Lung Toxicity of Condensed Aerosol from E-CIG Liquids: Influence of the Flavor and the In Vitro Model Used
Abstract
:1. Introduction
2. Material and Methods
2.1. Source of Refill Fluids
2.2. E-CIG Condensed Aerosol (CAs)
2.3. Lung Cell Monocultures
2.4. In Vitro Model of the Alveolar-Blood Barrier (ABB): 3D Co-Culture of Epithelial and Endothelial Cells
2.5. Cell Viability
2.6. Transepithelial Electrical Resistance (TEER) Measurements
2.7. Cytokines Release: IL-8 and MCP-1
2.8. Statistical Analysis
3. Results and Discussion
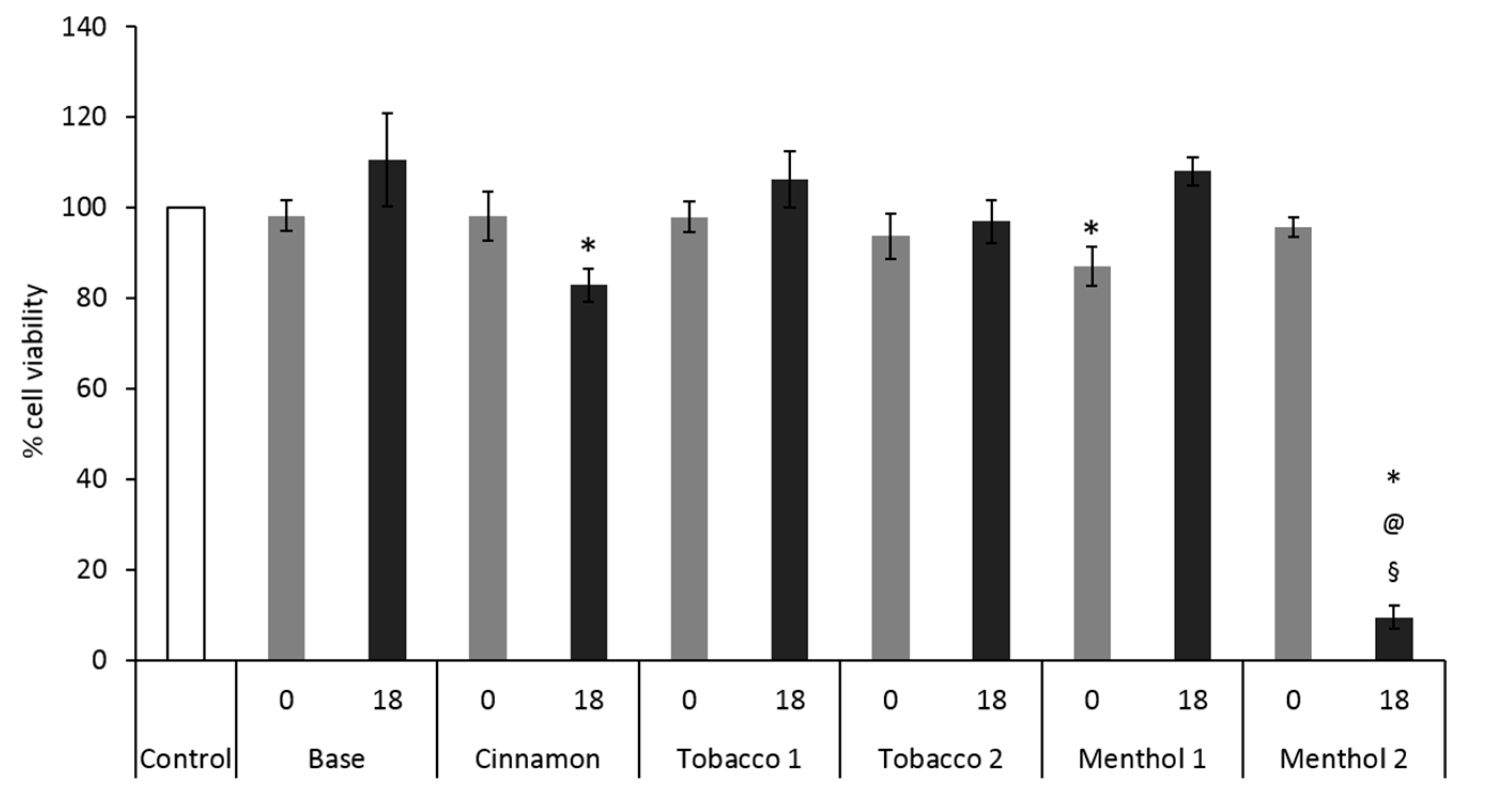
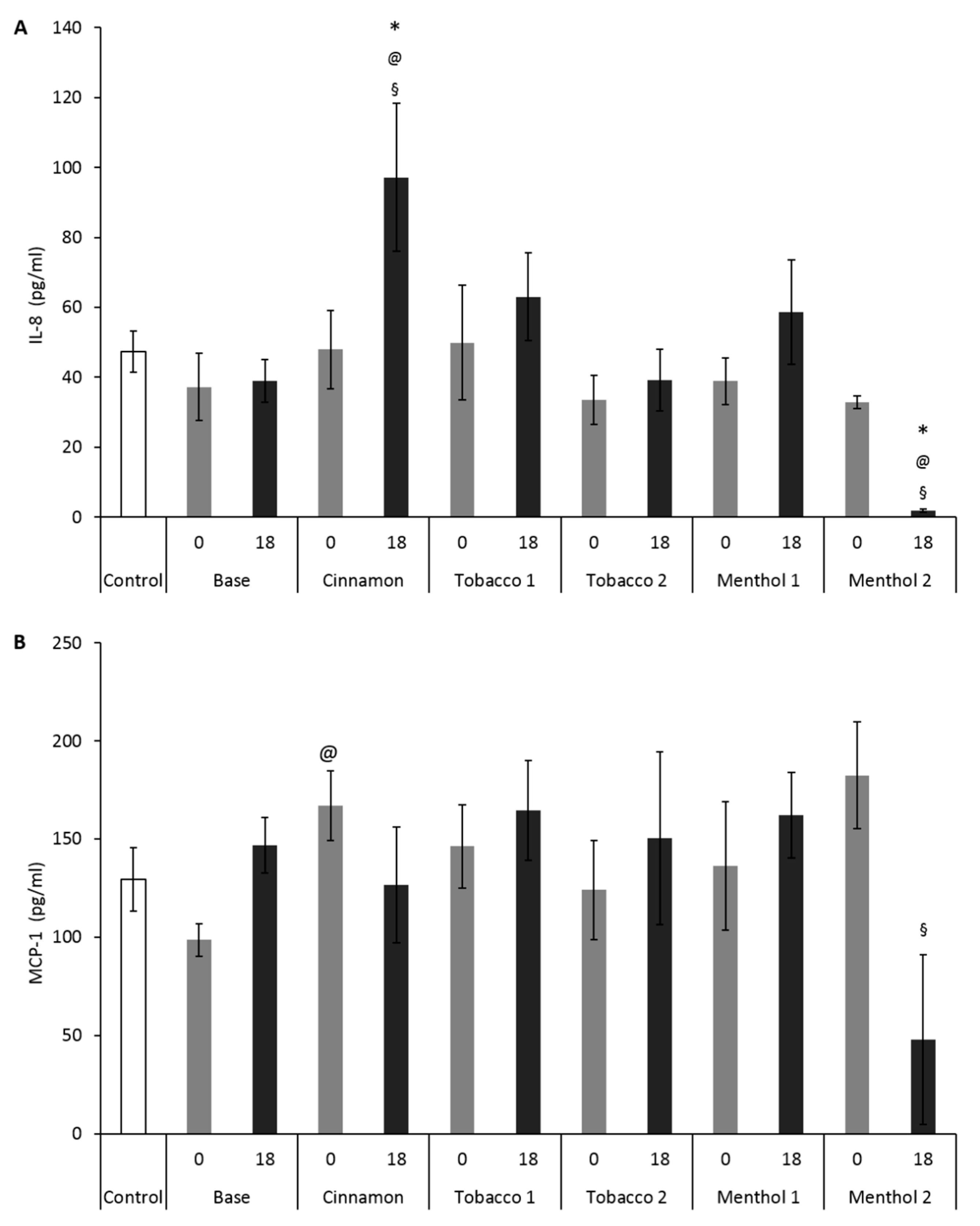
3.1. Comparative Effects of CAs on Cells Monoculture
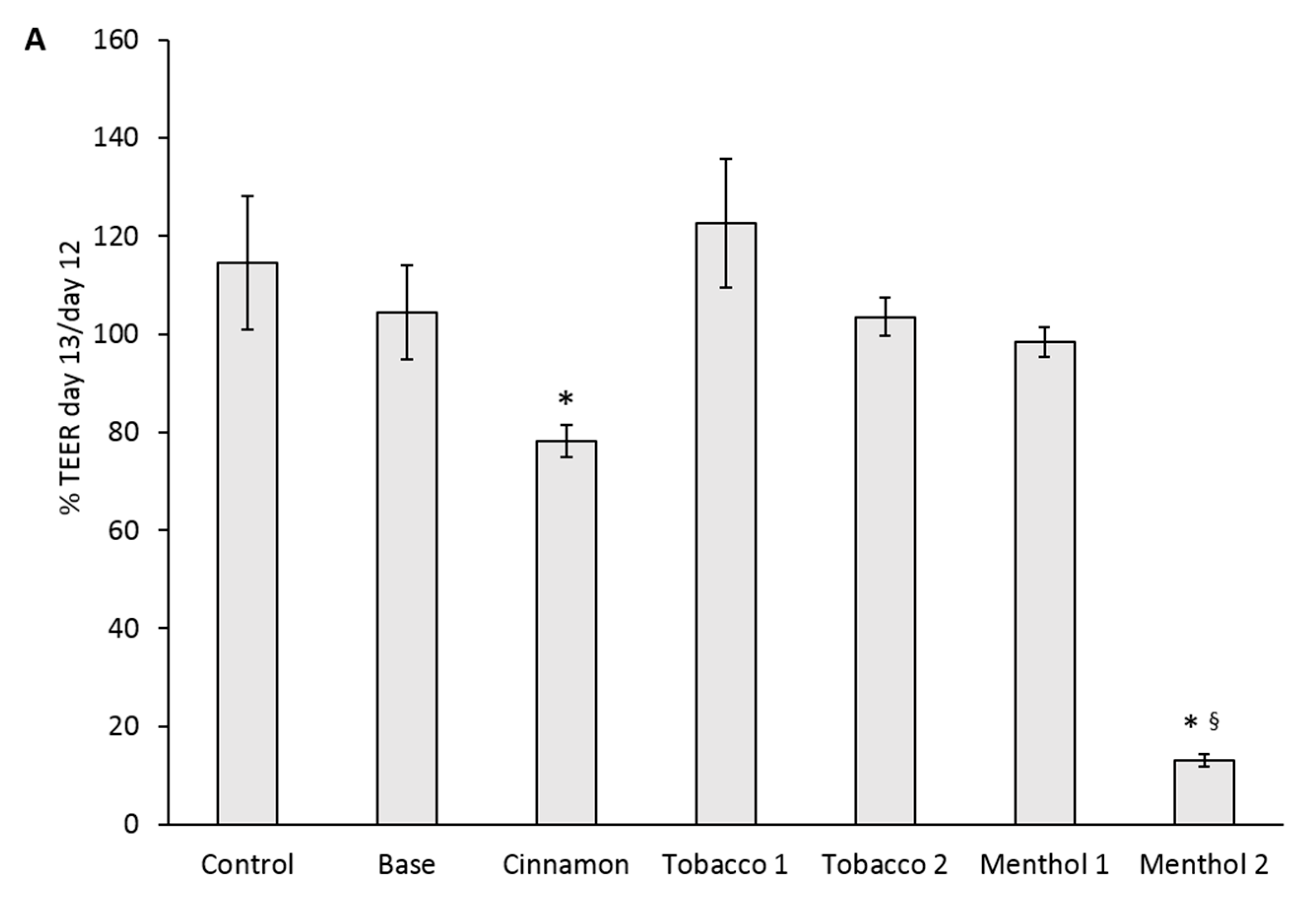
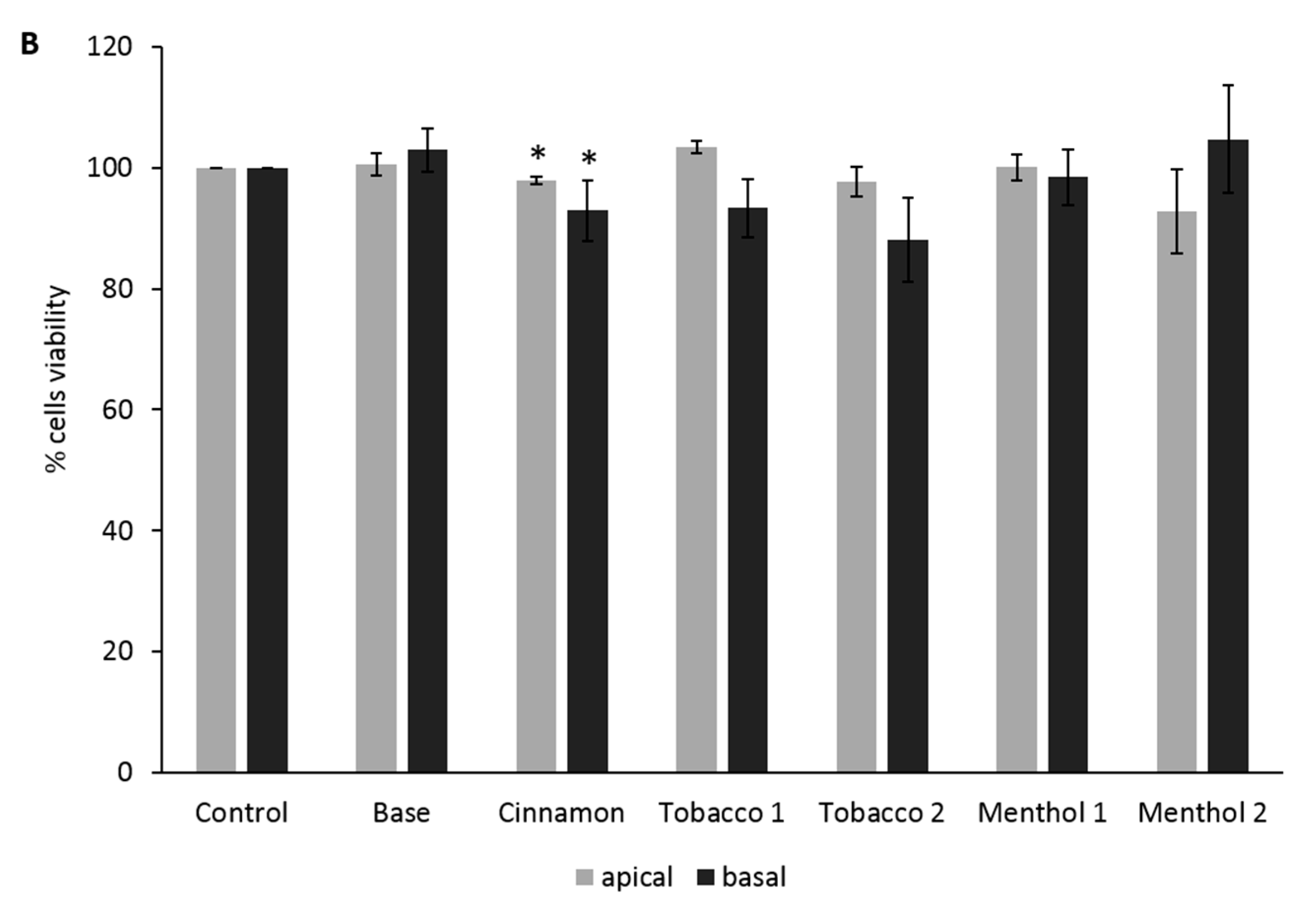
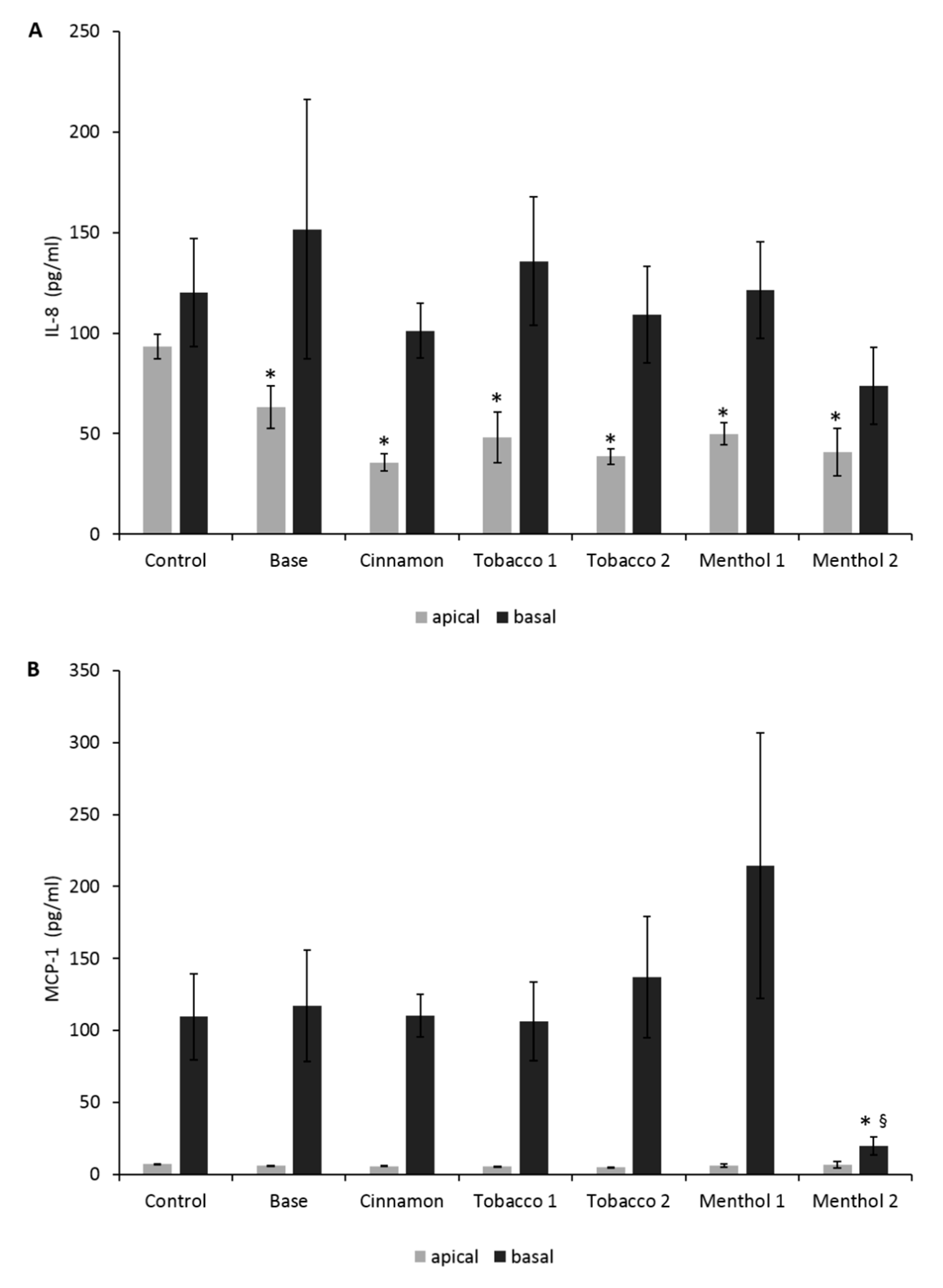
3.2. Comparative Effects of CAs on the ABB Model
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bahl, V.; Lin, S.; Xu, N.; Davis, B.; Wang, Y.H.; Talbot, P. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod. Toxicol. 2012, 34, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, F.; Muresan, X.M.; Sticozzi, C.; Gambari, R.; Montagner, G.; Forman, H.J.; Torricelli, C.; Maioli, E.; Valacchi, G. Comparative effects between electronic and cigarette smoke in human keratinocytes and epithelial lung cells. Toxicol. Vitr. 2014, 28, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Caponnetto, P.; Saitta, D.; Sweanor, D.; Polosa, R. What to consider when regulating electronic cigarettes: Pros, cons and unintended consequences. Int. J. Drug Policy 2015, 26, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Palazzolo, D.L.; Crow, A.P.; Nelson, J.M.; Johnson, R.A. Trace metals derived from electronic cigarette (ECIG) generated aerosol: Potential problem of ECIG devices that contain nickel. Front. Physiol. 2017, 7, 663. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Villarreal, A.; Bozhilov, K.; Lin, S.; Talbot, P. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS ONE 2013, 8, e57987. [Google Scholar] [CrossRef] [PubMed]
- Gillman, I.G.; Kistler, K.A.; Stewart, E.W.; Paolantonio, A.R. Effect of variable power levels on the yield of total aerosol mass and formation of aldehydes in e-cigarette aerosols. Regul. Toxicol. Pharmacol. 2016, 75, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Geiss, O.; Bianchi, I.; Barrero-Moreno, J. Correlation of volatile carbonyl yields emitted by e-cigarettes with the temperature of the heating coil and the perceived sensorial quality of the generated vapours. Int. J. Hyg. Environ. Health 2016, 219, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Behar, R.Z.; Davis, B.; Wang, Y.; Bahl, V.; Lin, S.; Talbot, P. Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicol. Vitr. 2014, 28, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Leigh, N.J.; Lawton, R.I.; Hershberger, P.A.; Goniewicz, M.L. Flavourings significantly affect inhalation toxicity of aerosol generated from electronic nicotine delivery systems (ENDS). Tob. Control 2016, 25, ii81–ii87. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, M.; Logue, J.M.; Montesinos, V.N.; Russell, M.L.; Litter, M.I.; Gundel, L.A.; Destaillats, H. Emissions from electronic cigarettes: Key parameters affecting the release of harmful chemicals. Environ. Sci. Technol. 2016, 50, 9644–9951. [Google Scholar] [CrossRef] [PubMed]
- Goniewicz, M.L.; Gupta, R.; Lee, Y.H.; Reinhardt, S.; Kim, S.; Kim, B.; Kosmider, L.; Sobczak, A. Nicotine levels in electronic cigarette refill solutions: A comparative analysis of products from the US, Korea, and Poland. Int. J. Drug Policy 2015, 26, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Morean, M.E.; Kong, G.; Cavallo, D.A.; Camenga, D.R.; Krishnan-Sarin, S. Nicotine concentration of e-cigarettes used by adolescents. Drug Alcohol Depend. 2016, 167, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Kress, M. RAI Gives Sneak Peek of VUSE’s Next Generation. Available online: https://csnews.com/rai-gives-sneak-peek-vuses-next-generation (accessed on 9 October 2017).
- Farsalinos, K.; Romagna, G.; Allifranchini, E.; Ripamonti, E.; Bocchietto, E.; Todeschi, S.; Tsiapras, D.; Kyrzopoulos, S.; Voudris, V. Comparison of the cytotoxic potential of cigarette smoke and electronic cigarette vapour extract on cultured myocardial cells. Int. J. Environ. Res. Public Health 2013, 10, 5146–5162. [Google Scholar] [CrossRef] [PubMed]
- Neilson, L.; Mankus, C.; Thorne, D.; Jackson, G.; DeBay, J.; Meredith, C. Development of an in vitro cytotoxicity model for aerosol exposure using 3D reconstructed human airway tissue; application for assessment of e-cigarette aerosol. Toxicol. In Vitro 2015, 29, 1952–1962. [Google Scholar] [CrossRef] [PubMed]
- Putzhammer, R.; Doppler, C.; Jakschitz, T.; Heinz, K.; Förste, J.; Danzl, K.; Messner, B.; Bernhard, D. Vapours of US and EU market leader electronic cigarette brands and liquids are cytotoxic for human vascular endothelial cells. PLoS ONE 2016, 11, e0157337. [Google Scholar] [CrossRef] [PubMed]
- Scheffler, S.; Dieken, H.; Krischenowski, O.; Förster, C.; Branscheid, D.; Aufderheide, M. Evaluation of E-cigarette liquid vapor and mainstream cigarette smoke after direct exposure of primary human bronchial epithelial cells. Int. J. Environ. Res. Public Health 2015, 12, 3915–3925. [Google Scholar] [CrossRef] [PubMed]
- Polosa, R.; Caruso, M.; Guarino, F. Comments on scheffler et al. Cytotoxic evaluation of E-liquid aerosol using different lung derived cell models. Int. J. Environ. Res. Public Health, 2015, 12, 12466–12474. Int. J. Environ. Res. Public Health 2016, 13, 108. [Google Scholar] [CrossRef]
- Bakand, S. Cell culture techniques essential for toxicity testing of inhaled materials and nanomaterials in vitro. J. Tissue Sci. Eng. 2016, 7, 1–5. [Google Scholar] [CrossRef]
- Klein, S.G.; Serchi, T.; Hoffmann, L.; Blömeke, B.; Gutleb, A.C. An improved 3D tetraculture system mimicking the cellular organisation at the alveolar barrier to study the potential toxic effects of particles on the lung. Part. Fibre Toxicol. 2013, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Mills, N.L.; Donaldson, K.; Hadoke, P.W.; Boon, N.A.; MacNee, W.; Cassee, F.R.; Sandström, T.; Blomberg, A.; Newby, D.E. Adverse cardiovascular effects of air pollution. Nat. Clin. Pract. Cardiovasc. Med. 2009, 6, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Longhin, E.; Gualtieri, M.; Capasso, L.; Bengalli, R.; Mollerup, S.; Holme, J.A.; Øvrevik, J.; Casadei, S.; Di Benedetto, C.; Parenti, P.; et al. Physico-chemical properties and biological effects of diesel and biomass particles. Environ. Pollut. 2016, 215, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.G.; Cambier, S.; Hennen, J.; Legay, S.; Serchi, T.; Nelissen, I.; Chary, A.; Moschini, E.; Krein, A.; Blömeke, B.; et al. Endothelial responses of the alveolar barrier in vitro in a dose-controlled exposure to diesel exhaust particulate matter. Part. Fibre Toxicol. 2017, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Fuoco, F.C.; Buonanno, G.; Stabile, L.; Vigo, P. Influential parameters on particle concentration and size distribution in the mainstream of e-cigarettes. Environ. Pollut. 2014, 184, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Manigrasso, M.; Buonanno, G.; Stabile, L.; Morawska, L.; Avino, P. Particle doses in the pulmonary lobes of electronic and conventional cigarette users. Environ. Pollut. 2015, 202, 24–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawkins, L.; Turner, J.; Roberts, A.; Soar, K. “Vaping” profiles and preferences: An online survey of electronic cigarette users. Addiction 2013, 108, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Bengalli, R.; Mantecca, P.; Camatini, M.; Gualtieri, M. Effect of nanoparticles and environmental particles on a cocultures model of the air-blood barrier. Biomed. Res. Int. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, L.T.; Mehta, N.Y.; Sweeney, C.T.; Schwartz, S.S.; Vogler, G.P.; Jarvis, M.J.; West, R.J. Filter ventilation and nicotine content of tobacco in cigarettes from Canada, the United Kingdom, and the United States. Tob. Control 1998, 7, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Rubin, H. Synergistic mechanisms in carcinogenesis by polycyclic aromatic hydrocarbons and by tobacco smoke: A bio-historical perspective with updates. Carcinogenesis 2001, 22, 1903–1930. [Google Scholar] [CrossRef] [PubMed]
- Higham, A.; Rattray, N.J.W.; Dewhurst, J.A.; Trivedi, D.K.; Fowler, S.J.; Goodacre, R.; Singh, D. Electronic cigarette exposure triggers neutrophil inflammatory responses. Respir. Res. 2016, 17, 56. [Google Scholar] [CrossRef] [PubMed]
- Gerszten, R.E.; Garcia-Zepeda, E.A.; Lim, Y.-C.; Yoshida, M.; Ding, H.A.; Gimbrone, M.A., Jr.; Luster, A.D.; Luscinskas, F.W.; Rosenzweig, A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature 1999, 398, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Balharry, D.; Sexton, K.; BéruBé, K.A. An in vitro approach to assess the toxicity of inhaled tobacco smoke components: Nicotine, cadmium, formaldehyde and urethane. Toxicology 2008, 244, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Shields, P.G.; Berman, M.; Brasky, T.M.; Freudenheim, J.L.; Mathe, E.; McElroy, J.P.; Song, M.-A.; Wewers, M.D. A review of pulmonary toxicity of electronic cigarettes in the context of smoking: A focus on inflammation. Cancer Epidemiol. Biomarkers Prev. 2017, 26, 1175–1191. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, A.; Nastrucci, C.; Cesario, A.; Russo, P. Nicotine: Specific role in angiogenesis, proliferation and apoptosis. Crit. Rev. Toxicol. 2012, 42, 68–89. [Google Scholar] [CrossRef] [PubMed]
- Hiemstra, P.S.; Bals, R. Basic science of electronic cigarettes: Assessment in cell culture and in vivo models. Respir. Res. 2016, 17, 127. [Google Scholar] [CrossRef] [PubMed]

| Identifier | Nicotine (mg/mL) | E-Liquid Composition (PG:VG:Water:Blend) | Blend Composition |
|---|---|---|---|
| Base | 0 | 50:40:10:0 | N.A. |
| 18 | |||
| Cinnamon | 0 | 50:40:7:3 | Propylene glycol (57-55-6) 80% Water (7732-18-5) 18% Cinnamaldehyde (104-55-2) 1.5% Cinnamon oil (84961-46-6) 0.5% |
| 18 | |||
| Tobacco 1 | 0 | 50:40:7:3 | Propylene glycol (57-55-6) 90% Water (7732-18-5) < 5% Dimethylhydroxy Furanone (3658-77-3) < 3% Triacetin (102-76-1) < 3% Cocoa Extract (84649-99-0) < 1% Hepta-2,4-dienal trans trans (4313-03-5) < 1% Racementhol (89-78-1) < 1% others < 0.1% |
| 18 | |||
| Tobacco 2 | 0 | 50:40:7:3 | Propylene glycol (57-55-6) 90% Water (7732-18-5) < 5% Triacetin (102-76-1) < 3% Methylcycopentenolone (80-71-7) <1% Vanillin (121-33-5) < 1% Ethyl Lactate (97-64-3) < 1% Ethyl Vanillin (121-32-4) < 1% others < 0.1% |
| 18 | |||
| Menthol 1 | 0 | 50:40:3:7 | Propylene glycol (57-55-6) 90% Menthol (89-78-1) 10% |
| 18 | |||
| Menthol 2 | 0 | 50:40:4:6 | Propylene glycol (57-55-6) 90% Menthol (89-78-1) 5% Carvone (99-49-0) 5% |
| 18 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bengalli, R.; Ferri, E.; Labra, M.; Mantecca, P. Lung Toxicity of Condensed Aerosol from E-CIG Liquids: Influence of the Flavor and the In Vitro Model Used. Int. J. Environ. Res. Public Health 2017, 14, 1254. https://doi.org/10.3390/ijerph14101254
Bengalli R, Ferri E, Labra M, Mantecca P. Lung Toxicity of Condensed Aerosol from E-CIG Liquids: Influence of the Flavor and the In Vitro Model Used. International Journal of Environmental Research and Public Health. 2017; 14(10):1254. https://doi.org/10.3390/ijerph14101254
Chicago/Turabian StyleBengalli, Rossella, Emanuele Ferri, Massimo Labra, and Paride Mantecca. 2017. "Lung Toxicity of Condensed Aerosol from E-CIG Liquids: Influence of the Flavor and the In Vitro Model Used" International Journal of Environmental Research and Public Health 14, no. 10: 1254. https://doi.org/10.3390/ijerph14101254






