Airborne or Fomite Transmission for Norovirus? A Case Study Revisited
Abstract
:1. Introduction
2. Methods
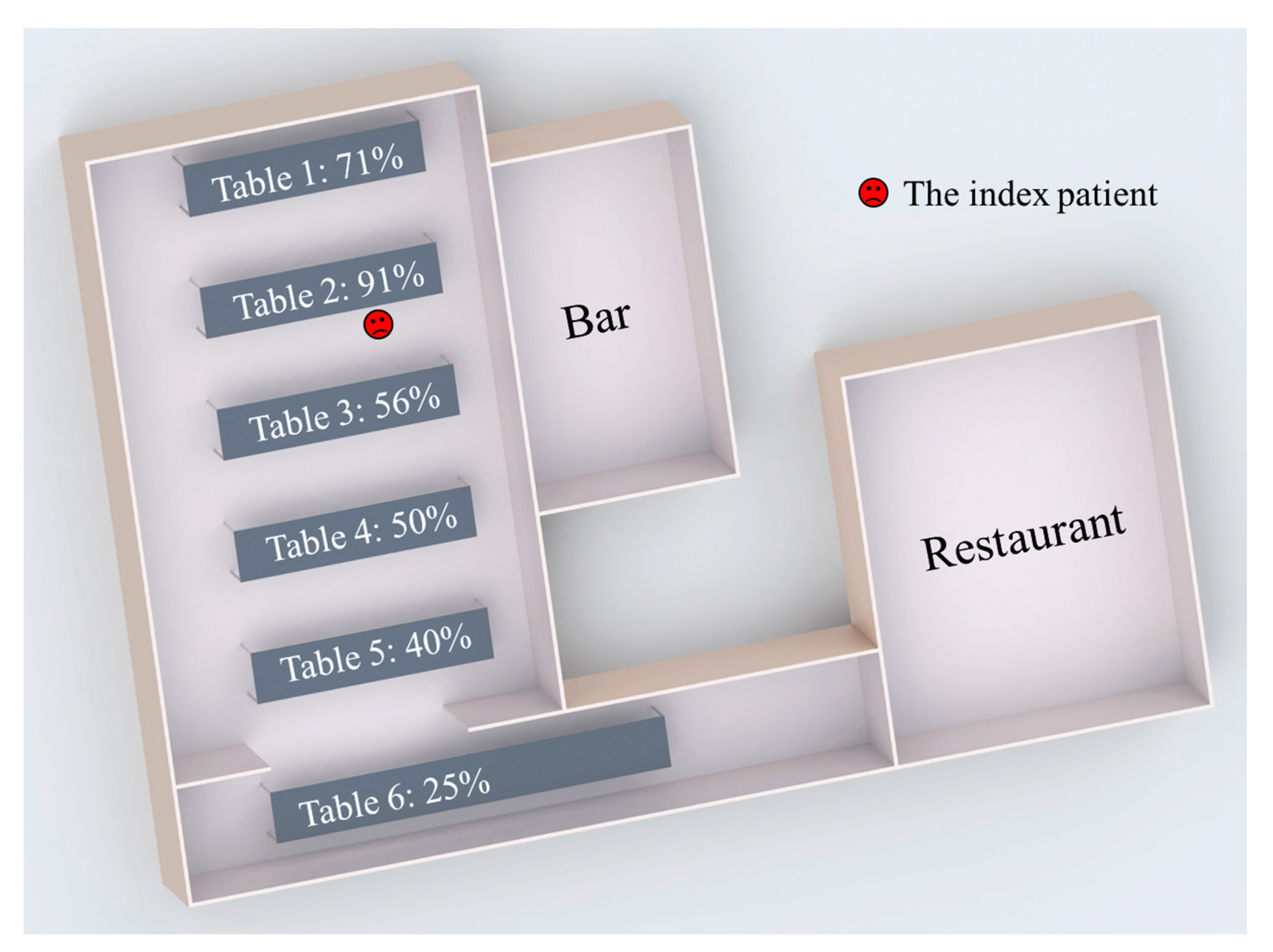
2.1. Outbreak
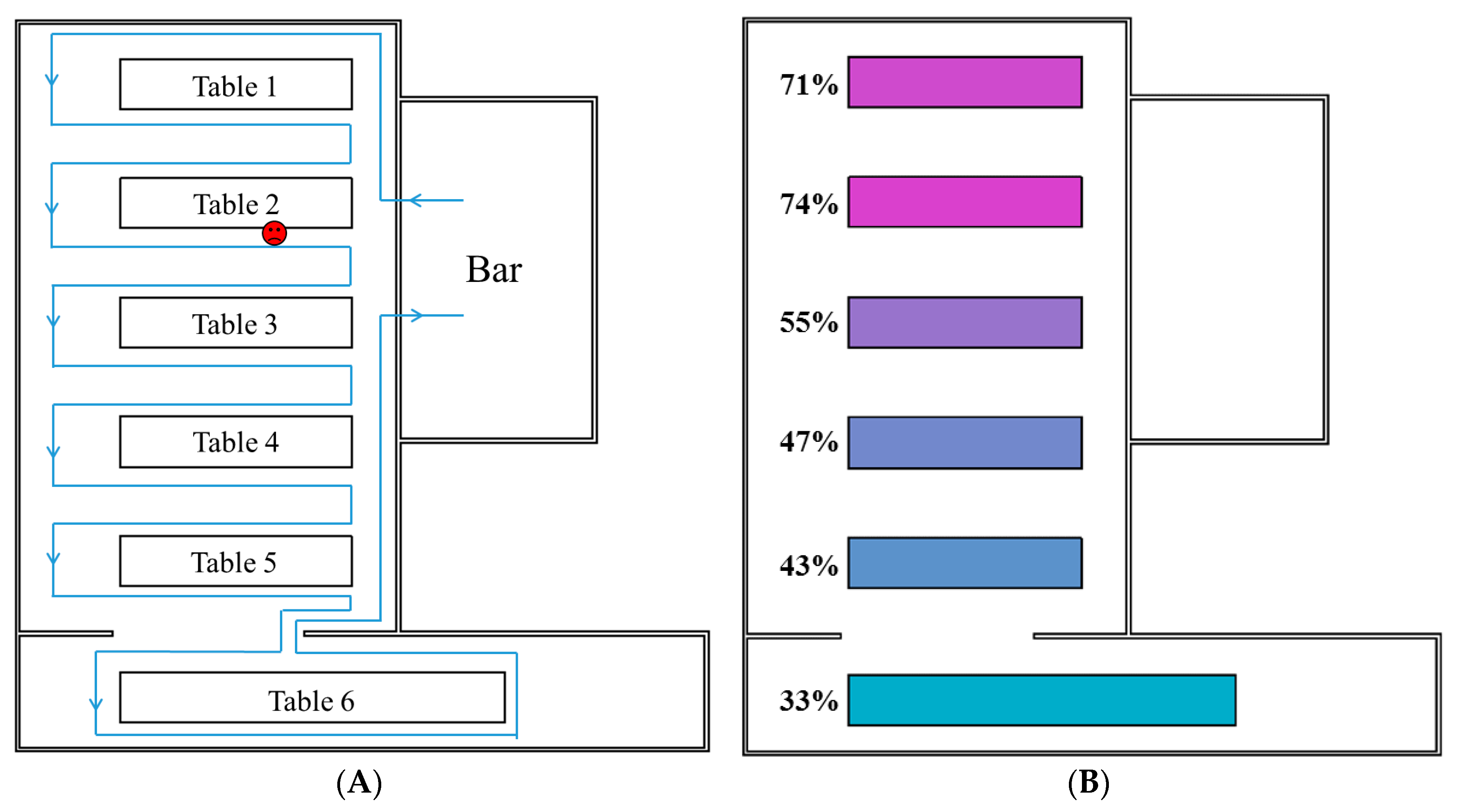
2.2. Multi-Agent Modelling Framework
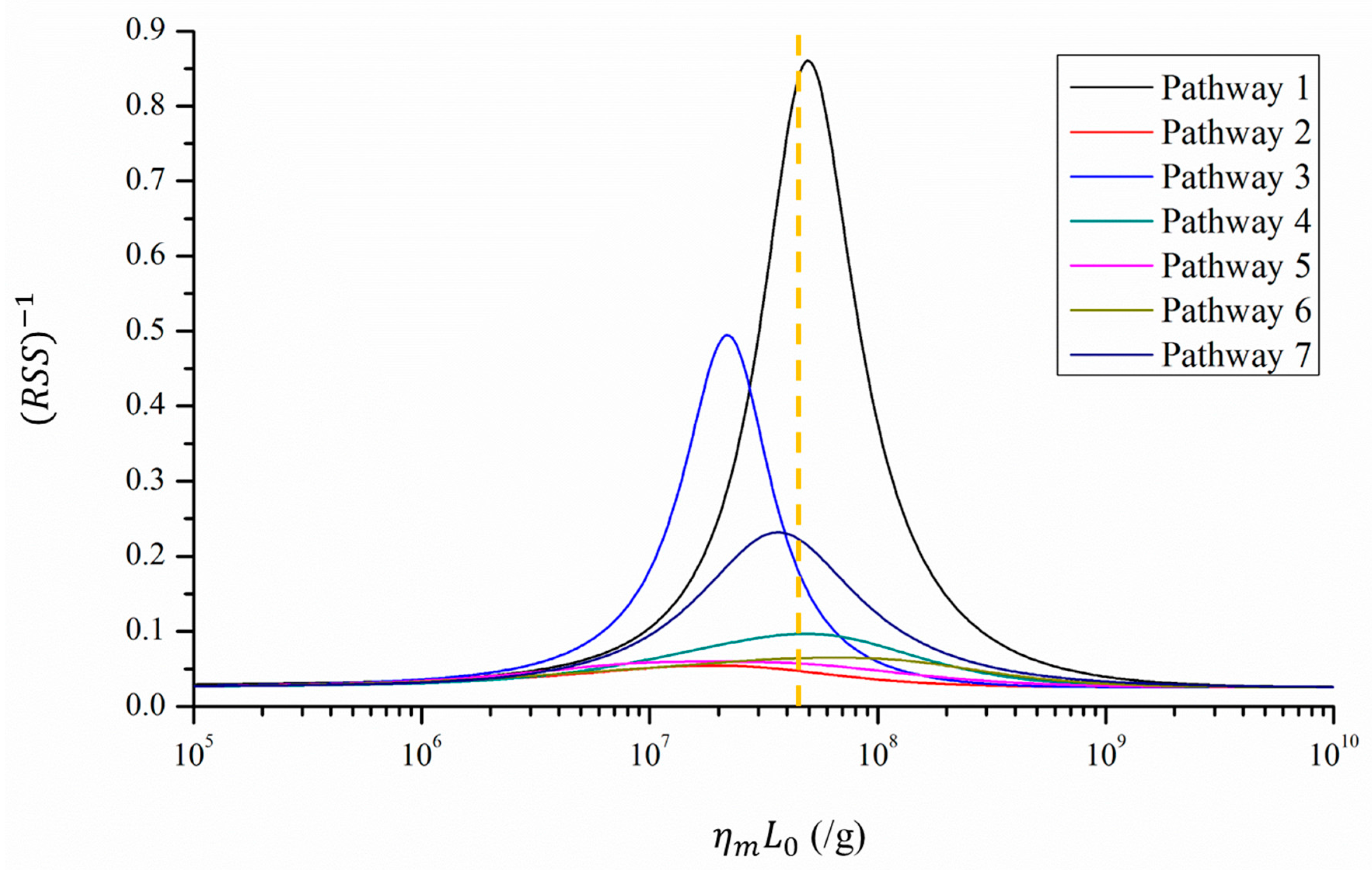
2.3. Least-Square Fitting
3. Results
3.1. Spatial Patterns of the Predicted Infection Risk Distributions
3.2. Fitness between Predicted Infection Risk and Reported Attack Rates
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ahmed, S.M.; Hall, A.J.; Robinson, A.E.; Verhoef, L.; Premkumar, P.; Parashar, U.D.; Koopmans, M.; Lopman, B.A. Global prevalence of norovirus in cases of gastroenteritis: A systematic review and meta-analysis. Lancet Infect. Dis. 2014, 14, 725–730. [Google Scholar] [CrossRef]
- Lopman, B. Global Burden of Norovirus and Prospects for Vaccine Development. CDC Foundation 2015. Available online: https://www.cdc.gov/norovirus/downloads/global-burden-report.pdf (accessed on 9 September 2017).
- Bartsch, S.M.; Lopman, B.A.; Ozawa, S.; Hall, A.J.; Lee, B.Y. Global economic burden of norovirus gastroenteritis. PLoS ONE 2016, 11, e0151219. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.C.; Sung, J.J.; Lam, R.K.; Chan, P.K.; Lee, N.L.; Lai, R.W.; Leung, W.K. Fecal viral load and norovirus-associated gastroenteritis. Emerg. Infect. Dis. 2006, 12, 1278–1280. [Google Scholar] [CrossRef] [PubMed]
- Clay, S.; Maherchandani, S.; Malik, Y.S.; Goyal, S.M. Survival on uncommon fomites of feline calicivirus, a surrogate of noroviruses. Am. J. Infect. Control 2006, 34, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Teunis, P.F.M.; Moe, C.L.; Liu, P.; Miller, S.E.; Lindesmith, L.; Baric, R.S.; Le Pendu, J.; Calderon, R.L. Norwalk virus: How infectious is it? J. Med. Virol. 2008, 80, 1468–1476. [Google Scholar] [CrossRef] [PubMed]
- Karst, S.M.; Wobus, C.E.; Lay, M.; Davidson, J.; Virgin, H.W. STAT1-dependent innate immunity to a Norwalk-like virus. Science 2003, 299, 1575–1578. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Norovirus Worldwide. 2016. Available online: https://www.cdc.gov/norovirus/worldwide.html (accessed on 9 September 2017).
- Centers for Disease Control and Prevention. Clinical Overview. 2013. Available online: https://www.cdc.gov/norovirus/hcp/clinical-overview.html (accessed on 9 September 2017).
- Nazaroff, W.W. Norovirus, gastroenteritis, and indoor environmental quality. Indoor Air 2011, 21, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.M.; Brosseau, L.M. Aerosol transmission of infectious disease. J. Occup. Environ. Med. 2015, 57, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Makison Booth, C. Vomiting Larry: A simulated vomiting system for assessing environmental contamination from projectile vomiting related to norovirus infection. J. Infect. Prev. 2014, 15, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.; Jones, M. The potential spread of infection caused by aerosol contamination of surfaces after flushing a domestic toilet. J. Appl. Microbiol. 2005, 99, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Nenonen, N.P.; Hannoun, C.; Svensson, L.; Torén, K.; Andersson, L.-M.; Westin, J.; Bergström, T. Norovirus GII. 4 detection in environmental samples from patient rooms during nosocomial outbreaks. J. Clin. Microbiol. 2014, 52, 2352–2358. [Google Scholar] [CrossRef] [PubMed]
- Masclaux, F.G.; Hotz, P.; Gashi, D.; Savova-Bianchi, D.; Oppliger, A. Assessment of airborne virus contamination in wastewater treatment plants. Environ. Res. 2014, 133, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Bonifait, L.; Charlebois, R.; Vimont, A.; Turgeon, N.; Veillette, M.; Longtin, Y.; Jean, J.; Duchaine, C. Detection and quantification of airborne norovirus during outbreaks in healthcare facilities. Clin. Infect. Dis. 2015, 61, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Southam, D.; Dolovich, M.; O’Byrne, P.; Inman, M. Distribution of intranasal instillations in mice: Effects of volume, time, body position, and anesthesia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 282, L833–L839. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, L.A.; Murphy, J.J.; Kaplan, J.E.; Pinsky, P.F.; Chacon, D.; Walmsley, S.; Schonberger, L.B.; Phillips, A.; Forward, K.; Goldman, C.; et al. 25-to 30-nm virus particle associated with a hospital outbreak of acute gastroenteritis with evidence for airborne transmission. Am. J. Epidemiol. 1988, 127, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Gellert, G.A.; Waterman, S.H.; Ewert, D.; Oshiro, L.; Giles, M.P.; Monroe, S.S.; Gorelkin, L.; Glass, R.I. An outbreak of acute gastroenteritis caused by a small round structured virus in a geriatric convalescent facility. Infect. Control Hosp. Epidemiol. 1990, 11, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, P.; Walker, M.; Rees, A. Airborne transmission of a small round structured virus. Lancet 1994, 343, 171. [Google Scholar] [CrossRef]
- Marks, P.; Vipond, I.; Carlisle, D.; Deakin, D.; Fey, R.; Caul, E. Evidence for airborne transmission of Norwalk-like virus (NLV) in a hotel restaurant. Epidemiol. Infect. 2000, 124, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Marks, P.; Vipond, I.; Regan, F.; Wedgwood, K.; Fey, R.; Caul, E. A school outbreak of Norwalk-like virus: Evidence for airborne transmission. Epidemiol. Infect. 2003, 131, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.-W.; Schmid, D.; Schwarz, K.; Pichler, A.-M.; Klein, H.; König, C.; de Martin, A.; Allerberger, F. A non-foodborne norovirus outbreak among school children during a skiing holiday, Austria, 2007. Wien. Klin. Wochenschr. 2009, 121, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Lin, Q.; Chen, C.; Zhang, J.; Zhang, H.; Hao, C. Epidemiology of norovirus gastroenteritis outbreaks in two primary schools in a city in eastern China. Am. J. Infect. Control 2013, 41, e107–e109. [Google Scholar] [CrossRef] [PubMed]
- Yu, I.T.; Li, Y.; Wong, T.W.; Tam, W.; Chan, A.T.; Lee, J.H.; Leung, D.Y.; Ho, T. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N. Engl. J. Med. 2004, 350, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, X.; Yu, I.T.; Wong, T.W.; Qian, H. Role of air distribution in SARS transmission during the largest nosocomial outbreak in Hong Kong. Indoor Air 2005, 15, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Li, Y.; Wong, T.-W.; Hui, D.S.C. Role of fomites in SARS transmission during the largest hospital outbreak in Hong Kong. PLoS ONE 2017, 12, e0181558. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Li, Y.; Sung, M.; Wei, J.; Yang, Z. A study of the probable transmission routes of MERS-CoV during the first hospital outbreak in the Republic of Korea. Indoor Air 2017. [Google Scholar] [CrossRef] [PubMed]
- Pittet, D.; Dharan, S.; Touveneau, S.; Sauvan, V.; Perneger, T.V. Bacterial contamination of the hands of hospital staff during routine patient care. Arch. Intern. Med. 1999, 159, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-M.; Wu, F.-M.; Kim, H.-K.; Doyle, M.P.; Michaels, B.S.; Williams, L.K. A comparison of hand washing techniques to remove Escherichia coli and caliciviruses under natural or artificial fingernails. J. Food Prot. 2003, 66, 2296–2301. [Google Scholar] [CrossRef] [PubMed]
- Kemeny, J.G.; Snell, J.L. Finite Markov Chains; van Nostrand, D., Ed.; Springer: Princeton, NJ, USA, 1960; Volume 356. [Google Scholar]
- Gao, X. Relative Effectiveness of Ventilation in Community Indoor Environments for Controlling Infection; The University of Hong Kong: Hong Kong, China, 2011. [Google Scholar]
- Draper, N.R.; Smith, H.; Pownell, E. Applied Regression Analysis; Wiley: New York, NY, USA, 1966; Volume 3. [Google Scholar]
- Johnson, J.B.; Omland, K.S. Model selection in ecology and evolution. Trends Ecol. Evol. 2004, 19, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Thebault, A.; Teunis, P.F.M.; Le Pendu, J.; Le Guyader, F.S.; Denis, J.-B. Infectivity of GI and GII noroviruses established from oyster related outbreaks. Epidemics 2013, 5, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Li, Y.; Xiao, S.; Yang, X.; Lin, C.; Norris, S.L.; Wei, D.; Hu, Z.; Ji, S. Logistic growth of a surface contamination network and its role in disease spread. Sci. Rep. 2017, 7, 14826. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.P.; Wein, L.M. Quantifying the routes of transmission for pandemic influenza. Bull. Math. Biol. 2008, 70, 820–867. [Google Scholar] [CrossRef] [PubMed]
- Riley, E.; Murphy, G.; Riley, R. Airborne spread of measles in a suburban elementary school. Am. J. Epidemiol. 1978, 107, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Li, Y.; Xiao, S.; Lin, C.; Norris, S.L.; Wei, D.; Hu, Z.; Ji, S. Route of transmission of influenza A H1N1, SARS CoV and norovirus in air cabins—A comparative analysis. Indoor Air. (accepted).

| Parameter | Reported Data | Pathway | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| Minimum RSS | N.A. | 1.16 | 18.26 | 2.02 | 10.33 | 16.67 | 15.35 | 4.32 | |
| 1 (/g) | Unknown | 107.69 | 107.28 | 107.34 | 107.69 | 107.26 | 107.81 | 107.56 | |
| Average attack rate/infection risks at the six tables | Table 1 | 71% | 73% | 18% | 74% | 30% | 48% | 34% | 52% |
| Table 2 | 91% | 76% | 45% | 73% | 63% | 55% | 67% | 67% | |
| Table 3 | 56% | 58% | 32% | 43% | 59% | 35% | 62% | 52% | |
| Table 4 | 50% | 49% | 43% | 42% | 59% | 33% | 53% | 51% | |
| Table 5 | 40% | 46% | 52% | 42% | 60% | 19% | 67% | 51% | |
| Table 6 | 25% | 35% | 62% | 44% | 54% | 33% | 57% | 51% | |
| Overall | 63% | 73% | 18% | 74% | 30% | 48% | 34% | 52% | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, S.; Tang, J.W.; Li, Y. Airborne or Fomite Transmission for Norovirus? A Case Study Revisited. Int. J. Environ. Res. Public Health 2017, 14, 1571. https://doi.org/10.3390/ijerph14121571
Xiao S, Tang JW, Li Y. Airborne or Fomite Transmission for Norovirus? A Case Study Revisited. International Journal of Environmental Research and Public Health. 2017; 14(12):1571. https://doi.org/10.3390/ijerph14121571
Chicago/Turabian StyleXiao, Shenglan, Julian W. Tang, and Yuguo Li. 2017. "Airborne or Fomite Transmission for Norovirus? A Case Study Revisited" International Journal of Environmental Research and Public Health 14, no. 12: 1571. https://doi.org/10.3390/ijerph14121571




