu, F.; et al. Performance of the Interferon Gamma Release Assays in Tuberculosis Disease in Children Five Years Old or Less. PLoS ONE 2015, 10, e0143820. [Google Scholar] [CrossRef] [PubMed]
- Nakielna, E.M.; Cragg, R.; Grzybowski, S. Lifelong follow-up of inactive tuberculosis: Its value and limitations. Am. Rev. Respir. Dis. 1975, 112, 765–772. [Google Scholar] [PubMed]
- Sallakci, N.; Coskun, M.; Berber, Z.; Gürkan, F.; Kocamaz, H.; Uysal, G.; Bhuju, S.; Yavuzer, U.; Singh, M.; Yeğin, O. Interferon-γ+874T—A polymorphism is associated with tuberculosis and gamma interferon response. Tuberculosis 2007, 87, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Petruccioli, E.; Chiacchio, T.; Pepponi, I.; Vanini, V.; Urso, R.; Cuzzi, G.; Barcellini, L.; Cirillo, D.M.; Palmieri, F.; Ippolito, G.; et al. First characterization of the CD4 and CD8 T-cell responses to QuantiFERON-TB Plus. J. Infect. 2016, 73, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Chiacchio, T.; Petruccioli, E.; Vanini, V.; Cuzzi, G.; Pinnetti, C.; Sampaolesi, A.; Antinori, A.; Girardi, E.; Goletti, D. Polyfunctional T-cells and effector memory phenotype are associated with active TB in HIV-infected patients. J. Infect. 2014, 69, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Day, C.L.; Abrahams, D.A.; Lerumo, L.; van Rensburg, E.J.; Stone, L.; O’rie, T.; Pienaar, B.; de Kock, M.; Kaplan, G.; Mahomed, H.; et al. Functional capacity of Mycobacterium tuberculosis-specific T cell responses in humans is associated with mycobacterial load. J. Immunol. 2011, 187, 2222–2232. [Google Scholar] [CrossRef] [PubMed]
- Rozot, V.; Patrizia, A.; Vigano, S.; Mazza-Stalder, J.; Idrizi, E.; Day, C.L.; Perreau, M.; Lazor-Blanchet, C.; Ohmiti, K.; Goletti, D.; et al. Combined use of Mycobacterium tuberculosis-specific CD4 and CD8 T-cell responses is a powerful diagnostic tool of active tuberculosis. Clin. Infect. Dis. 2015, 60, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Rozot, V.; Vigano, S.; Mazza-Stalder, J.; Idrizi, E.; Day, C.L.; Perreau, M.; Lazor-Blanchet, C.; Petruccioli, E.; Hanekom, W.; Goletti, D.; et al. Mycobacterium tuberculosis-specific CD8+ T cells are functionally and phenotypically different between latent infection and active disease. Eur. J. Immunol. 2013, 43, 1568–1577. [Google Scholar] [CrossRef] [PubMed]
- Barcellini, L.; Borroni, E.; Brown, J.; Brunetti, E.; Campisi, D.; Castellotti, P.F.; Codecasa, L.R.; Cugnata, F.; Di Serio, C.; Ferrarese, M.; et al. First evaluation of QuantiFERON-TB Gold Plus performance in contact screening. Eur. Respir. J. 2016, 48, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Sasaki, Y.; Nagai, H.; Ishikawa, S.; Takamori, M.; Sakashita, K.; Saito, T.; Fukushima, K.; Igarashi, Y.; Aono, A.; et al. Evaluation of QuantiFERON-TB Gold Plus for Detection of Mycobacterium tuberculosis infection in Japan. Sci. Rep. 2016, 6, 30617. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, H.; Avsar, K.; Göres, R.; Mavi, S.C.; Hofmann-Thiel, S. Equal sensitivity of the new generation QuantiFERON-TB Gold plus in direct comparison with the previous test version QuantiFERON-TB Gold IT. Clin. Microbiol. Infect. 2016, 22, 701–703. [Google Scholar] [CrossRef] [PubMed]
- Igari, H.; Watanabe, A.; Sato, T. Booster phenomenon of Quanti-FERON-TB Gold after prior intradermal PPD injection. Int. J. Tuberc. Lung Dis. 2007, 11, 788–791. [Google Scholar] [PubMed]
- Leyten, E.M.; Prins, C.; Bossink, A.W.; Thijsen, S.; Ottenhoff, T.H.; van Dissel, J.T.; Arend, S.M. Effect of tuberculin skin testing on a Mycobacterium tuberculosis-specific interferon-gamma assay. Eur. Respir. J. 2007, 29, 1212–1216. [Google Scholar] [CrossRef] [PubMed]
- Ndzi, E.N.; Nkenfou, C.N.; Gwom, L.C.; Fainguem, N.; Fokam, J.; Pefura, Y. The pros and cons of the QuantiFERON test for the diagnosis of tuberculosis, prediction of disease progression, and treatment monitoring. Int. J. Mycobacteriol. 2016, 5, 177–184. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total N = 266 | Cases n = 133 | Controls n = 133 | p Value * |
|---|---|---|---|---|
| Age (year) (median) (IQR) | 58.6 (49.6–71.2) | 56.2 (47.3–70.4) | 62.3 (51.2–72.1) | 0.17 |
| Sex | 0.19 | |||
| Male (%) | 180 (68) | 85 (64) | 95 (71) | |
| Female (%) | 86 (32) | 48 (36) | 38 (29) | |
| QFT-GIT test | 0.001 | |||
| Positive (%) | 162 (61) | 95 (71) | 48 (36) | |
| Negative (%) | 104 (39) | 38 (29) | 85 (64) | |
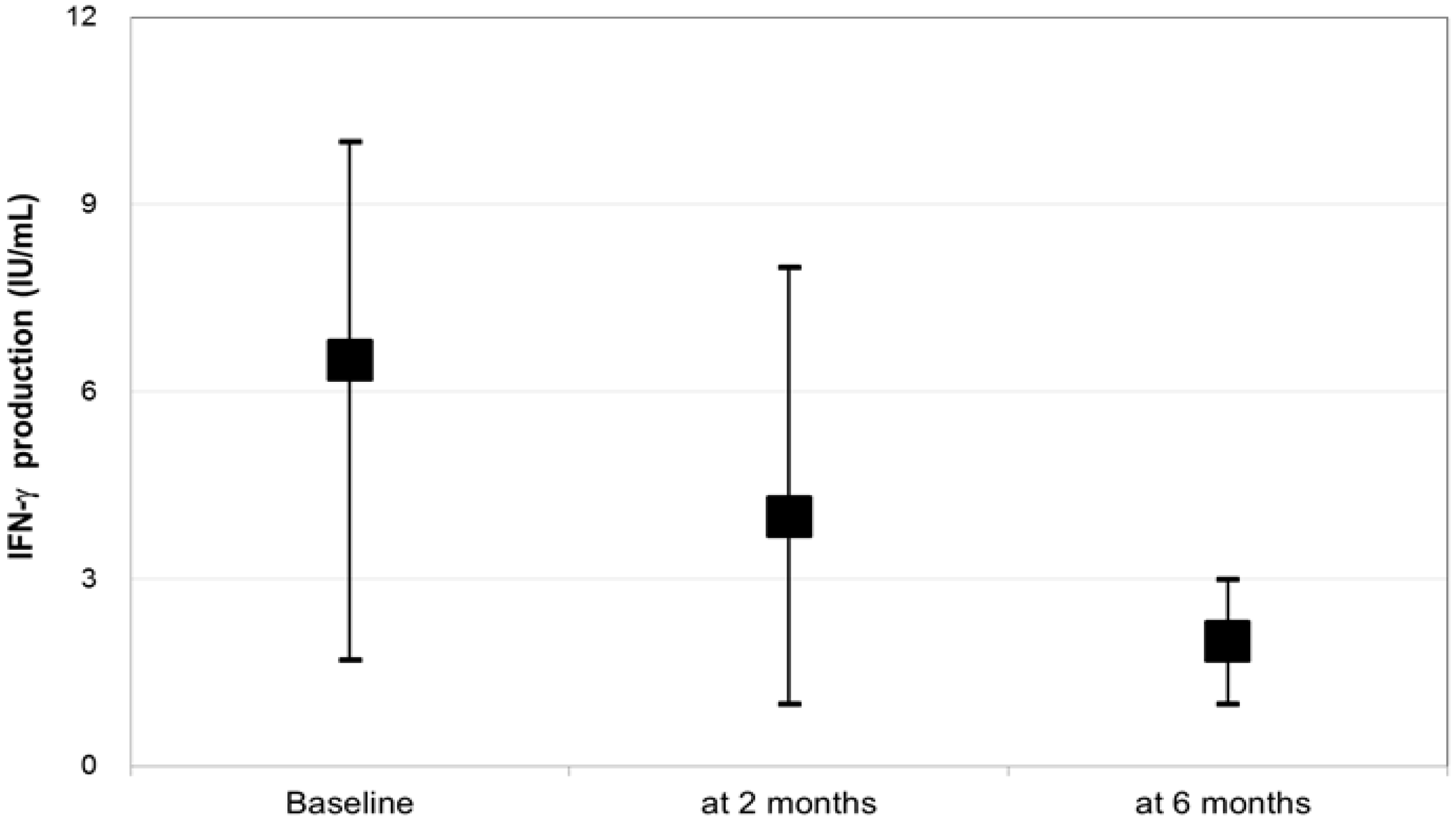
| Median IFN-γ response (IU/mL) (IQR) | 3.54 (0.51–10.0) | 6.32 (1.01–10.0) | 0.76 (0.01–10.0) | <0.001 |
| QFT-GIT Test Cut-Off Value (IU/mL) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| ≥0.35 | 74.4 | 66.2 | 68.8 | 72.1 |
| ≥0.20 | 78.2 | 63.6 | 68.4 | 74.6 |
| ≥0.10 | 80.5 | 57.1 | 65.2 | 74.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, P.-C.; Wang, P.-H.; Chen, K.-T. Use of the QuantiFERON-TB Gold In-Tube Test in the Diagnosis and Monitoring of Treatment Efficacy in Active Pulmonary Tuberculosis. Int. J. Environ. Res. Public Health 2017, 14, 236. https://doi.org/10.3390/ijerph14030236
Chang P-C, Wang P-H, Chen K-T. Use of the QuantiFERON-TB Gold In-Tube Test in the Diagnosis and Monitoring of Treatment Efficacy in Active Pulmonary Tuberculosis. International Journal of Environmental Research and Public Health. 2017; 14(3):236. https://doi.org/10.3390/ijerph14030236
Chicago/Turabian StyleChang, Ping-Chin, Pin-Hui Wang, and Kow-Tong Chen. 2017. "Use of the QuantiFERON-TB Gold In-Tube Test in the Diagnosis and Monitoring of Treatment Efficacy in Active Pulmonary Tuberculosis" International Journal of Environmental Research and Public Health 14, no. 3: 236. https://doi.org/10.3390/ijerph14030236





