Variations of Escherichia coli O157:H7 Survival in Purple Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Collection and Characterization
2.2. Bacterial Strains
2.3. Survivals of Escherichia coli O157:H7 in Soils
2.4. Survival Data Modeling
2.5. Statistical Analysis
3. Results and Discussion
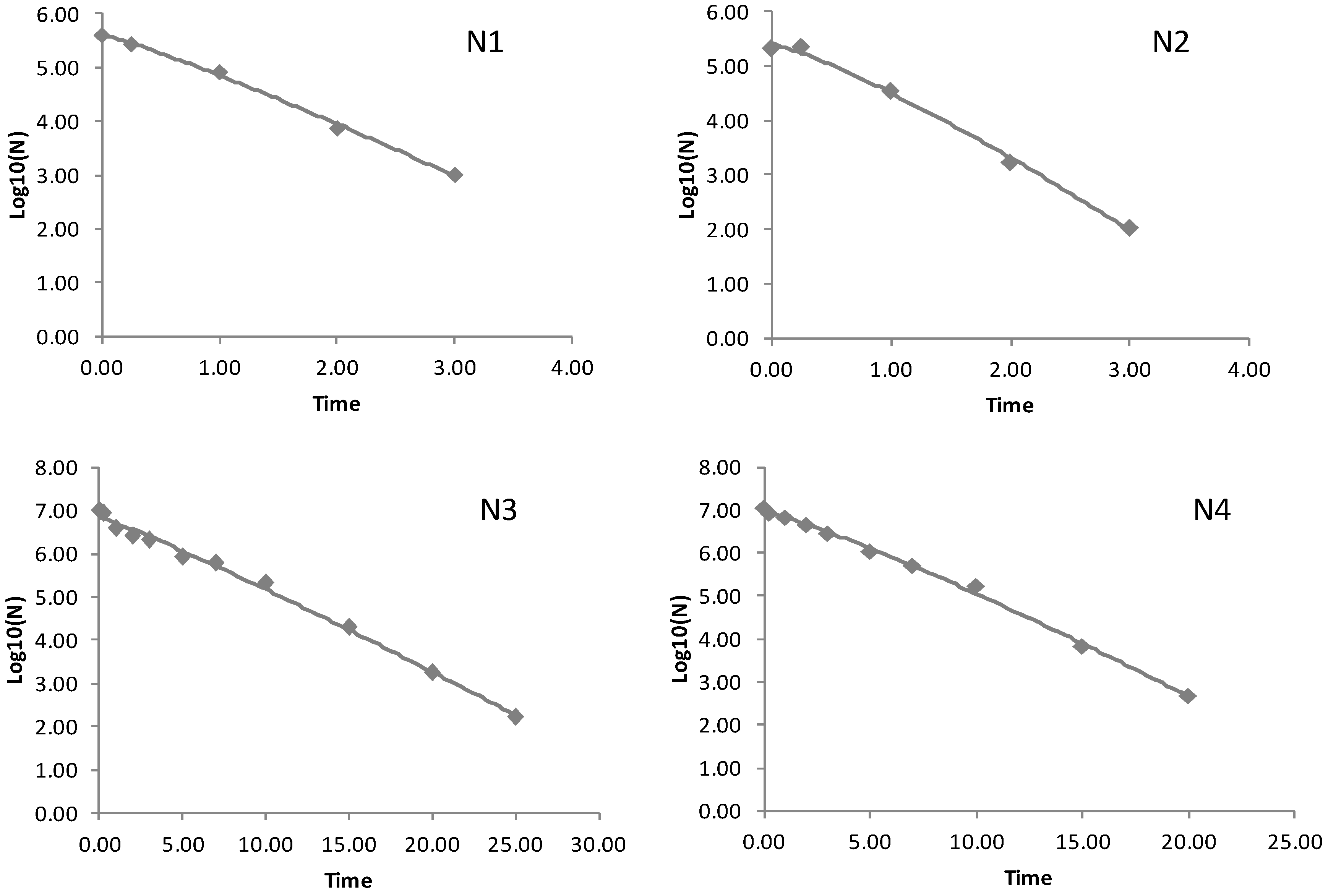
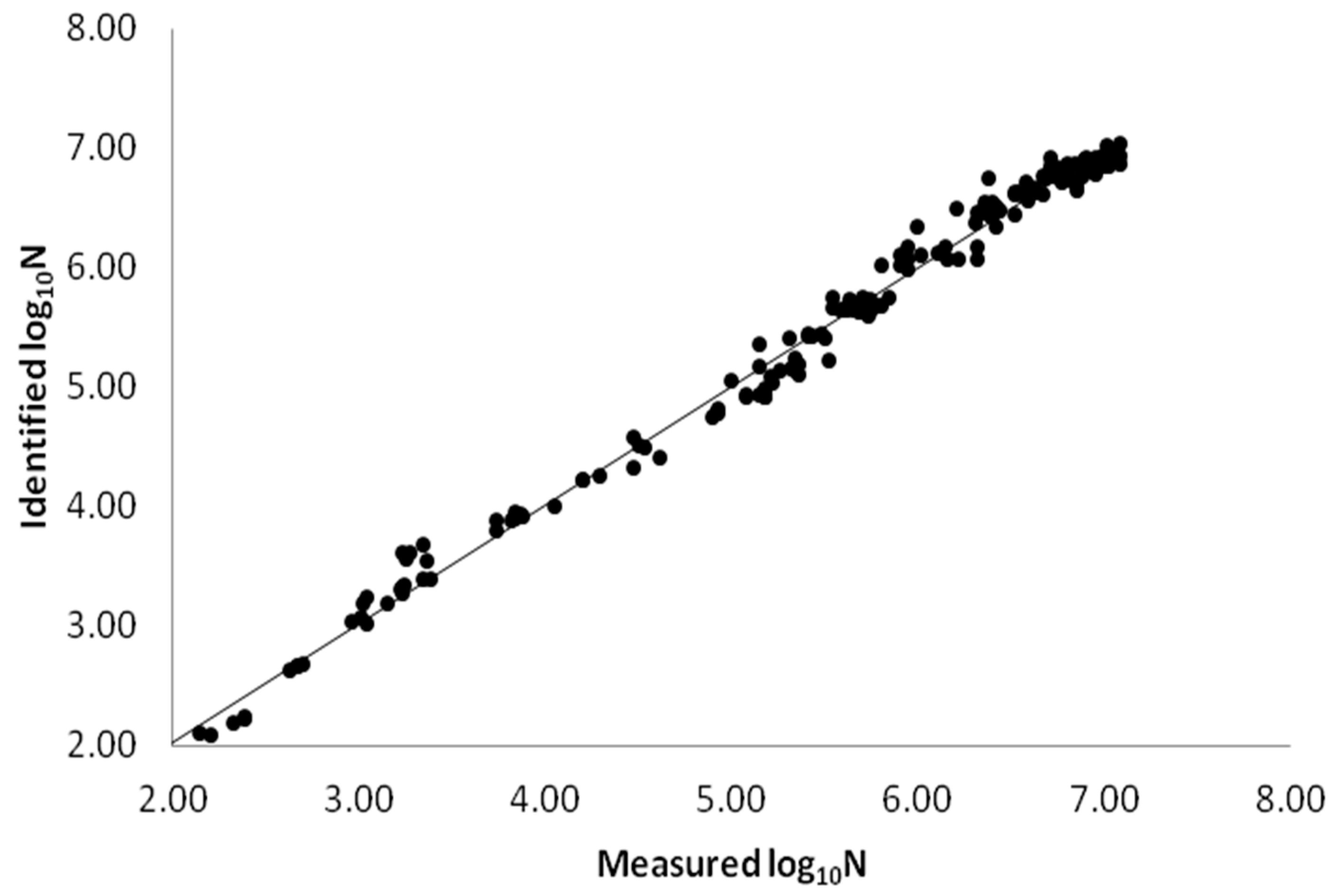
3.1. Model Performance
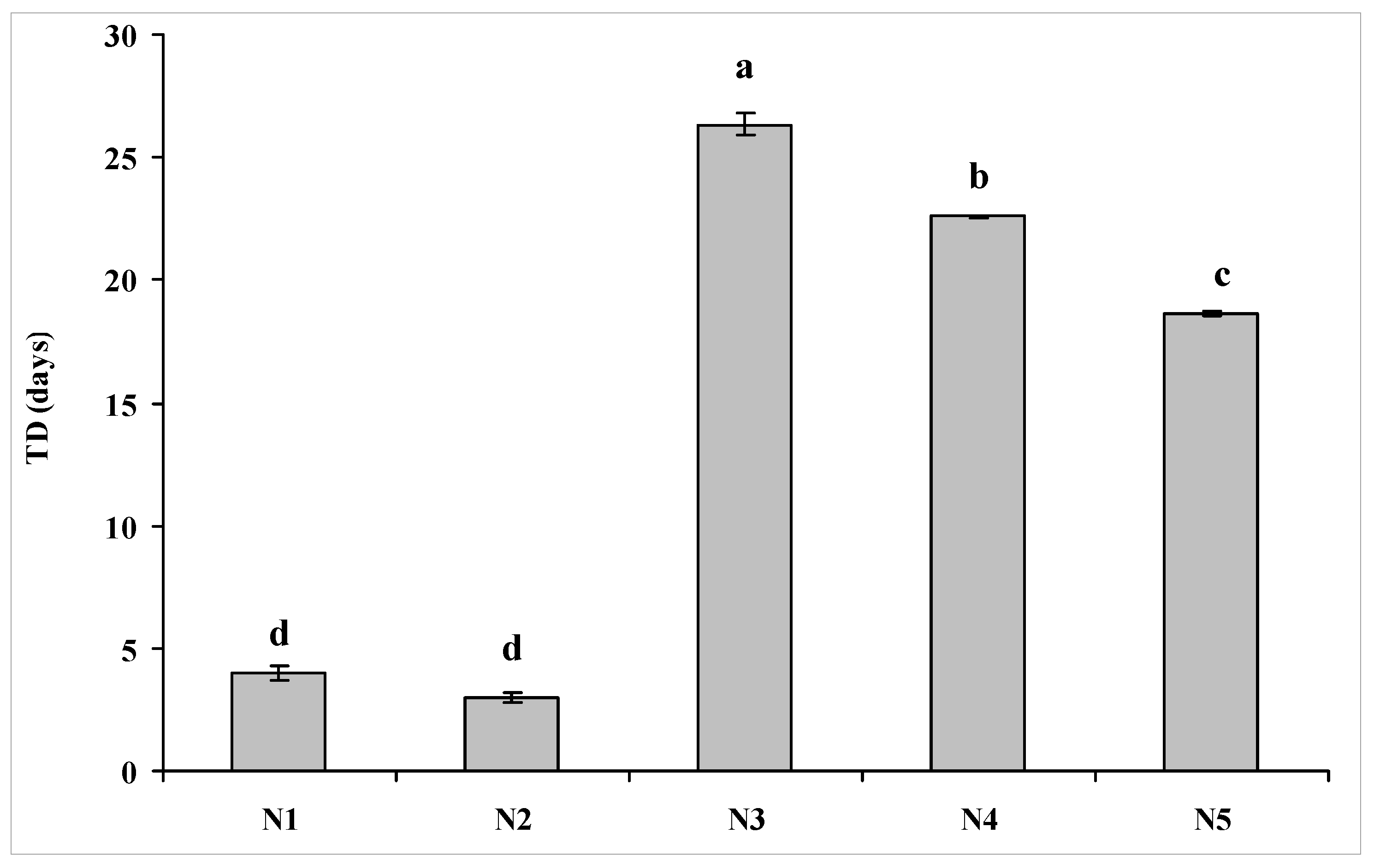
3.2. Fate Behavior of Escherichia coli O157:H7 in Soils
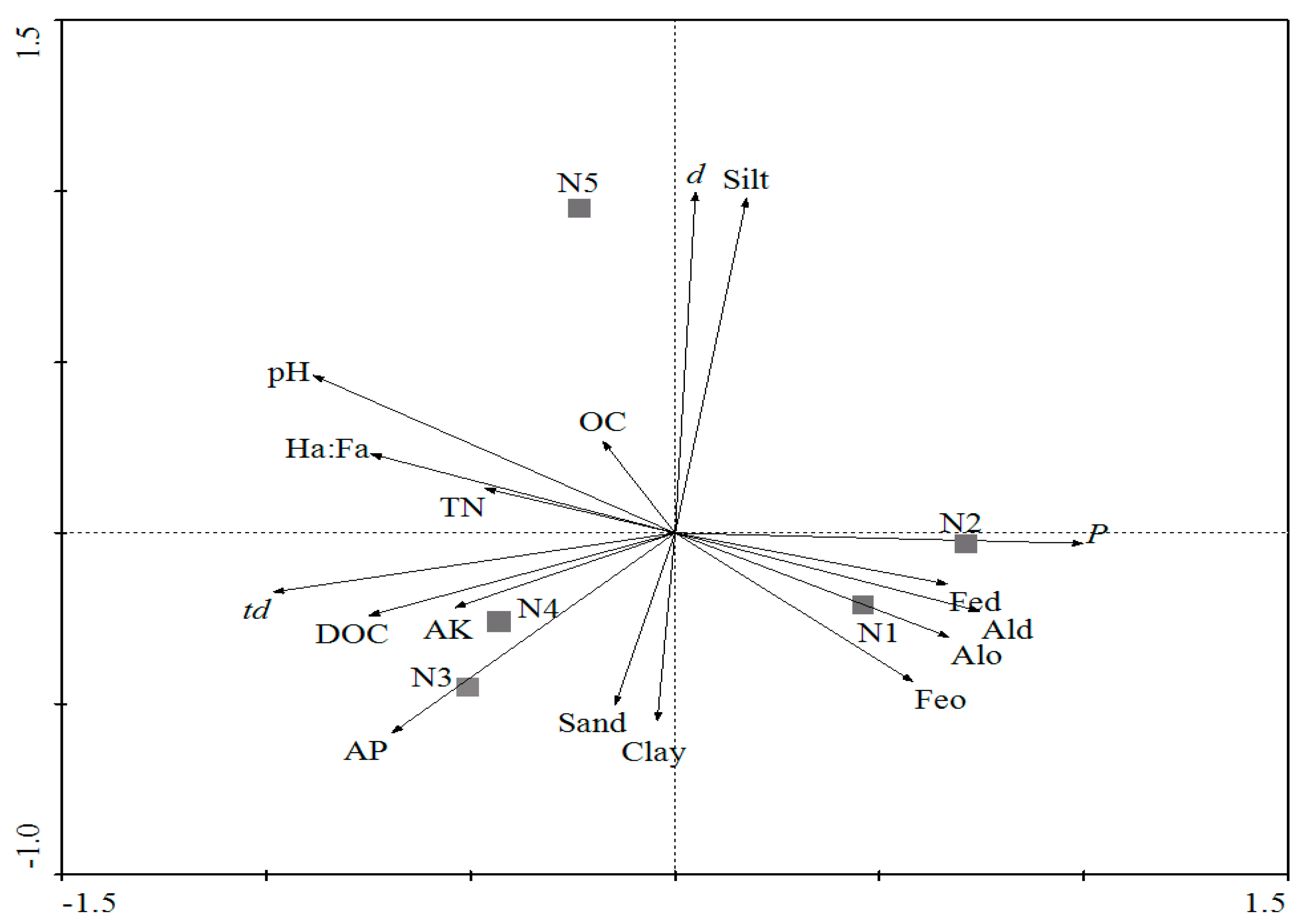
3.3. RDA Analysis of Survival Data on Soil Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Keene, W.E.; McAnulty, J.M.; Hoesly, F.C.; Williams, L.P., Jr.; Hedberg, K.; Oxman, G.L.; Barrett, T.J.; Pfaller, M.A.; Fleming, D.W. A swimming-associated outbreak of hemorrhagic colitis caused by Escherichia coli O157:H7 and Shigella sonnei. N. Engl. J. Med. 1994, 331, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Feng, P. Escherichia coli serotype O157:H7: Novel vehicles of infection and emergence of phenotypic variants. Emerg. Infect. Dis. 1995, 1, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Viñas, I.; Usall, J.; Anguera, M.; Abadias, M. Presence and survival of Escherichia coli O157:H7 on lettuce leaves and in soil treated with contaminated compost and irrigation water. Int. J. Food. Microbiol. 2012, 156, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Semenov, A.V.; van Overbeek, L.; van Bruggen, A.H. Percolation and survival of Escherichia coli O157:H7 and Salmonella enterica serovar Typhimurium in soil amended with contaminated dairy manure or slurry. Appl. Environ. Microb. 2009, 75, 3206–3215. [Google Scholar] [CrossRef] [PubMed]
- Solomon, E.B.; Yaron, S.; Matthews, K.R. Transmission of Escherichia coli O157:H7 from contaminated manure and irrigation water to lettuce plant tissue and its subsequent internalization. Appl. Environ. Microb. 2002, 68, 397–400. [Google Scholar]
- Eppinger, M.; Mammel, M.K.; Leclerc, J.E.; Ravel, J.; Cebula, T.A. Genomic anatomy of Escherichia coli O157:H7 outbreaks. Proc. Natl. Acad. Sci. USA 2011, 108, 20142–20147. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Morgan, J.; Doyle, M.P. Fate of Escherichia coli O157:H7 in manure-amended soil. Appl. Environ. Microb. 2002, 68, 2605–2609. [Google Scholar] [CrossRef]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.-A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States-major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Avery, L.; Killham, K.; Jones, D. Survival of Escherichia coli O157:H7 in the rhizosphere of maize grown in waste-amended soil. J. Appl. Microbiol. 2007, 102, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Franz, E.; Semenov, AV.; Termorshuizen, A.J.; De Vos, O.; Bokhorst, J.G.; Van Bruggen, A.H. Manure-amended soil characteristics affecting the survival of E. coli O157:H7 in 36 Dutch soils. Environ. Microbiol. 2008, 10, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Ongeng, D.; Vasquez, G.; Muyanja, C.; Ryckeboer, J.; Geeraerd, A.; Springael, D. Transfer and internalisation of Escherichia coli O157:H7 and Salmonella enterica serovar Typhimurium in cabbage cultivated on contaminated manure-amended soil under tropical field conditions in Sub-Saharan Africa. Int. J. Food Microbiol. 2011, 145, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Semenov, A.V.; van Overbeek, L.; Termorshuizen, A.J.; van Bruggen, A.H. Influence of aerobic and anaerobic conditions on survival of Escherichia coli O157:H7 and Salmonella enterica serovar Typhimurium in Luria-Bertani broth, farm-yard manure and slurry. J. Environ. Manag. 2011, 92, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ibekwe, A.M.; Yang, C.H.; Crowley, D.E. Influence of bacterial communities based on 454-pyrosequencing on the survival of Escherichia coli O157:H7 in soils. FEMS Microbiol. Ecol. 2013, 84, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Ongeng, D.; Muyanja, C.; Geeraerd, A.; Springael, D.; Ryckeboer, J. Survival of Escherichia coli O157:H7 and Salmonella enterica serovar Typhimurium in manure and manure-amended soil under tropical climatic conditions in Sub-Saharan Africa. J. Appl. Microbiol. 2011, 110, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, H.; Wu, L.; Lou, J.; Wu, J.; Brookes, P.C.; Xu, J. Survival of Escherichia coli O157:H7 in soils from Jiangsu Province, China. PLoS ONE 2013, 8, e81178. [Google Scholar] [CrossRef] [PubMed]
- Van Elsas, J.D.; Semenov, A.V.; Costa, R.; Trevors, J.T. Survival of Escherichia coli in the environment: Fundamental and public health aspects. ISME J. 2010, 5, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ibekwe, A.M.; Crowley, D.E.; Yang, C.-H. Persistence of Escherichia coli O157:H7 in major leafy green producing soils. Environ. Sci. Technol. 2012, 46, 12154–12161. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Sun, D.; Luo, Y.; Zhou, D.; He, R.; Mao, J.; Luo, Y. The fertility of purple soil in relation to the characteristics of parent material in Sichuan Basin. Acta Pedol. Sin. 1984, 21, 123–133. [Google Scholar]
- Wang, H.; Shi, X.; Yu, D.; Weindorf, D.C.; Huang, B.; Sun, W.; Ritsema, C.J.; Milne, E. Factors determining soil nutrient distribution in a small-scaled watershed in the purple soil region of Sichuan Province, China. Soil Till. Res. 2009, 105, 300–306. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, T.; You, X.; Gao, M. Nutrient release from weathering of purplish rocks in the Sichuan Basin, China. Pedosphere 2008, 18, 257–264. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, H.; Zhang, C.; Luan, R.; Tu, G. Molecular epidemiolog y, antimicrobial and disinfectant resistance of Escherichia coli O157:H7 in Sichuan. J. Hygiene Res. 2005, 5, 028. [Google Scholar]
- Agricultural Chemistry Committee of China. Conventional Methods of Soil and Agricultural Chemistry Analysis; Science Press: Beijing, China, 1983. (In Chinese) [Google Scholar]
- Ter Braak, C.J.; Prentice, I.C. A theory of gradient analysis. Adv. Ecol. Res. 1988, 18, 271–317. [Google Scholar]
- Peleg, M. Microbial survival curves: Interpretation, mathematical modeling, and utilization. Comments Theor. Biol. 2003, 8, 4–5. [Google Scholar] [CrossRef]
- Van Boekel, M.A. On the use of the Weibull model to describe thermal inactivation of microbial vegetative cells. Int. J. Food. Microbiol. 2002, 74, 139–159. [Google Scholar] [CrossRef]
- Crane, B.S.; Moore, J. Modeling enteric bacterial die-off: A review. Water Air Soil Poll. 1986, 27, 411–439. [Google Scholar] [CrossRef]
- Franz, E.; van Diepeningen, A.D.; de Vos, O.J.; van Bruggen, A.H. Effects of cattle feeding regimen and soil management type on the fate of Escherichia coli O157:H7 and Salmonella enterica serovar Typhimurium in manure, manure-amended soil, and lettuce. Appl. Environ. Microb. 2005, 71, 6165–6174. [Google Scholar] [CrossRef] [PubMed]
- Yee, N.; Fein, J.B.; Daughney, C.J. Experimental study of the pH, ionic strength, and reversibility behavior of bacteria–mineral adsorption. Geochim. Cosmochim. Acta 2000, 64, 609–617. [Google Scholar] [CrossRef]
- Wu, H.; Jiang, D.; Cai, P.; Rong, X.; Dai, K.; Liang, W.; Huang, Q. Adsorption of Pseudomonas putida on soil particle size fractions: Effects of solution chemistry and organic matter. J. Soil Sediments 2012, 12, 143–149. [Google Scholar] [CrossRef]
- Cai, P.; Huang, Q.; Walker, S.L. Deposition and Survival of Escherichia coli O157:H7 on Clay Minerals in a Parallel Plate Flow System. Environ. Sci. Technol. 2013, 47, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xing, X.; Xu, Z. Relationship between pH value and available nutrients of purple soil in panxi tobacco-growing areas. Soil Fertil. Sci. Chin. 2007, 6, 004. [Google Scholar]
- Aciego Pietri, J.C.; Brookes, P.C. Relationships between soil pH and microbial properties in a UK arable soil. Soil Biol. Biochem. 2008, 40, 1856–1861. [Google Scholar] [CrossRef]
- Artz, R.R.; Killham, K. Survival of Escherichia coli O157:H7 in private drinking water wells: Influences of protozoan grazing and elevated copper concentrations. FEMS Microbiol. Lett. 2002, 216, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Pandey, S.D.; Misra, V. Stability constants of metal–humic acid complexes and its role in environmental detoxification. Ecotoxicol. Environ. Saf. 2000, 47, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Dalang, F.; Buffle, J.; Haerdi, W. Study of the influence of fulvic substances on the adsorption of copper (II) ions at the kaolinite surface. Environ. Sci. Technol. 1984, 18, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Robert, M.; Huang, P.; Berthelin, J.; Bollag, J.; McGill, W.; Page, A. Aluminum toxicity: A major stress for microbes in the environment. In Environmental Impact of Soil Component Interactions: Volume 2: Metals, other Inorganics, and Microbial Activities; CRC Press: Boca Raton, FL, USA, 1995; pp. 227–242. [Google Scholar]
- Avery, L.; Williams, A.; Killham, K.; Jones, D. Survival of Escherichia coli O157:H7 in waters from lakes, rivers, puddles and animal-drinking troughs. Sci. Total Environ. 2008, 389, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S. Interaction of aluminum and iron oxides and clay minerals and their effect on soil physical properties: A review. Commun. Soil Sci. Plan. 1989, 20, 1181–1207. [Google Scholar] [CrossRef]
- Chen, G.; Zhu, H. Bacterial adhesion to silica sand as related to Gibbs energy variations. Colloid. Surf. B 2005, 44, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Park, S.J.; Han, Y.U.; Park, J.A.; Kim, S.B. Bacterial attachment and detachment in aluminum-coated quartz sand in response to ionic strength change. Water Environ. Res. 2010, 82, 499–505. [Google Scholar] [CrossRef] [PubMed]
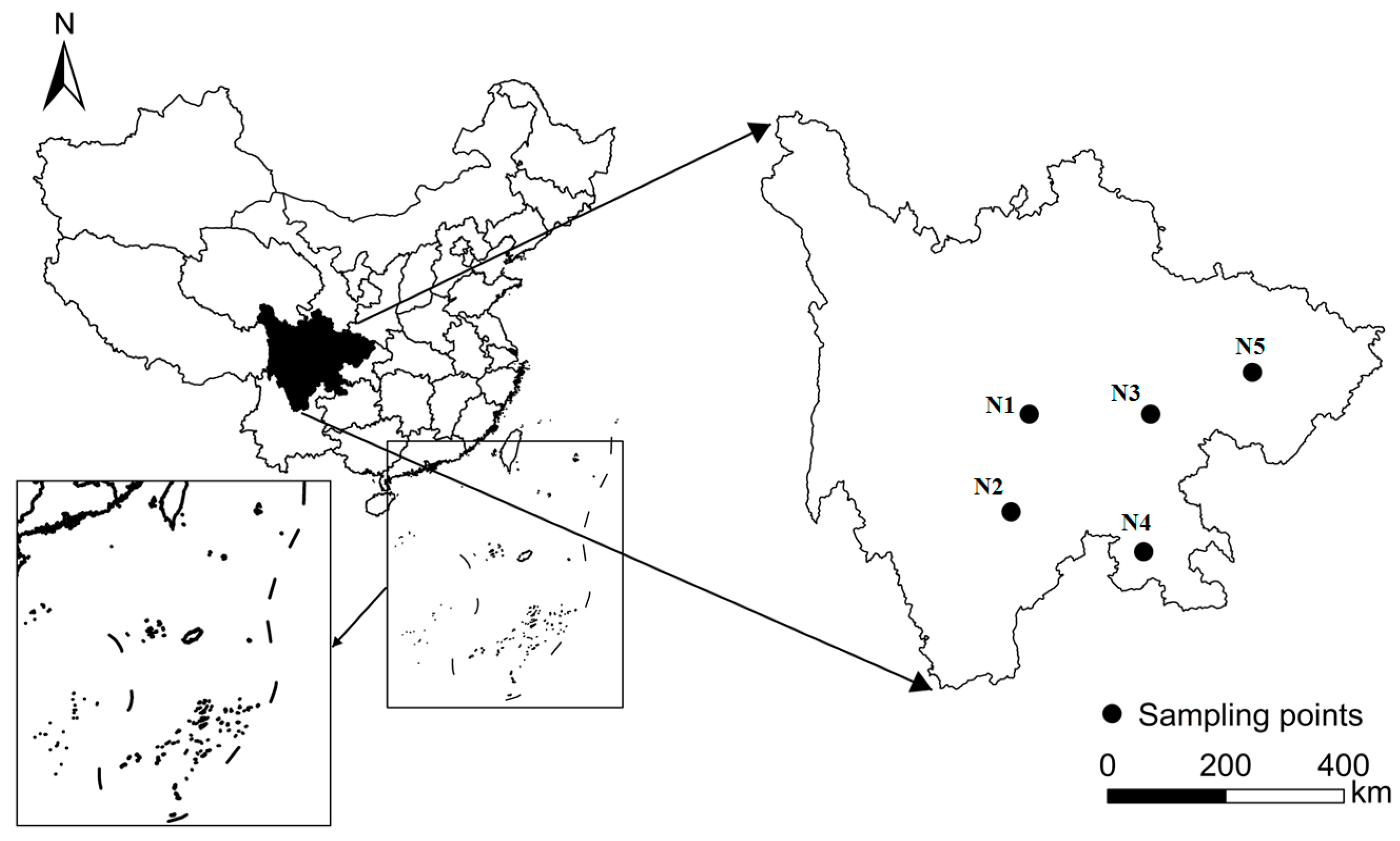

| Soil Code | pH | DOC | AK | AP | OC g kg−1 | TN | Clay | Silt | Sand | Ha/Fa | Fed | Ald | Feo | Alo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg·kg−1 | % | g·kg−1 | ||||||||||||
| N1 | 4.56 | 9.52 | 112.54 | 0.26 | 8.6 | 0.89 | 38.30 | 37.27 | 24.43 | 0.16 | 44.14 | 11.34 | 4.38 | 4.92 |
| N2 | 3.89 | 28.11 | 48.76 | 1.69 | 12.8 | 0.88 | 25.53 | 39.00 | 35.47 | 0.36 | 23.34 | 5.43 | 1.70 | 2.47 |
| N3 | 6.63 | 62.79 | 349.89 | 15.97 | 16.3 | 1.77 | 29.93 | 33.03 | 37.04 | 0.66 | 19.18 | 2.54 | 1.66 | 1.48 |
| N4 | 6.64 | 38.94 | 51.47 | 7.62 | 6.9 | 0.65 | 35.07 | 33.83 | 31.10 | 0.42 | 8.00 | 1.10 | 0.55 | 1.80 |
| N5 | 7.84 | 32.65 | 143.94 | 0.68 | 14.4 | 1.38 | 25.82 | 48.08 | 26.10 | 0.58 | 17.10 | 1.97 | 0.33 | 1.31 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Hu, S.; Yang, W. Variations of Escherichia coli O157:H7 Survival in Purple Soils. Int. J. Environ. Res. Public Health 2017, 14, 1246. https://doi.org/10.3390/ijerph14101246
Zhang T, Hu S, Yang W. Variations of Escherichia coli O157:H7 Survival in Purple Soils. International Journal of Environmental Research and Public Health. 2017; 14(10):1246. https://doi.org/10.3390/ijerph14101246
Chicago/Turabian StyleZhang, Taoxiang, Suping Hu, and Wenhao Yang. 2017. "Variations of Escherichia coli O157:H7 Survival in Purple Soils" International Journal of Environmental Research and Public Health 14, no. 10: 1246. https://doi.org/10.3390/ijerph14101246




