Preparation and Application of Carboxylated Graphene Oxide Sponge in Dye Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of GO-COOH Sponge
2.3. Adsorption Isotherm
2.4. Kinetics and Thermodynamics
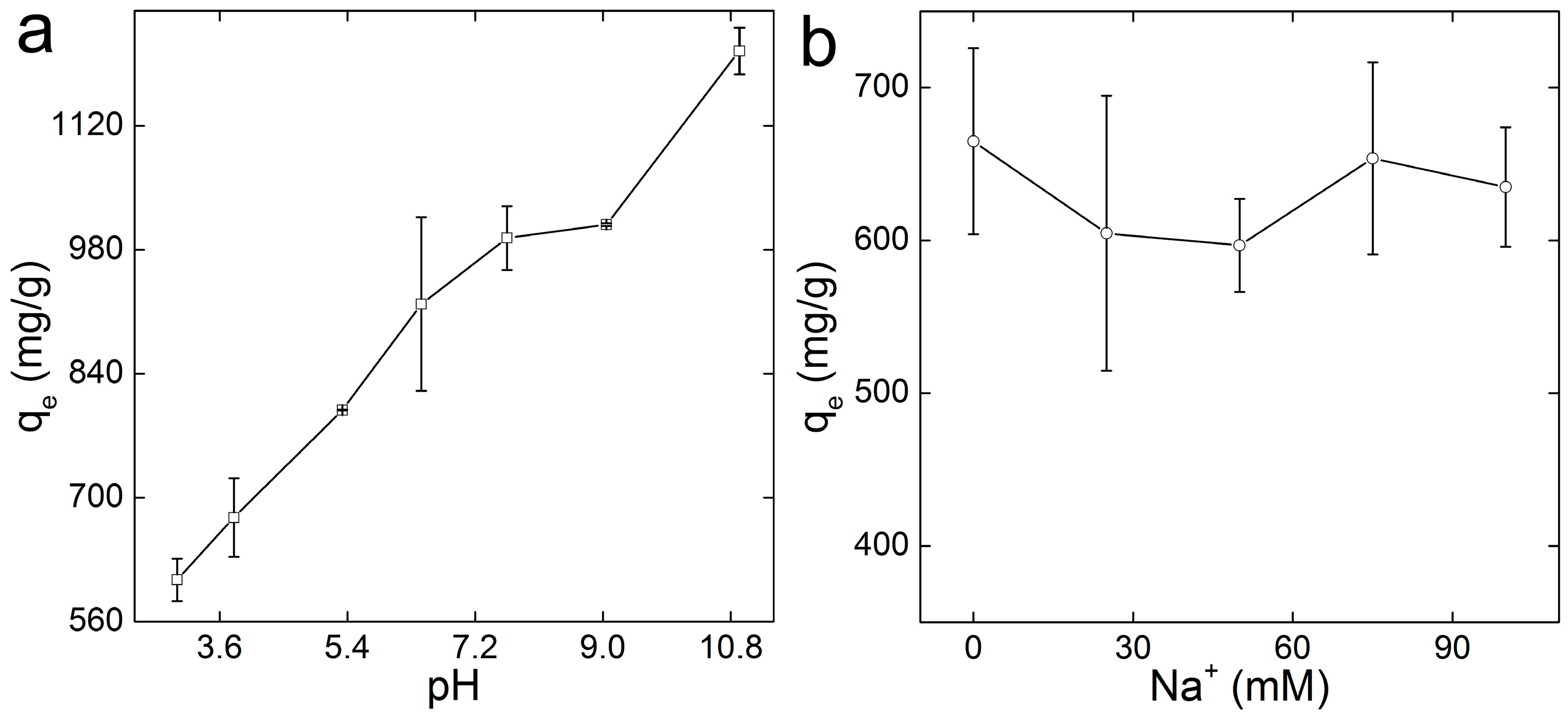
2.5. Influence of pH and Ionic Strength
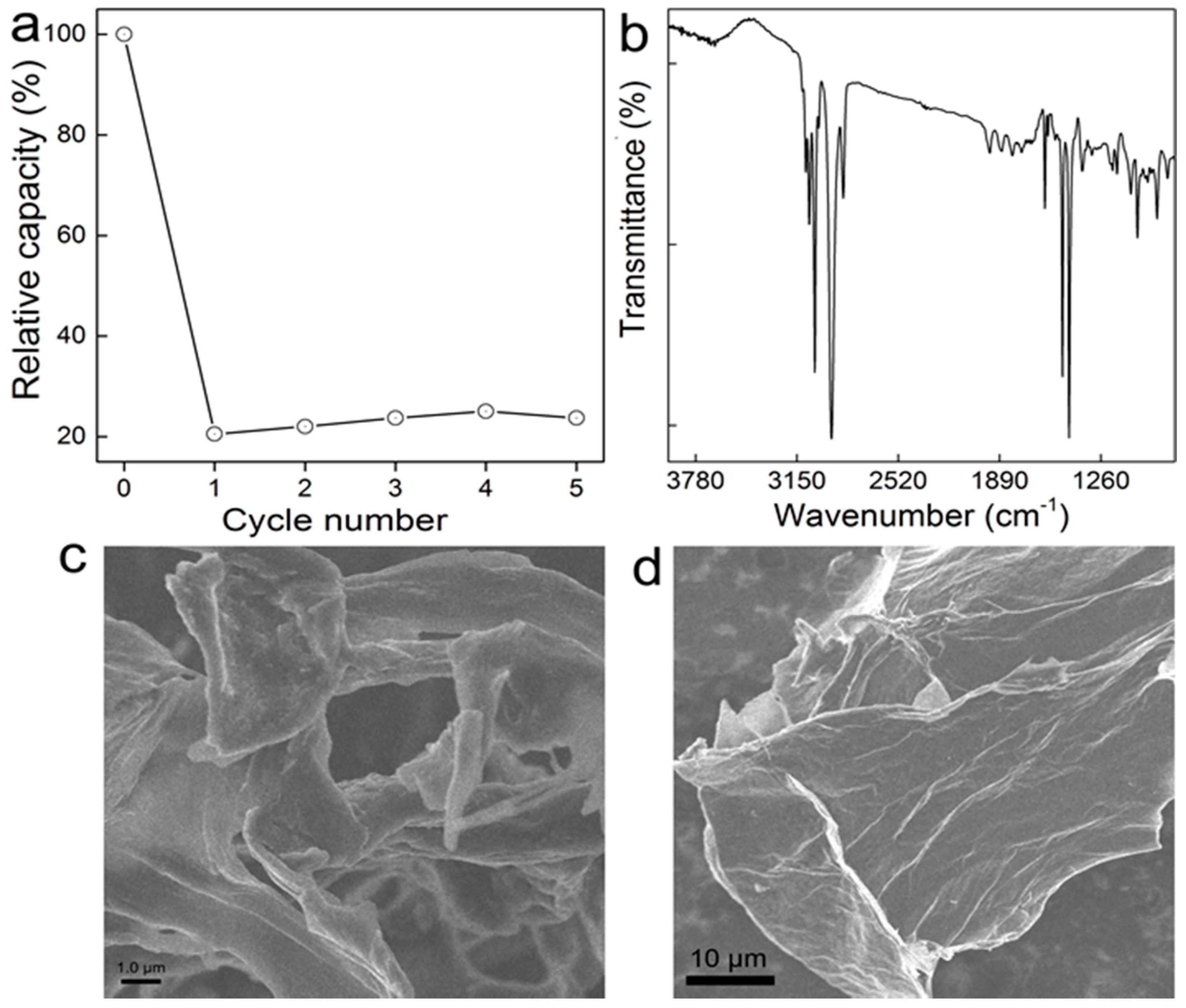
2.6. Regeneration
3. Results and Discussion
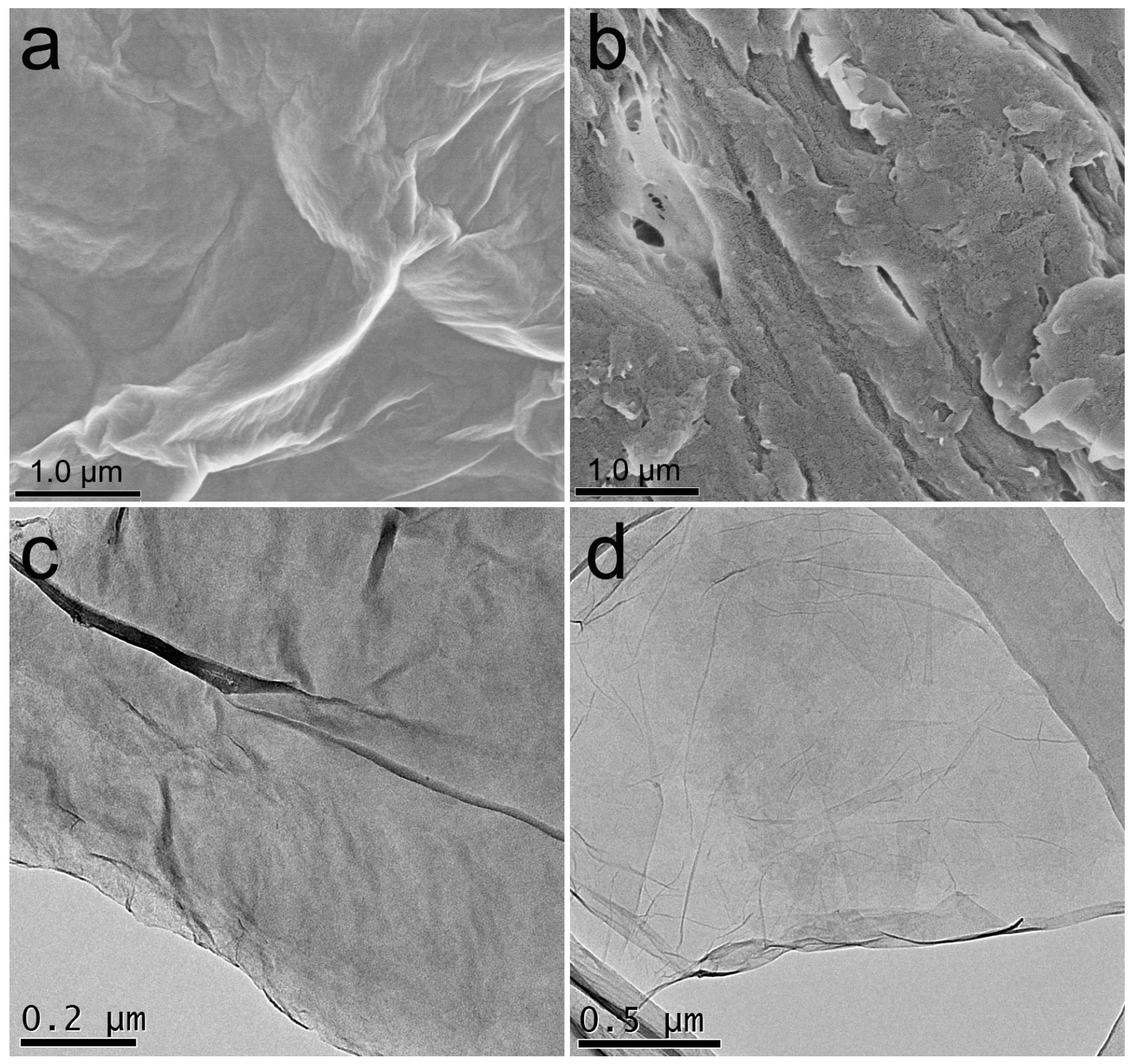
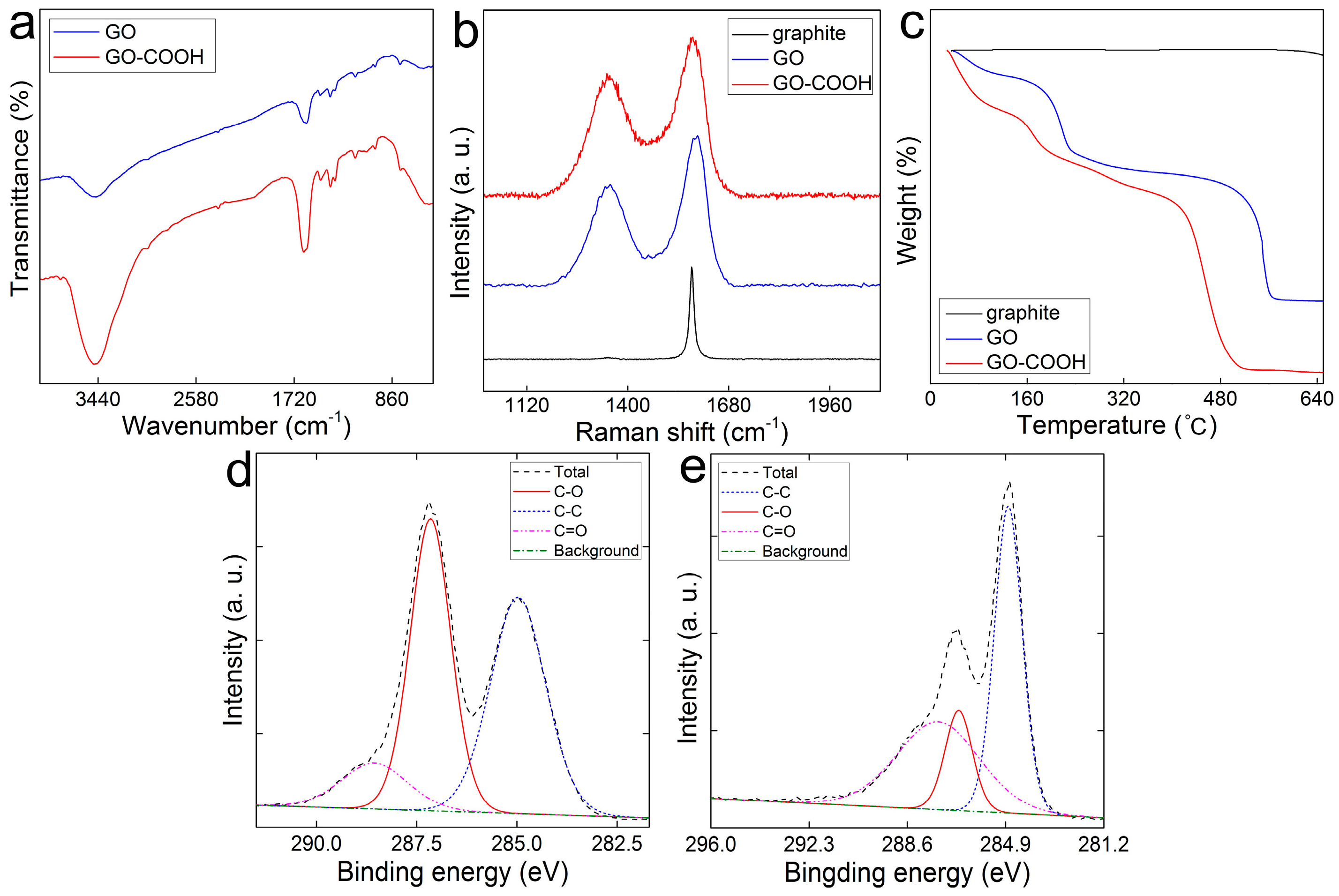
3.1. Characterization of GO-COOH Sponge
3.2. Adsorption of MB on GO-COOH Sponge
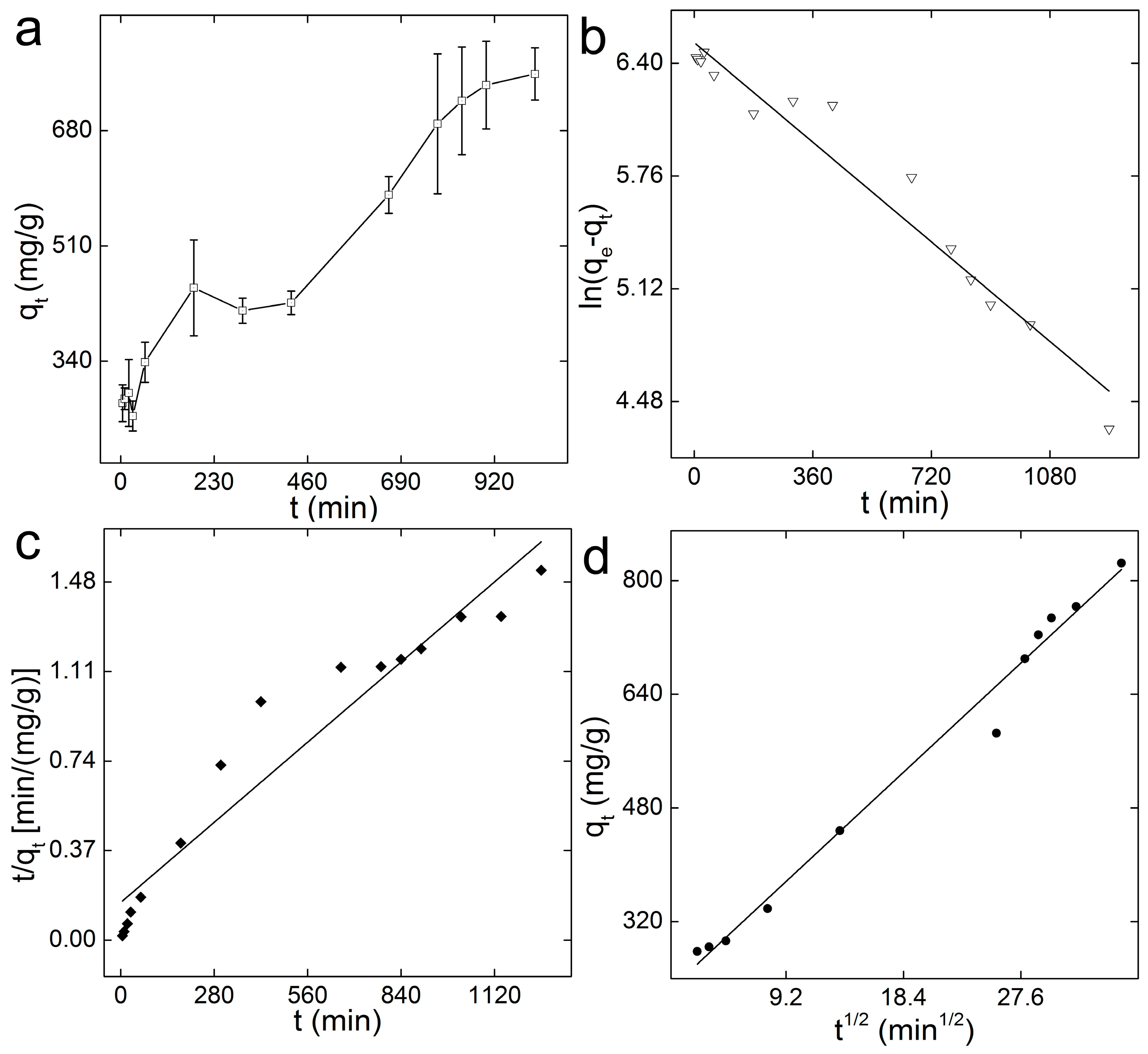
3.3. Adsorption Kinetics and Thermodynamics
3.4. Influence of pH and Ionic Strength
3.5. Recycling of GO-COOH
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Croce, R.; Cina, F.; Lombardo, A.; Crispeyn, G.; Cappelli, C.I.; Vian, M.; Maiorana, S.; Benfenati, E.; Baderna, D. Aquatic toxicity of several textile dye formulations: Acute and chronic assays with daphnia magna and raphidocelis subcapitata. Ecotox. Environ. Saf. 2017, 144, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Gürses, A.; Açıkyıldız, M.; Güneş, K.; Gürses, M.S. Colorants in health and environmental aspects. Dyes Pigments 2016, 69–83. [Google Scholar] [CrossRef]
- Vacchi, F.I.; Von der Ohe, P.C.; de Albuquerque, A.F.; Vendemiatti, J.A.D.; Azevedo, C.C.J.; Honorio, J.G.; da Silva, B.F.; Zanoni, M.V.B.; Henry, T.B.; Nogueira, A.J. Occurrence and risk assessment of an azo dye—The case of Disperse Red 1. Chemosphere 2016, 156, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Rocha, O.P.; Cesila, C.A.; Christovam, E.M.; Barros, S.B.D.M.; Zanoni, M.V.B.; de Oliveira, D.P. Ecotoxicological risk assessment of the “Acid Black 210” dye. Toxicology 2017, 376, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Tai, S.Y.; Hsieh, H.M.; Huang, S.P.; Wu, M.T. Hair dye use, regular exercise, and the risk and prognosis of prostate cancer: Multicenter case-control and case-only studies. BMC Cancer 2016, 16, 242. [Google Scholar] [CrossRef] [PubMed]
- Naushad, M.; Ahamad, T.; Al-Maswari, B.M.; Alqadami, A.A.; Alshehri, S.M. Nickel ferrite bearing nitrogen-doped mesoporous carbon as efficient adsorbent for the removal of highly toxic metal ion from aqueous medium. Chem. Eng. J. 2017, 330, 1351–1360. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Charara, M.; Abu Tarboush, B.J.; Ahmad, M.N.M.; Kurniawan, T.A.; Naushad, M.; Walker, G.M.; Mangwandi, C. Mechanism analysis of tartrazine biosorption onto masau stones; a low cost by-product from semi-arid regions. J. Mol. Liq. 2017, 242, 478–483. [Google Scholar] [CrossRef]
- Daneshvar, E.; Vazirzadeh, A.; Niazi, A.; Kousha, M.; Naushad, M.; Bhatnagar, A. Desorption of Methylene blue dye from brown macroalga: Effects of operating parameters, isotherm study and kinetic modeling. J. Clean. Prod. 2017, 152, 443–453. [Google Scholar] [CrossRef]
- Unuabonah, E.I.; Adebowale, K.O.; Dawodu, F.A. Equilibrium, kinetic and sorber design studies on the adsorption of aniline blue dye by sodium tetraborate-modified kaolinite clay adsorbent. J. Hazard. Mater. 2008, 157, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wei, S.Y.; Kaila, C.; Su, X.; Wu, J.; Karki, A.B.; Young, D.P.; Guo, Z.H. Carbon-stabilized iron nanoparticles for environmental remediation. Nanoscale 2010, 2, 917–919. [Google Scholar] [CrossRef] [PubMed]
- Mu’azu, N.D.; Jarrah, N.; Zubair, M.; Alagha, O. Removal of Phenolic Compounds from Water Using Sewage Sludge-Based Activated Carbon Adsorption: A Review. Int. J. Environ. Res. Public Health 2017, 14, 1094. [Google Scholar] [CrossRef] [PubMed]
- Moyo, F.; Tandlich, R.; Wilhelmi, B.S.; Balaz, S. Sorption of hydrophobic organic compounds on natural sorbents and organoclays from aqueous and non-aqueous solutions: A mini-review. Int. J. Environ. Res. Public Health 2014, 11, 5020–5048. [Google Scholar] [CrossRef] [PubMed]
- Albadarin, A.B.; Collins, M.N.; Naushad, M.; Shirazian, S.; Walker, G.; Mangwandi, C. Activated lignin-chitosan extruded blends for efficient adsorption of methylene blue. Chem. Eng. J. 2017, 307, 264–272. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Naushad, M.; Abdalla, M.A.; Khan, M.R.; Alothman, Z.A. Adsorptive Removal of Toxic Dye Using Fe3O4-TSC Nanocomposite: Equilibrium, Kinetic, and Thermodynamic Studies. J. Chem. Eng. Data 2016, 61, 3806–3813. [Google Scholar] [CrossRef]
- Pathania, D.; Katwal, R.; Sharma, G.; Naushad, M.; Khan, M.R.; Al-Muhtaseb, A.H. Novel guar gum/Al2O3 nanocomposite as an effective photocatalyst for the degradation of malachite green dye. Int. J. Biol. Macromol. 2016, 87, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Naushad, M.; Kumar, A.; Rana, S.; Sharma, S.; Bhatnagar, A.; Stadler, F.J.; Ghfar, A.A.; Khan, M.R. Efficient removal of coomassie brilliant blue R-250 dye using starch/poly (alginic acid-Cl-acrylamide) nanohydrogel. Process Saf. Environ. 2017, 109, 301–310. [Google Scholar] [CrossRef]
- Yang, S.-T.; Chang, Y.L.; Wang, H.F.; Liu, G.B.; Chen, S.; Wang, Y.W.; Liu, Y.F.; Cao, A.N. Folding/aggregation of graphene oxide and its application in Cu2+ removal. J. Colloid Interf. Sci. 2010, 351, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-T.; Chen, S.; Chang, Y.L.; Cao, A.N.; Liu, Y.F.; Wang, H.F. Removal of methylene blue from aqueous solution by graphene oxide. J. Colloid Interf. Sci. 2011, 359, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.F.; Xie, Y.L.; Pang, L.C.; Li, M.; Song, X.H.; Wen, J.G.; Zhao, H.Y. Preparation of reduced graphene oxide/poly (acrylamide) nanocomposite and its adsorption of Pb(II) and methylene blue. Langmuir 2013, 29, 10727–10736. [Google Scholar] [CrossRef] [PubMed]
- Bele, S.; Samanidou, V.; Deliyanni, E. Effect of the reduction degree of graphene oxide on the adsorption of bisphenol A. Chem. Eng. Res. Des. 2016, 109, 573–585. [Google Scholar] [CrossRef]
- Yan, H.; Tao, X.; Yang, Z.; Li, K.; Yang, H.; Li, A.M.; Cheng, R.S. Effects of the oxidation degree of graphene oxide on the adsorption of methylene blue. J. Hazard. Mater. 2014, 268, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Mu, Y.Y.; Wang, P.; Liu, X.T. Effect of oxidation degree on the synthesis and adsorption property of magnetite/graphene nanocomposites. Appl. Surf. Sci. 2015, 359, 188–195. [Google Scholar] [CrossRef]
- Zhao, L.Q.; Yu, B.W.; Xue, F.M.; Xie, J.R.; Zhang, X.L.; Wu, R.H.; Wang, R.J.; Hu, Z.Y.; Yang, S.-T.; Luo, J.B. Facile hydrothermal preparation of recyclable S-doped graphene sponge for Cu2+ adsorption. J. Hazard. Mater. 2015, 286, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.Z.; Shang, K.; Sun, B.; Chen, L.J.; Ai, S.Y. Decoration of surface-carboxylated graphene oxide with luminescent Sm3+-complexes. J. Mater. Sci. 2014, 49, 2672–2679. [Google Scholar] [CrossRef]
- Park, K.W. Carboxylated graphene oxide–Mn2O3 nanorod composites for their electrochemical characteristics. J. Mater. Chem. A 2014, 2, 4292–4298. [Google Scholar] [CrossRef]
- Lv, S.S.; Wei, J.; Jiang, F.; Wang, S. Adsorption-decolorization of four ionic dyes by carboxylated graphene. Chin. J. Chem. 2013, 30, 1215–1221. [Google Scholar] [CrossRef]
- Sun, Y.B.; Yang, S.B.; Chen, Y.; Ding, C.C.; Cheng, W.C.; Wang, X.K. Adsorption and desorption of U (VI) on functionalized graphene oxides: A combined experimental and theoretical study. Environ. Sci. Technol. 2015, 49, 4255–4262. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Wang, Z.W.; Gao, Y.T.; Wang, Z.F.; Yan, L.L.; Zhang, H.J.; Zhao, J. Electrochemical research of cadmium on carboxylated graphene electrode. Chem. Res. Appl. 2013, 25, 1078–1084. [Google Scholar]
- Zhao, L.Q.; Dong, P.J.; Xie, J.R.; Li, J.Y.; Wu, L.X.; Yang, S.-T.; Luo, J.B. Porous graphene oxide-chitosan aerogel for tetracycline removal. Mater. Res. Express 2014, 1, 015601. [Google Scholar] [CrossRef]
- McAllister, M.J.; Li, J.L.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Herrera-Alonso, M.; Milius, D.L.; Car, R.; Prud’homme, R.K. Single sheet functionalized graphene by oxidation and thermal expansion of graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Valerga Jimenez, P.S. Thermal decomposition of graphite oxidation products DSC studies of internal combustion of graphite oxide. Mater. Res. Bull. 1987, 22, 601–608. [Google Scholar] [CrossRef]
- Liu, P.G.; Gong, K.C.; Xiao, P.; Xiao, M. Preparation and characterization of poly (vinyl acetate)-intercalated graphite oxide nanocomposite. J. Mater. Chem. 2000, 10, 933–935. [Google Scholar] [CrossRef]
- Mallakpour, S.; Abdolmaleki, A.; Borandeh, S. Covalently functionalized graphene sheets with biocompatible natural amino acids. Appl. Surf. Sci. 2014, 307, 533–542. [Google Scholar] [CrossRef]
- Shen, J.F.; Li, T.; Shi, M.; Li, N.; Ye, M.X. Polyelectrolyte-assisted one-step hydrothermal synthesis of Ag-reduced graphene oxide composite and its antibacterial properties. Mater. Sci. Eng. C 2012, 32, 2042–2047. [Google Scholar] [CrossRef]
- Pei, F.Y.; Liu, Y.L.; Zhang, L.; Wang, S.P.; Xu, S.G.; Cao, S.K. TiO2 nanocomposite with reduced graphene oxide through facile blending and its photocatalytic behaviour for hydrogen evolution. Mater. Res. Bull. 2013, 48, 2824–2831. [Google Scholar] [CrossRef]
- Yao, Y.J.; Miao, S.D.; Yu, S.M.; Ma, L.P.; Sun, H.Q.; Wang, S.B. Fabrication of Fe3O4/SiO2 core/shell nanoparticles attached to graphene oxide and its use as an adsorbent. J. Colloid Interf. Sci. 2012, 379, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.W.; Chen, L.Y.; Wu, R.H.; Liu, X.Y.; Li, H.L.; Yang, H.; Ming, Z.; Bai, Y.T.; Yang, S.-T. Effect of reduction degree on the adsorption properties of graphene sponge for dyes. Mater. Res. Express 2017, 4, 045008. [Google Scholar] [CrossRef]
- Yang, S.-T.; Luo, J.B.; Liu, J.H.; Zhou, Q.H.; Wan, J.; Ma, C.; Liao, R.; Wang, H.F.; Liu, Y.F. Graphene oxide/chitosan composite for methylene blue adsorption. Nanosci. Nanotechnol. Lett. 2013, 5, 372–376. [Google Scholar] [CrossRef]
- Yu, B.W.; Zhang, X.L.; Xie, J.R.; Wu, R.H.; Liu, X.Y.; Li, H.L.; Chen, F.; Yang, H.; Ming, Z.; Yang, S.-T. Magnetic graphene sponge for the removal of methylene blue. Appl. Surf. Sci. 2015, 351, 765–771. [Google Scholar] [CrossRef]
- Bai, S.; Shen, X.P.; Zhong, X.; Liu, Y.; Zhu, G.X.; Xu, X.; Xu, X. One-pot solvothermal preparation of magnetic reduced graphene oxide-ferrite hybrids for organic dye removal. Carbon 2012, 50, 2337–2346. [Google Scholar] [CrossRef]
- Wu, Z.B.; Zhong, H.; Yuan, X.Z.; Wang, H.; Wang, L.L.; Chen, X.H.; Zeng, G.M.; Wu, Y. Adsorptive removal of methylene blue by rhamnolipid-functionalized graphene oxide from wastewater. Water Res. 2014, 67, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.L.; Luo, C.N.; Li, X.J.; Lu, F.G.; Qiu, H.M.; Sun, M. Fabrication of novel magnetic chitosan grafted with graphene oxide to enhance adsorption properties for methyl blue. J. Hazard. Mater. 2012, 215, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Deng, J.; Lei, B.; He, A.; Zhang, X.; Ma, L.; Li, S.; Zhao, C.S. Toward 3D graphene oxide gels based adsorbents for high-efficient water treatment via the promotion of biopolymers. J. Hazard. Mater. 2013, 263, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lv, H.D.; Yang, S.-T.; Luo, J.B. Preparation of graphene adsorbents and their applications in water purification. Rev. Inorg. Chem. 2013, 33, 139–160. [Google Scholar] [CrossRef]
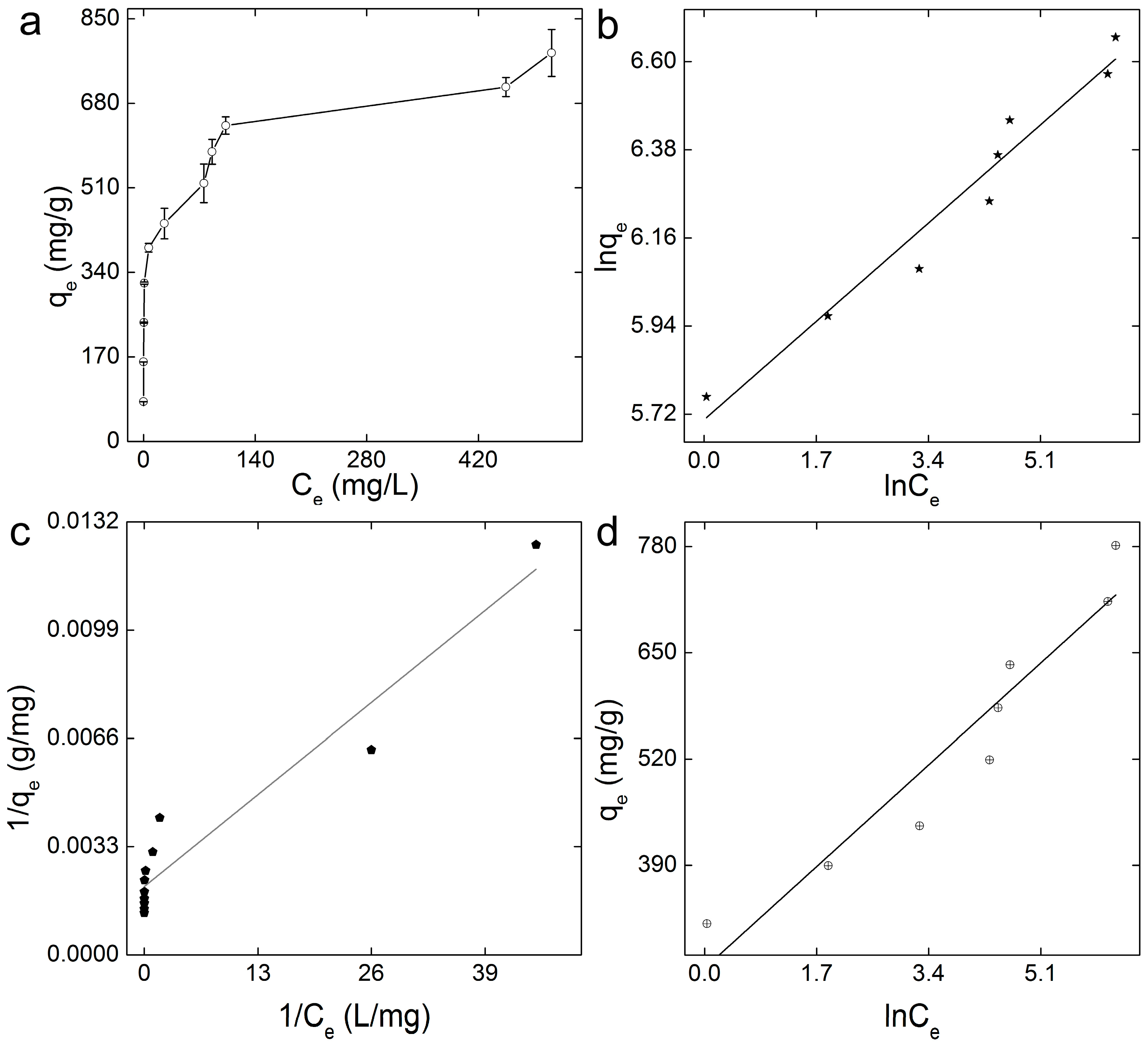

| Adsorption Model | Linear Fitting Equation | R |
|---|---|---|
| Langmuir | y = 0000216x + 0.00208 | 0.962 |
| Freundlich | y = 0.144x + 5.706 | 0.979 |
| Temkin | y = 73.26x + 263.75 | 0.956 |
| Adsorbent | qm (mg/g) | Ref. |
|---|---|---|
| GO sponge | 446 | [38] |
| GO-CS sponge | 468 | [39] |
| GO-Fe3O4 sponge | 526 | [40] |
| RGO sponge | 27 | [38] |
| RGO-MFe2O4 | 34.72 | [41] |
| RL-GO | 529.10 | [42] |
| MCGO | 95.16 | [43] |
| GO-biopolymer gel | 701.78 | [44] |
| GO-COOH sponge | 780 ± 47 | This study |
| T (K) | ΔG (kJ/mol) | ΔH (kJ/mol) | ΔS (J/(mol·K)) |
|---|---|---|---|
| 303 | −1.66 | 25.85 | 90.81 |
| 313 | −2.57 | ||
| 323 | −3.48 | ||
| 333 | −4.39 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Yang, S.-T.; Feng, S.; Ma, Q.; Peng, X.; Wu, D. Preparation and Application of Carboxylated Graphene Oxide Sponge in Dye Removal. Int. J. Environ. Res. Public Health 2017, 14, 1301. https://doi.org/10.3390/ijerph14111301
Zhao L, Yang S-T, Feng S, Ma Q, Peng X, Wu D. Preparation and Application of Carboxylated Graphene Oxide Sponge in Dye Removal. International Journal of Environmental Research and Public Health. 2017; 14(11):1301. https://doi.org/10.3390/ijerph14111301
Chicago/Turabian StyleZhao, Lianqin, Sheng-Tao Yang, Shicheng Feng, Qiang Ma, Xiaoling Peng, and Deyi Wu. 2017. "Preparation and Application of Carboxylated Graphene Oxide Sponge in Dye Removal" International Journal of Environmental Research and Public Health 14, no. 11: 1301. https://doi.org/10.3390/ijerph14111301





