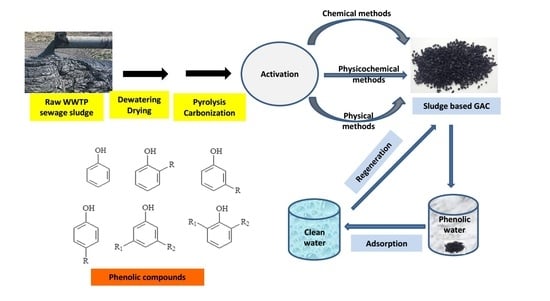
Removal of Phenolic Compounds from Water Using Sewage Sludge-Based Activated Carbon Adsorption: A Review
Abstract
:1. Introduction
2. Classifications of Phenolic Compounds
3. Production of Sewage Sludge and Its Potential Use as an Adsorbent
4. SBAC Production Processes
4.1. Drying of Dewatered Sludge and Its Pyrolysis and Carbonization
4.2. Physically Activated SBAC
4.3. Chemically Activated SBAC
4.3.1. ZnCl2 Activation
4.3.2. H2SO4 Activation
4.3.3. KOH/NaOH Activation
4.3.4. Other Activation Methods for SBAC Production
5. Adsorptive Characteristics of SBAC
5.1. Pore Structure of SBAC
5.2. Functional Groups on the Surface of SBAC
6. Adsorption of Phenolic Compounds on SBAC
6.1. Adsorptive Characteristics of Phenolic Compounds
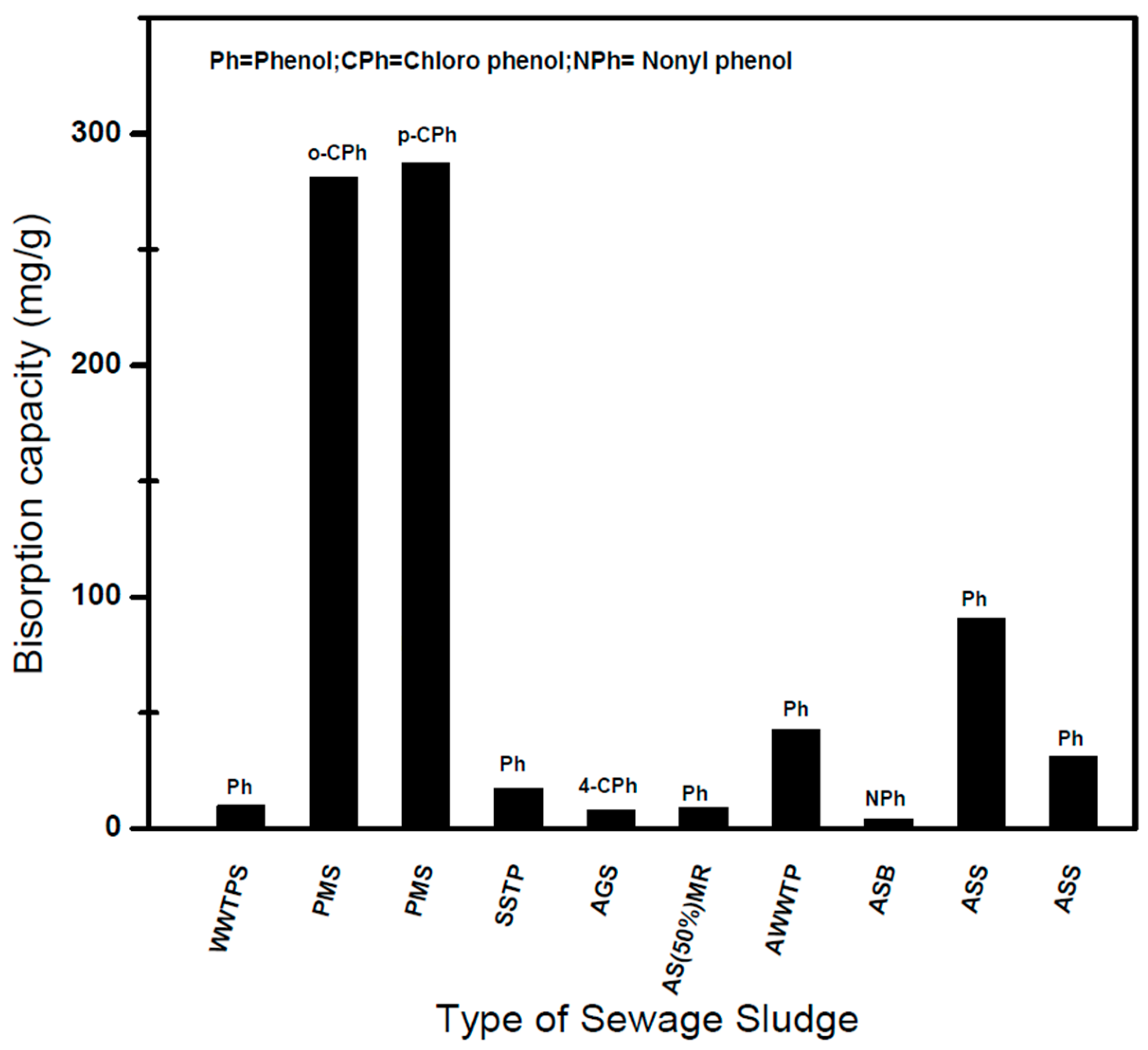
6.2. Adsorption of Phenolic Compounds on Dried Sludge
6.3. Adsorption of Phenolic Compounds on Physically Activated SBAC
6.4. Adsorption of Phenolic Compounds on Chemically Activated SBAC
6.5. Effect of Operation Conditions
7. Isotherms and Mechanisms of Phenolic Compound Adsorption on SBAC
7.1. Adsorption Isotherms
7.2. Mechanisms of Adsorption of Phenolic Compounds on SBAC
- Characteristics of the SBAC. These include the pore size distribution (surface area, pore volume), presence of oxygen functionalities on the carbon surface, ash content and others like mineral content.
- Characteristics of the adsorbate. These include the molecular size of the adsorbate, its pKa value, functional groups and polarity.
- Experimental conditions. These include the pH of the solution, temperature, ionic nature and concentration of the solution.
8. Regeneration of Spent SBAC
8.1. Thermal Regeneration of Spent SBAC
8.2. Chemical Regeneration of Spent SBAC
8.3. Electrochemical Regeneration of Spent SBAC
9. Sustainable SBAC Production and Utilization for PC Removal from Water
10. Conclusions
- Different SBACs exhibiting divergent physicochemical characteristics as well as adsorption performances for removal of PC from water were attributed to the diverse sources of SS as well as activation techniques employed for SBAC production.
- Although chemical activation techniques produce better SBAC textural properties and superior PC adsorptive performance compared to physical activation, t more research works are needed to harness the advances in material science to improve the functional groups and textural properties of SBACs as well as the low performance of physical activation methods.
- Investigation of new and novel chemical activation reagents and combined chemical and physical activation systems are rare. Thus, these need to be explored for producing better SBACs for improved effectiveness of PC removal from water.
- The Freundlich and Langmuir models were the most satisfactorily isotherm models that describe well the uptake of PC on both dried and activated SBAC.
- Even though practical industrial and large scale applications of water and wastewater GAC treatment processes are based on the continuous process hence rendering fixed bed studies more beneficial. However, very few studies have investigated the adsorption behavior of PC on SBACs using continuous modes for breakthrough curve analysis.
- Most of the investigated PC mainly included parent the phenol molecule and simple derivatives like chlorophenols, bromophenols and nitrophenols. Thus, the adsorption performances of SBACs for the removal of toxic compounds such as catechol, resorcinol, benzoquinone and several other PC of environmental significant in single and multi-systems are also important to be evaluated.
- Despite the established economic benefits of regeneration of spent SBACs using different techniques, studies that evaluate the regeneration potential of spent SBACs employed for adsorption of PC are very rare.
- Studies focusing on PC adsorptive performance on SBACs under continuous mode (that are more relevant for industrial applications) in both single and multi-pollutant aqueous systems to cover a wide range of PC of environmental concerns are lacking, thus they are recommended for future research.
- It is also recommended that the production processes and utilization of SBAC need to be economically re-evaluated and assessed within the realm of environmental sustainability via LCA analyses.
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AAS | Aerobic activated sludge |
| AGS | anaerobic granular sludge |
| AAS | Anaerobic activated sludge |
| ADWWTPS | Aerobically digested WWTP sludge |
| ADDWWTP | Anaerobically digested and dewatered sludge |
| AGCWS | Aerobic granular sludge from cosmetic factory |
| ASB | Activated sludge biomass |
| AS (50%) MR | Activated sludge (50%) immobilized in Mowital® B30H resin |
| ASS | Activated Sludge System |
| AWWTPS | Anaerobic wastewater treatment plant sludge |
| CFS | Cosmetic factory sludge |
| DAEDS | Dewatered aerobically digested sludge |
| DASS | Dried aerobic sewage sludge |
| DMADS | Dewatered anaerobically digested sludge |
| DSBS | Dewatered secondary biological sludge |
| MDSS | Mechanical dewatered sewage sludge |
| DUSS | Dewatered undigested sewage sludge |
| DRAWS | Dewatered raw sludge |
| FIS | Fertilizer industry sludge |
| GAC | Granular activated carbon(s) |
| LS | Limed sludge |
| LCA | Life cycle assessment |
| PC | Phenolic compounds |
| POES | Palm oil effluent sludge |
| SBET | Adsorbent specific surface area measured using Brunauer Emmett Teller (BET) method |
| SBAC | Sludge based activated carbon |
| SSTP | sewage sludge from sludge treatment plant |
| VLS | Viscous liquid sludge |
| WBS | Waste biological sludge |
| WWTP | Wastewater treatment plant |
| WWTPS | Wastewater treatment plant sludge |
References
- Vermerris, W.; Nicholson, R. Phenolic compounds and their effects on human health. In Phenolic Compound Biochemistry; Springer: Dordrecht, The Netherlands, 2006; pp. 235–255. [Google Scholar]
- Michałowicz, J.; Duda, W. Phenols—Sources and toxicity. Pol. J. Environ. Stud. 2007, 16, 347–362. [Google Scholar]
- Busca, G.; Berardinelli, S.; Resini, C.; Arrighi, L. Technologies for the removal of phenol from fluid streams: A short review of recent developments. J. Hazard. Mater. 2008, 160, 265–288. [Google Scholar] [CrossRef] [PubMed]
- Khalili, N.R.; Vyas, J.D.; Weangkaew, W.; Westfall, S.J.; Parulekar, S.J.; Sherwood, R. Synthesis and characterization of activated carbon and bioactive adsorbent produced from paper mill sludge. Sep. Purif. Technol. 2002, 26, 295–304. [Google Scholar]
- Reddy, S.S.; Kotaiah, B. Comparative evaluation of commercial and sewage sludge based activated carbons for the removal of textile dyes from aqueous solutions. J. Environ. Health Sci. Eng. 2006, 3, 239–246. [Google Scholar]
- Abussaud, B.; Asmaly, H.A.; Ihsanullah; Saleh, T.A.; Gupta, V.K.; laoui, T.; Ali Atieh, M. Sorption of phenol from waters on activated carbon impregnated with iron oxide, aluminum oxide and titanium oxide. J. Mol. Liq. 2016, 213, 351–359. [Google Scholar]
- Anisuzzaman, S.M.; Bono, A.; Krishnaiah, D.; Tan, Y.Z. A study on dynamic simulation of phenol adsorption in activated carbon packed bed column. J. King Saud Univ. Eng. Sci. 2016, 28, 47–55. [Google Scholar] [CrossRef]
- Goncalves, M.; Guerreiro, M.C.; Ramos, P.H.; Alves de Oliveira, L.C.; Sapag, K. Activated carbon prepared from coffee pulp: Potential adsorbent of organic contaminants in aqueous solution. Water Sci. Technol. 2013, 68, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Monsalvo, V.M.; Mohedano, A.F.; Rodriguez, J.J. Activated carbons from sewage sludge: Application to aqueous-phase adsorption of 4-chlorophenol. Desalination 2011, 277, 377–382. [Google Scholar] [CrossRef]
- Devi, P.; Saroha, A.K. Utilization of sludge based adsorbents for the removal of various pollutants: A review. Sci. Total Environ. 2017, 578, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B. Biochemistry of Phenolic Compounds; Academic Press: London, UK, 1964. [Google Scholar]
- Wang, X.; Zhu, N.; Yin, B. Preparation of sludge-based activated carbon and its application in dye wastewater treatment. J. Hazard. Mater. 2008, 153, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Hong, J.; Otaki, M.; Jolliet, O. Environmental and economic life cycle assessment for sewage sludge treatment processes in Japan. Waste Manag. 2009, 29, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Al-Malack, M.H.; Abuzaid, N.S.; Bukhari, A.A.; Essa, M.H. Characterization, utilization, and disposal of municipal sludge: The state of-the-art. Arab. J. Sci. Eng. 2002, 27, 3–27. [Google Scholar]
- Uggetti, E.; Ferrer, I.; Molist, J.; García, J. Technical, economic and environmental assessment of sludge treatment wetlands. Water Res. 2011, 45, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Fytili, D.; Zabaniotou, A. Utilization of sewage sludge in EU application of old and new methods—A review. Renew. Sustain. Energy Rev. 2008, 12, 116–140. [Google Scholar] [CrossRef]
- Hospido, A.; Moreira, T.; Martín, M.; Rigola, M.; Feijoo, G. Environmental evaluation of different treatment processes for sludge from urban wastewater treatments: Anaerobic digestion versus thermal processes (10 pp). Int J Life Cycle Assess. 2005, 10, 336–345. [Google Scholar] [CrossRef]
- Mohedano, A.; Monsalvo, V.; Bedia, J.; Lopez, J.; Rodriguez, J. Highly stable iron catalysts from sewage sludge for CWPO. J. Environ. Chem. Eng. 2014, 2, 2359–2364. [Google Scholar] [CrossRef]
- Dos Reis, G.S.; Adebayo, M.A.; Sampaio, C.H.; Lima, E.C.; Thue, P.S.; de Brum, I.A.S.; Dias, S.L.P.; Pavan, F.A. Removal of phenolic compounds from aqueous solutions using sludge-based activated carbons prepared by conventional heating and microwave-assisted pyrolysis. Water Air Soil Pollut. 2016, 228, 33. [Google Scholar] [CrossRef]
- Hadi, P.; Xu, M.; Ning, C.; Sze Ki Lin, C.; McKay, G. A critical review on preparation, characterization and utilization of sludge-derived activated carbons for wastewater treatment. Chem. Eng. J. 2015, 260, 895–906. [Google Scholar] [CrossRef]
- Smith, K.; Fowler, G.; Pullket, S.; Graham, N.J.D. Sewage sludge-based adsorbents: A review of their production, properties and use in water treatment applications. Water Res. 2009, 43, 2569–2594. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Yang, X.; Spinosa, L. Development of sludge-based adsorbents: Preparation, characterization, utilization and its feasibility assessment. J. Environ. Manag. 2015, 151, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Li, C.; Cai, Z.; Zhang, W.; Gao, H.; Chen, L.; Zeng, G.; Shu, X.; Zhao, Y. Study on activated carbon derived from sewage sludge for adsorption of gaseous formaldehyde. Bioresour. Technol. 2011, 102, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.J.; Serra, E.; Ros, A.; Balaguer, M.D.; Rigola, M. Carbonaceous adsorbents from sewage sludge and their application in a combined activated sludge-powdered activated carbon (AS-PAC) treatment. Carbon 2004, 42, 1389–1394. [Google Scholar] [CrossRef]
- Rio, S.; Faur-Brasquet, C.; Le Coq, L.; Le Cloirec, P. Structure characterization and adsorption properties of pyrolyzed sewage sludge. Environ. Sci. Technol. 2005, 39, 4249–4257. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Muyibi, S.A.; Mansor, M.F.; Wahid, R. Removal of phenol by activated carbons prepared from palm oil mill effluent sludge. J. Environ. Sci. 2006, 18, 446–452. [Google Scholar]
- Mohamed, E.F.; Andriantsiferana, C.; Wilhelm, A.-M.; Delmas, H. Competitive adsorption of phenolic compounds from aqueous solution using sludge-based activated carbon. Environ. Technol. 2011, 32, 1325–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Wu, Y.; Feng, L.; Zhang, L. Surface properties of SAC and its adsorption mechanisms for phenol and nitrobenzene. Bioresour. Technol. 2012, 113, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Pirzadeh, K.; Ghoreyshi, A.A. Phenol removal from aqueous phase by adsorption on activated carbon prepared from paper mill sludge. Desalination Water Treat. 2014, 52, 6505–6518. [Google Scholar] [CrossRef]
- Bousba, S.; Meniai, A. Adsorption of 2-chlorophenol onto sewage sludge based adsorbent: Equilibrium and kinetic study. ChemEng 2013, 35. [Google Scholar] [CrossRef]
- Masomi, M.; Ghoreyshi, A.; Najafpour, G.; Mohamed, A. Adsorption of phenolic compounds onto the activated carbon synthesized from pulp and paper mill sludge: Equilibrium isotherm, kinetics, thermodynamics and mechanism studies. Int. J. Eng. Trans. A Basics 2014, 27, 1485–1494. [Google Scholar]
- Uggetti, E.; Ferrer, I.; Llorens, E.; García, J. Sludge treatment wetlands: A review on the state of the art. Bioresour. Technol. 2010, 101, 2905–2912. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, E.; Eddy, H.P.; Tchobanoglous, G. Wastewater Engineering: Treatment, Disposal and Reuse; McGraw-Hill: New York, NY, USA, 1991. [Google Scholar]
- Lin, Q.; Cheng, H.; Chen, G. Preparation and characterization of carbonaceous adsorbents from sewage sludge using a pilot-scale microwave heating equipment. J. Anal. Appl. Pyrolysis 2012, 93, 113–119. [Google Scholar] [CrossRef]
- Bagreev, A.; Bandosz, T.J.; Locke, D.C. Pore structure and surface chemistry of adsorbents obtained by pyrolysis of sewage sludge-derived fertilizer. Carbon 2001, 39, 1971–1979. [Google Scholar] [CrossRef]
- Inguanzo, M.; Menendez, J.; Fuente, E.; Pis, J. Reactivity of pyrolyzed sewage sludge in air and CO2. J. Anal. Appl. Pyrolysis 2001, 58, 943–954. [Google Scholar] [CrossRef]
- Zhai, Y.; Wei, X.; Zeng, G. Effect of pyrolysis temperature and hold time on the characteristic parameters of adsorbent derived from sewage sludge. J. Environ. Sci. (China) 2003, 16, 683–686. [Google Scholar]
- Lu, G.Q.; Low, J.C.F.; Liu, C.Y.; Lua, A.C. Surface area development of sewage sludge during pyrolysis. Fuel 1995, 74, 344–348. [Google Scholar] [CrossRef]
- Monsalvo, V.M.; Mohedano, A.F.; Rodriguez, J.J. Adsorption of 4-chlorophenol by inexpensive sewage sludge-based adsorbents. Chem. Eng. Res. Des. 2012, 90, 1807–1814. [Google Scholar] [CrossRef]
- Ros, A.; Montes-Moran, M.A.; Fuente, E.; Nevskaia, D.M.; Martin, M.J. Dried sludges and sludge-based chars for H2S removal at low temperature: Influence of sewage sludge characteristics. Environ. Sci. Technol. 2006, 40, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Inguanzo, M.; Domínguez, A.; Menéndez, J.A.; Blanco, C.G.; Pis, J.J. On the pyrolysis of sewage sludge: The influence of pyrolysis conditions on solid, liquid and gas fractions. J. Anal. Appl. Pyrolysis 2002, 63, 209–222. [Google Scholar] [CrossRef]
- Lebigue, C.J.; Andriantsiferana, C.; Ayral, C.; Mohamed, E.; Wilhelm, A.-M.; Delmas, H.; Le Coq, L.; Gerente, C.; Smith, K.M.; Pullket, S.; et al. Application of sludge-based carbonaceous materials in a hybrid water treatment process based on adsorption and catalytic wet air oxidation. J. Environ. Manag. 2010, 91, 2432–2439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, J. Characterization of Activated Carbon Produced from Coffee Residues by Chemical and Physical Activation. Master’s Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 2011. [Google Scholar]
- Zou, J.; Dai, Y.; Wang, X.; Ren, Z.; Tian, C.; Pan, K.; Li, S.; Abuobeidah, M.; Fu, H. Structure and adsorption properties of sewage sludge-derived carbon with removal of inorganic impurities and high porosity. Bioresour. Technol. 2013, 142, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Ros, A.; Lillo-Ródenas, M.; Fuente, E.; Montes-Morán, M.; Martin, M.; Linares-Solano, A. High surface area materials prepared from sewage sludge-based precursors. Chemosphere 2006, 65, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Jindarom, C.; Meeyoo, V.; Kitiyanan, B.; Rirksomboon, T.; Rangsunvigit, P. Surface characterization and dye adsorptive capacities of char obtained from pyrolysis/gasification of sewage sludge. Chem. Eng. J. 2007, 133, 239–246. [Google Scholar] [CrossRef]
- Fitzmorris, K.B.; Lima, I.M.; Marshall, W.E.; Reimers, R.S. Anion and cation leaching or desorption from activated carbons from municipal sludge and poultry manure as affected by pH. Water Environ. Res. 2006, 78, 2324–2329. [Google Scholar] [CrossRef] [PubMed]
- Fitzmorris, K.B.; Lima, I.M.; Marshall, W.E.; Reimers, R.S. Anion and cation removal from solution using activated carbons from municipal sludge and poultry manure. J. Residuals Sci. Technol. 2006, 3, 161–167. [Google Scholar]
- Rio, S.; Le Coq, L.; Faur, C.; Le Cloirec, P. Production of porous carbonaceous adsorbent from physical activation of sewage sludge: Application to wastewater treatment. Water Sci. Technol. 2006, 53, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Rio, S.; Le Coq, L.; Faur, C.; Lecomte, D.; Le Cloirec, P. Preparation of adsorbents from sewage sludge by steam activation for industrial emission treatment. Process Saf. Environ. Prot. 2006, 84, 258–264. [Google Scholar] [CrossRef]
- Rozada, F.; Otero, M.; Morán, A.; García, A.I. Activated carbons from sewage sludge and discarded tyres: Production and optimization. J. Hazard. Mater. 2005, 124, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A. Removal of bromophenols from water using industrial wastes as low cost adsorbents. J. Hazard. Mater. 2007, 139, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.R.N.; Stüber, F.; Smith, K.M.; Fabregat, A.; Bengoa, C.; Font, J.; Fortuny, A.; Pullket, S.; Fowler, G.D.; Graham, N.G.D. Sewage sludge based catalysts for catalytic wet air oxidation of phenol: Preparation, characterisation and catalytic performance. Appl. Catal. B Environ. 2011, 101, 306–316. [Google Scholar] [CrossRef]
- Stüber, F.; Smith, K.M.; Mendoza, M.B.; Marques, R.R.N.; Fabregat, A.; Bengoa, C.; Font, J.; Fortuny, A.; Pullket, S.; Fowler, G.D.; et al. Sewage sludge based carbons for catalytic wet air oxidation of phenolic compounds in batch and trickle bed reactors. Appl. Catal. B Environ. 2011, 110, 81–89. [Google Scholar] [CrossRef]
- Patrick, J.W. Porosity in Carbons: Characterization and Applications; Edward Arnold: London, UK, 1995. [Google Scholar]
- Gu, L.; Wang, Y.; Zhu, N.; Zhang, D.; Huang, S.; Yuan, H.; Lou, Z.; Wang, M. Preparation of sewage sludge based activated carbon by using Fenton’s reagent and their use in 2-Naphthol adsorption. Bioresour. Technol. 2013, 146, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Ye, C.; Zhu, T.; Lou, Z.; Yuan, H.; Zhu, N. Preparation of activated carbon from wet sludge by electrochemical-NaClO activation. Environ. Technol. 2014, 35, 2685–2691. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.H.; Chen, X.G.; Jeyaseelan, S.; Graham, N. Optimising the preparation of activated carbon from digested sewage sludge and coconut husk. Chemosphere 2001, 44, 45–51. [Google Scholar] [CrossRef]
- Otero, M.; Rozada, F.; Calvo, L.F.; García, A.I.; Morán, A. Elimination of organic water pollutants using adsorbents obtained from sewage sludge. Dyes Pigment. 2003, 57, 55–65. [Google Scholar] [CrossRef]
- Bousba, S.; Meniai, A.H. Removal of phenol from water by adsorption onto sewage sludge based adsorbent. Chem. Eng. Trans. 2014, 40. [Google Scholar] [CrossRef]
- Kong, L.; Xiong, Y.; Sun, L.; Tian, S.; Xu, X.; Zhao, C.; Luo, R.; Yang, X.; Shih, K.; Liu, H. Sorption performance and mechanism of a sludge-derived char as porous carbon-based hybrid adsorbent for benzene derivatives in aqueous solution. J. Hazard. Mater. 2014, 274, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, M.; Fuertes, A.B.; Mokaya, R. High density hydrogen storage in superactivated carbons from hydrothermally carbonized renewable organic materials. Energy Environ. Sci. 2011, 4, 1400–1410. [Google Scholar] [CrossRef]
- Cheng, F.; Luo, H.; Hu, L.; Yu, B.; Luo, Z.; Fidalgo de Cortalezzi, M. Sludge carbonization and activation: From hazardous waste to functional materials for water treatment. J. Environ. Chem. Eng. 2016, 4, 4574–4586. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Li, L.; Shi, X.; Wang, Z. Preparation and analysis of activated carbon from sewage sludge and corn stalk. Adv. Powder Technol. 2016, 27, 684–691. [Google Scholar] [CrossRef]
- Gu, L.; Li, C.; Wen, H.; Zhou, P.; Zhang, D.; Zhu, N.; Tao, H. Facile synthesis of magnetic sludge-based carbons by using Electro-Fenton activation and its performance in dye degradation. Bioresour. Technol. 2017, 241, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Namazi, A.B.; Grant Allen, D.; Jia, C.Q. Microwave-assisted pyrolysis and activation of pulp mill sludge. Biomass Bioenergy 2015, 73, 217–224. [Google Scholar] [CrossRef]
- Antunes, E.; Schumann, J.; Brodie, G.; Jacob, M.V.; Schneider, P.A. Biochar produced from biosolids using a single-mode microwave: Characterisation and its potential for phosphorus removal. J. Environ. Manag. 2017, 196, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, G.S.; Wilhelm, M.; de Almeida Silva, T.C.; Rezwan, K.; Sampaio, C.H.; Lima, E.C.; de Souza, S.M.A.G.U. The use of design of experiments for the evaluation of the production of surface rich activated carbon from sewage sludge via microwave and conventional pyrolysis. Appl. Therm. Eng. 2016, 93, 590–597. [Google Scholar] [CrossRef]
- Puchana-Rosero, M.J.; Adebayo, M.A.; Lima, E.C.; Machado, F.M.; Thue, P.S.; Vaghetti, J.C.P.; Umpierres, C.S.; Gutterres, M. Microwave-assisted activated carbon obtained from the sludge of tannery-treatment effluent plant for removal of leather dyes. Colloids Surf. A Physicochem. Eng. Asp. 2016, 504, 105–115. [Google Scholar] [CrossRef]
- Kirschhöfer, F.; Sahin, O.; Becker, G.C.; Meffert, F.; Nusser, M.; Anderer, G.; Kusche, S.; Klaeusli, T.; Kruse, A.; Brenner-Weiss, G. Wastewater treatment—Dsorption of organic micropollutants on activated HTC-carbon derived from sewage sludge. Water Sci. Technol. 2016, 73, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Simões dos Reis, G.; Sampaio, C.H.; Lima, E.C.; Wilhelm, M. Preparation of novel adsorbents based on combinations of polysiloxanes and sewage sludge to remove pharmaceuticals from aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp. 2016, 497, 304–315. [Google Scholar] [CrossRef]
- Alvarez, J.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M. Preparation of adsorbents from sewage sludge pyrolytic char by carbon dioxide activation. Process Saf. Environ. Prot. 2016, 103, 76–86. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Chan, J.C.; Hameed, B.H.; Lim, L.L.P. Adsorption behavior of cadmium ions onto phosphoric acid-impregnated microwave-induced mesoporous activated carbon. J. Water Process Eng. 2016, 14, 60–70. [Google Scholar] [CrossRef]
- Li, L.; Quinlivan, P.A.; Knappe, D.R.U. Effects of activated carbon surface chemistry and pore structure on the adsorption of organic contaminants from aqueous solution. Carbon 2002, 40, 2085–2100. [Google Scholar] [CrossRef]
- Alam, M.Z.; Ameem, E.S.; Muyibi, S.A.; Kabbashi, N.A. The factors affecting the performance of activated carbon prepared from oil palm empty fruit bunches for adsorption of phenol. Chem. Eng. J. 2009, 155, 191–198. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Teng, H. Influence of mesopore volume and adsorbate size on adsorption capacities of activated carbons in aqueous solutions. Carbon 2000, 38, 863–869. [Google Scholar] [CrossRef]
- Ruiz, B.; Cabrita, I.; Mestre, A.S.; Parra, J.B.; Pires, J.; Carvalho, A.P.; Ania, C.O. Surface heterogeneity effects of activated carbons on the kinetics of paracetamol removal from aqueous solution. Appl. Surf. Sci. 2010, 256, 5171–5175. [Google Scholar] [CrossRef]
- Dąbrowski, A.; Podkościelny, P.; Hubicki, Z.; Barczak, M. Adsorption of phenolic compounds by activated carbon—A critical review. Chemosphere 2005, 58, 1049–1070. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Castilla, C.; Rivera-Utrilla, J. Carbon materials as adsorbents for the removal of pollutants from the aqueous phase. MRS Bull. 2001, 26, 890–894. [Google Scholar]
- Coughlin, R.W.; Ezra, F.S. Role of surface acidity in the adsorption of organic pollutants on the surface of carbon. Environ. Sci. Technol. 1968, 2, 291–297. [Google Scholar]
- Vidic, R.D.; Tessmer, C.H.; Uranowski, L.J. Impact of surface properties of activated carbons on oxidative coupling of phenolic compounds. Carbon 1997, 35, 1349–1359. [Google Scholar] [CrossRef]
- Franz, M.; Arafat, H.A.; Pinto, N.G. Effect of chemical surface heterogeneity on the adsorption mechanism of dissolved aromatics on activated carbon. Carbon 2000, 38, 1807–1819. [Google Scholar] [CrossRef]
- Yin, C.Y.; Aroua, M.K.; Daud, W.M.A.W. Review of modifications of activated carbon for enhancing contaminant uptakes from aqueous solutions. Sep. Purif. Technol. 2007, 52, 403–415. [Google Scholar] [CrossRef]
- Grant, T.M.; King, C.J. Mechanism of irreversible adsorption of phenolic compounds by activated carbons. Ind. Eng. Chem. Res. 1990, 29, 264–271. [Google Scholar] [CrossRef]
- Terzyk, A.P. Molecular properties and intermolecular forces—Factors balancing the effect of carbon surface chemistry in adsorption of organics from dilute aqueous solutions. J. Colloid Interface Sci. 2004, 275, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, F.; Cinquini, M.; Annuziata, R.; Siegel, J.S. Dominance of polar/.pi. over charge-transfer effects in stacked phenyl interactions. J. Am. Chem. Soc. 1993, 115, 5330–5331. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. Adsorption of phenolic compounds on low-cost adsorbents: A review. Adv. Colloid Interface Sci. 2008, 143, 48–67. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-H.; Juang, R.-S. Adsorption of phenol and its derivatives from water using synthetic resins and low-cost natural adsorbents: A review. J. Environ. Manag. 2009, 90, 1336–1349. [Google Scholar] [CrossRef] [PubMed]
- Aksu, Z.; Akpinar, D. Competitive biosorption of phenol and chromium (VI) from binary mixtures onto dried anaerobic activated sludge. Biochem. Eng. J. 2001, 7, 183–193. [Google Scholar] [CrossRef]
- Gao, R.; Wang, J. Effects of pH and temperature on isotherm parameters of chlorophenols biosorption to anaerobic granular sludge. J. Hazard. Mater. 2007, 145, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Mu’azu, N.D.; Al-Malack, M.H. Influence of some operating parameters on electro-oxidation of phenol using boron doped diamond anode and graphite cathode. J. Environ. Sci. Technol. 2012, 5, 460. [Google Scholar]
- Aksu, Z.; Yener, J. A comparative adsorption/biosorption study of mono-chlorinated phenols onto various sorbents. Waste Manag. 2001, 21, 695–702. [Google Scholar] [CrossRef]
- Kennedy, K.J.; Pham, T.T. Effect of anaerobic sludge source and condition on biosorption of PCP. Water Res. 1995, 29, 2360–2366. [Google Scholar] [CrossRef]
- Aksu, Z.; Yener, J. The usage of dried activated sludge and fly ash wastes in phenol biosorption/adsorption: Comparison with granular activated carbon. J. Environ. Sci. Health Part A 1999, 34, 1777–1796. [Google Scholar] [CrossRef]
- Aksu, Z.; Akpınar, D. Modelling of simultaneous biosorption of phenol and nickel(II) onto dried aerobic activated sludge. Sep. Purif. Technol. 2000, 21, 87–99. [Google Scholar] [CrossRef]
- Moura, M.N.; Martín, M.J.; Burguillo, F.J. A comparative study of the adsorption of humic acid, fulvic acid and phenol onto Bacillus subtilis and activated sludge. J. Hazard. Mater. 2007, 149, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Arslan, C.S.; Dursun, A.Y. Biosorption of phenol on dried activated sludge: Effect of temperature. Sep. Sci. Technol. 2008, 43, 3251–3268. [Google Scholar] [CrossRef]
- Sulaymon, A.H.; Abbood, D.W.; Ali, A.H. A comparative adsorption/biosorption for the removal of phenol and lead onto granular activated carbon and dried anaerobic sludge. Desalination Water Treat. 2012, 51, 2055–2067. [Google Scholar] [CrossRef]
- Thawornchaisit, U.; Pakulanon, K. Application of dried sewage sludge as phenol biosorbent. Bioresour. Technol. 2007, 98, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Aksu, Z.; Gönen, F. Biosorption of phenol by immobilized activated sludge in a continuous packed bed: Prediction of breakthrough curves. Process Biochem. 2004, 39, 599–613. [Google Scholar] [CrossRef]
- Bouki, C.; Dvorakova, M.; Diamadopoulos, E. Adsorption of nonylphenol on activated sludge biomass under aseptic conditions. CLEAN Soil Air Water 2010, 38, 516–520. [Google Scholar] [CrossRef]
- Kennedy, K.J.; Lu, J.; Mohn, W.W. Biosorption of chlorophenols to anaerobic granular sludge. Water Res. 1992, 26, 1085–1092. [Google Scholar] [CrossRef]
- Aksu, Z.; Yener, J. Investigation of the biosorption of phenol and monochlorinated phenols on the dried activated sludge. Process Biochem. 1998, 33, 649–655. [Google Scholar] [CrossRef]
- Hadi, P.; Barford, J.; McKay, G. Toxic heavy metal capture using a novel electronic waste-based material—Mechanism, modeling and comparison. Environ. Sci. Technol. 2013, 47, 8248–8255. [Google Scholar] [CrossRef] [PubMed]
- Hadi, P.; Ning, C.; Ouyang, W.; Lin, C.S.K.; Hui, C.-W.; McKay, G. Conversion of an aluminosilicate-based waste material to high-value efficient adsorbent. Chem. Eng. J. 2014, 256, 415–420. [Google Scholar] [CrossRef]
- Toles, C.A.; Marshall, W.E.; Johns, M.M. Surface functional groups on acid-activated nutshell carbons. Carbon 1999, 37, 1207–1214. [Google Scholar] [CrossRef]
- László, K. Adsorption from aqueous phenol and 2,3,4-trichlorophenol solutions on nanoporous carbon prepared from poly(ethylene terephthalate). In Adsorption and Nanostructure; Dékány, I., Ed.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 5–12. [Google Scholar]
- László, K.; Podkościelny, P.; Da̧browski, A. Heterogeneity of polymer-based active carbons in adsorption of aqueous solutions of phenol and 2,3,4-trichlorophenol. Langmuir 2003, 19, 5287–5294. [Google Scholar] [CrossRef]
- Wu, J.; Yu, H.-Q. Biosorption of phenol and chlorophenols from aqueous solutions by fungal mycelia. Process Biochem. 2006, 41, 44–49. [Google Scholar] [CrossRef]
- Shukla, A.; Zhang, Y.-H.; Dubey, P.; Margrave, J.L.; Shukla, S.S. The role of sawdust in the removal of unwanted materials from water. J. Hazard. Mater. 2002, 95, 137–152. [Google Scholar] [CrossRef]
- Li, W.-H.; Yue, Q.-Y.; Gao, B.-Y.; Ma, Z.-H.; Li, Y.-J.; Zhao, H.-X. Preparation and utilization of sludge-based activated carbon for the adsorption of dyes from aqueous solutions. Chem. Eng. J. 2011, 171, 320–327. [Google Scholar] [CrossRef]
- Ng, C.; Losso, J.N.; Marshall, W.E.; Rao, R.M. Freundlich adsorption isotherms of agricultural by-product-based powdered activated carbons in a geosmin–water system. Bioresour. Technol. 2002, 85, 131–135. [Google Scholar] [CrossRef]
- Song, X.; Zhang, Y.; Yan, C.; Jiang, W.; Chang, C. The Langmuir monolayer adsorption model of organic matter into effective pores in activated carbon. J. Colloid Interface Sci. 2013, 389, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Sips, R. On the structure of a catalyst surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Redlich, O.; Peterson, D.L. A useful adsorption isotherm. J. Phys. Chem. 1959, 63, 1024. [Google Scholar] [CrossRef]
- Wu, F.-C.; Liu, B.-L.; Wu, K.-T.; Tseng, R.-L. A new linear form analysis of Redlich–Peterson isotherm equation for the adsorptions of dyes. Chem. Eng. J. 2010, 162, 21–27. [Google Scholar] [CrossRef]
- Derylo-Marczewska, A.; Swiatkowski, A.; Biniak, S.; Walczyk, M. Effect of properties of chemically modified activated carbon and aromatic adsorbate molecule on adsorption from liquid phase. Colloids Surf. A Physicochem. Eng. Asp. 2008, 327, 1–8. [Google Scholar] [CrossRef]
- Przepiórski, J. Enhanced adsorption of phenol from water by ammonia-treated activated carbon. J. Hazard. Mater. 2006, 135, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Sorial, G.A. The effect of functional groups on oligomerization of phenolics on activated carbon. J. Hazard. Mater. 2007, 148, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Gupta, V.K.; Jain, S.; Suhas. Removal of chlorophenols using industrial wastes. Environ. Sci. Technol. 2004, 38, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Leong, M.L.; Lee, K.M.; Lai, S.O.; Ooi, B.S. Sludge characteristics and performances of the sequencing batch reactor at different influent phenol concentrations. Desalination 2011, 270, 181–187. [Google Scholar] [CrossRef]
- Moreno-Castilla, C. Adsorption of organic molecules from aqueous solutions on carbon materials. Carbon 2004, 42, 83–94. [Google Scholar] [CrossRef]
- Liu, Q.-S.; Zheng, T.; Wang, P.; Jiang, J.-P.; Li, N. Adsorption isotherm, kinetic and mechanism studies of some substituted phenols on activated carbon fibers. Chem. Eng. J. 2010, 157, 348–356. [Google Scholar] [CrossRef]
- Mattson, J.A.; Mark, H.B., Jr.; Malbin, M.D.; Weber, W.J., Jr.; Crittenden, J.C. Surface chemistry of active carbon: Specific adsorption of phenols. J. Colloid Interface Sci. 1969, 31, 116–130. [Google Scholar] [CrossRef]
- Mohammad, E. Removal of Organic Compounds from Water by Adsorption and Photocatalytic Oxidation; University de Toulouse: Toulouse, France, 2011. [Google Scholar]
- Liu, P.K.T.; Wagner, N.J. Thermal regeneration of granular activated carbon. Environ. Prog. 1985, 4, 136–141. [Google Scholar] [CrossRef]
- Leng, C.-C.; Pinto, N.G. An investigation of the mechanisms of chemical regeneration of activated carbon. Ind. Eng. Chem. Res. 1996, 35, 2024–2031. [Google Scholar] [CrossRef]
- Narbaitz, R.M.; Karimi-Jashni, A. Electrochemical reactivation of granular activated carbon: Impact of reactor configuration. Chem. Eng. J. 2012, 197, 414–423. [Google Scholar] [CrossRef]
- San Miguel, G.; Lambert, S.; Graham, N. Thermal regeneration of granular activated carbons using inert atmospheric conditions. Environ. Technol. 2002, 23, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Narbaitz, R.M.; Karimi-Jashni, A. Electrochemical regeneration of granular activated carbons loaded with phenol and natural organic matter. Environ. Technol. 2009, 30, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Garg, A. Primary sewage sludge-derived activated carbon: Characterisation and application in wastewater treatment. Clean Technol. Environ. Policy 2015, 17, 1619–1631. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Rivera-Utrilla, J.; Joly, J.P.; López-Ramón, M.V.; Ferro-García, M.A.; Carrasco-Marín, F. Thermal regeneration of an activated carbon exhausted with different substituted phenols. Carbon 1995, 33, 1417–1423. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Rivera-Utrilla, J.; López-Ramón, M.V.; carrasco-Marín, F. Adsorption of some substituted phenols on activated carbons from a bituminous coal. Carbon 1995, 33, 845–851. [Google Scholar] [CrossRef]
- Martin, R.J.; Ng, W.J. The repeated exhaustion and chemical regeneration of activated carbon. Water Res. 1987, 21, 961–965. [Google Scholar] [CrossRef]
- Chiang, P.C.; Chang, E.E.; Wu, J.S. Comparison of chemical and thermal regeneration of aromatic compounds on exhausted activated carbon. Water Sci. Technol. 1997, 35, 279–285. [Google Scholar]
- Ferro-Garcia, M.A.; Rivera-Utrilla, J.; Bautista-Toledo, I.; Moreno-Castilla, C. Chemical and thermal regeneration of an activated carbon saturated with chlorophenols. J. Chem. Technol. Biotechnol. 1996, 67, 183–189. [Google Scholar] [CrossRef]
- Fan, S.; Wang, Y.; Wang, Z.; Tang, J.; Tang, J.; Li, X. Removal of methylene blue from aqueous solution by sewage sludge-derived biochar: Adsorption kinetics, equilibrium, thermodynamics and mechanism. J. Environ. Chem. Eng. 2017, 5, 601–611. [Google Scholar] [CrossRef]
- Martin, R.J.; Ng, W.J. Chemical regeneration of exhausted activated carbon—I. Water Res. 1984, 18, 59–73. [Google Scholar] [CrossRef]
- García-Otón, M.; Montilla, F.; Lillo-Rodenas, M.A.; Morallón, E.; Vázquez, J.L. Electrochemical regeneration of activated carbon saturated with toluene. J. Appl. Electrochem. 2005, 35, 319–325. [Google Scholar] [CrossRef]
- Taiwo, E.; Adesina, A. Electrochemical regeneration of a native activated carbon. Chem. Biochem. Eng. Q. 2005, 19, 269–273. [Google Scholar]
- Wang, L.; Balasubramanian, N. Electrochemical regeneration of granular activated carbon saturated with organic compounds. Chem. Eng. J. 2009, 155, 763–768. [Google Scholar] [CrossRef]
- Berenguer, R.; Marco-Lozar, J.; Quijada, C.; Cazorla-Amorós, D.; Morallon, E. Electrochemical regeneration and porosity recovery of phenol-saturated granular activated carbon in an alkaline medium. Carbon 2010, 48, 2734–2745. [Google Scholar] [CrossRef]
- Devi, P.; Saroha, A.K. Improvement in performance of sludge-based adsorbents by controlling key parameters by activation/modification: A critical review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1704–1743. [Google Scholar] [CrossRef]



| Sludge Type | Proximate Analysis | Ultimate Analysis | Ref. | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SBET (m2 g−1) | Ash Content (wt % of Dried Matter) | Carbon (wt %) | Volatile Matter | Moisture Content | Particle Size | pH | C | H | N | S | O | Heavy Metals Cu Ni Pb Zn Hg | ||||||
| PMS | 20 | 34 | 600 um | - | 34 | 5 | - | 0.24 | 41 | [4] | ||||||||
| ADWWTPS | 22 | 57.7 | 8.5 | 9.3 | 0.5 | 24.0 | [24] | |||||||||||
| VLS | 2.9 | 22.0 | 39.4 | 5.6 | 6.4 | 0.9 | 19.8 | 306 | 76 | 64 | 634 | <5 | [25] | |||||
| LS (40% lime) | 4.8 | 57 | 27.9 | 3.5 | 2.9 | 0.9 | 18.7 | 201 | 32 | 49 | 320 | <5 | [25] | |||||
| POES | 34,000 mg/L | 125 um | 4.7 | 0.89 | 2.3 | [26] | ||||||||||||
| AGCWS | <3 | 23 | 48.7 | 0.1–0.25 | 6.9 | 48.7 | 7.5 | 9.4 | 0.6 | 10.8 | [9] | |||||||
| Municipal DRAWS | 20.4 | 41 | 65.9 | 10 mm | [27] | |||||||||||||
| Municipal DUSS | 32.6 | 6.8 | 60.6 | 82 | 3 mm | [28] | ||||||||||||
| PMS | 36.4 | 44 | 0.33 | 0.3 mm | [29] | |||||||||||||
| WWTP | 0.96 | 43.95 | 2.81 | 53.24 | 2.32 | 8.14 | [30] | |||||||||||
| PMS | 36.4 | 25 mm | 44.8 | 0.4 | [31] | |||||||||||||
| Sewage Treatment | Source of Sludge | Total Solids (%) |
|---|---|---|
| Centrifuge | Activated sludge | 14–20 |
| Anaerobic digester | 15–35 | |
| Aerobic digester | 8–10 | |
| Vacuum filter | Activated sludge | 12–18 |
| Anaerobic digester (mixture) | 17–23 | |
| Belt press | Activated sludge | 12–18 |
| Anaerobic digester (mixture) | 17–23 | |
| Anaerobic digester | 12–30 | |
| Aerobic digester | 12–25 | |
| Filter press | Activated sludge | 27–33 |
| Anaerobic digester (mixture) | 29–35 |
| Sludge Type | Carbonization Conditions | Physical Activation Conditions | Pre/Post Treatment | Textural Properties | Target Compound | Uptake (mg/g) | Ref. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) | t (h) | HR (min−1) | atm | T (°C) | t (h) | HR (min−1) | atm | SBET (m2/g) | Vmicro (cm3/g) | Vmeso (cm3/g) | Dp (nm) | pH | |||||
| VLS LS | 600 | 1 | Na Na na | N2 N2 | Not activated Not activated | 59 | 0.025 | 8.9 | Phenol Phenol | 170 | [25] | ||||||
| 1000 | 1 | 96 | 0.036 | 10.6 | 182 | ||||||||||||
| 600 | 1 | 33 | 0.01 | 12.4 | 161 | ||||||||||||
| 800 | 1 | 62 | 0.015 | 12.5 | 185 | ||||||||||||
| AGCWS | 300 | 0.5 | 10 | N2 | Not Activated | Washed, dried/washed | 10 | - | 7.6 | - | [39] | ||||||
| 450 | 20 | ||||||||||||||||
| 600 | 38 | ||||||||||||||||
| 750 | 44 | ||||||||||||||||
| AWWTPS | 500 Microwave heating, 980 W | 1 | - | N2 | Not Activated | Washed and dried/None | 641 | - | - | - | 7 | Hydroquinone | 1218.3 | [19] | |||
| 0.2 | - | N2 | 540 | 1202.1 | |||||||||||||
| AWWTPS AWWTPS + tyres (1:1) | 650 | 0.5 | 40 | na | Not activated | Washed and dried/None | 60 | 0.04 | 0.05 | Phenol | 9.8 | [51] | |||||
| 650 | 0.5 | 40 | na | Not activated | Washed and dried/None | 59 | 0.03 | 0.08 | Phenol | 10.1 | |||||||
| POES | 300 | 0.5 | air | 150 | 2 | - | H2O | Washed and Dried/None | Phenol | - | [26] | ||||||
| 500 | 0.5 | air | - | - | |||||||||||||
| 800 | 0.5 | air | - | 12.078 | |||||||||||||
| - | - | - | - | - | |||||||||||||
| FIS | 500 | 1 | Air | - | - | - | - | Dried/1 M HCl | 380 | - | - | - | 4-bromophenol 2-bromophenol 2,4-dibromophenol | 40.7 170.4 190.2 | [52] | ||
| DMADS DRAWS DSBS | 900 900 900 900 600 | 1 | 10 10 10 10 | N2 N2 N2 N2 N2 | - 838 - 838 875 | - 1.21 - 1.34 1 | - 0.7 g - 0.7 g 1.5 L | - Steam - Steam CO2 | Sterilized-dried/None Sterilized-dried/None Sterilized-dried/None | 125 155 180 265 90 | 0.05 0.06 0.07 0.11 0.03 | 0.11 0.08 | 4.4 4.5 2.7 3.5 2.5 | Phenol | 94 117.5 131 150 112 | [42] | |
| DMADS DRAWS | 1000 - 950 250/500/1000 - | 0.5 | 5 10 5 - | N2 N2 - | - 838 838 925 838 838 838 - 900 925 | - 1.21 1.21 1 1.34 1.4 1.21 - 1.67 1 | - 10 °C 10 °C 10 °C 10 °C - 10 °C 10 °C | - Steam Steam CO2 Steam Steam Steam - Steam CO2 | Sterilized-dried/None Sterlized-dried/HCL (0 & 1 pH) Sterilized-dried/None Sterilized-dried/soaked in RO-24 h Sterilized-dried/HCL(3%) Sterilized-dried/None Sterilized-dried/None Sterilized-dried/None | 153.4 179.3 na 169.1 268.9 497.4 269.11 8.1/12.1/150.1 214.4 227.8 | 8.9 7.6 8.1 6.1/7.3/9.8 10.1 8.4 | Phenol | 28.4% CWAO 62.7% 58.6% 65% 93% 62% 88/56/51 69.3 68.6 | [53] | |||
| DRAWS | 1000 | na | 10 | N2 | 838 | 1.33 | 0.7 g with nitrogen | steam | Sterilized with steam/washed | 265 | 0.11 | 0.17 | 3.5 | Phenol | 244.66 | [27] | |
| P-Chloro phenol | 216.2 | ||||||||||||||||
| p-nitro phenol | 235 | ||||||||||||||||
| DMADS | 950 | 0.5 | 10 | N2 | 838 | 1.21 | 10 °C | Steam | Sterilized-dried/None | 269.1 | - | - | - | 8.1 | Phenol | 0.65~(5 g/L) | [54] |
| o-Cresol | 1 | ||||||||||||||||
| o-chlorophenol | 0.82 | ||||||||||||||||
| p-nitrophenol | 0.06 | ||||||||||||||||
| AGCWS | None | 700/800 | 0.5 | 10 °C | CO2 | Washed/none | 11/20 | 0.01/0.02 | None | None | 4-chloro phenol | - | [9] | ||||
| 2 | 75/94 | 0.05/0.04 | 187/301.6 | ||||||||||||||
| 4 | 79/97 | 0.06/0.09 | 185.4/241.8 | ||||||||||||||
| 200/300/400 | 0.5 | 10 °C | Air | 7/13/15 | <0.01/0.01/0.03 | None | None | 4-chloro phenol | 31.2/170.8/145.8 | ||||||||
| 2 | 34/51/92 | 0.03/0.05/0.06 | 65.6/181.9/192.54 | ||||||||||||||
| 4 | 47/53/91 | 0.05/0.05/0.07 | 180.8/154.7/223.22 | ||||||||||||||
| Type of Sludge | Carbonisation Conditions | Chemical Activation Conditions | Pre/Post Treatment | Textural Properties | Target Compound | Uptake Capacity (mg/g) | Ref | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) | t (h) | HR (min−1) | atm | Reagents | T (°C) | t (h) | HR (min−1) | atm | SBET (m2/g) | Vmicro (cm3/g) | Vmeso (cm3/g) | Dp (nm) | pH | |||||
| ADDDS & coconut husk (1:2) | 500/600/700 | 2 | 15 | ZnCl2 (3 M) ZnCl2 (5 M) ZnCl2 (7 M) | 25 25 25 | 24 24 24 | Dried/Dried-carbonized-HCL | 448/648/425 | phenol | 5.2/5.2/4.0 | [58] | |||||||
| 600 | 2 | 10 | 750 | 6.7 | ||||||||||||||
| 500/600/700 | 2 | 15 | 725/648/525 | 5.2/5.9/4.4 | ||||||||||||||
| 600 | 2 | 10 | 867 | 5.9 | ||||||||||||||
| 500/600/700 | 2 | 15 | 660/700/550 | 4.9/5.7/4.3 | ||||||||||||||
| 600 | 2 | 10 | 690 | 7.0 | ||||||||||||||
| PMS | 800 | 2 | 20 °C | N2, 70 mL/min | ZnCl2:sludge = 3.5 | 85 | 8 | Na | Na | Dried/22 h light exposure-carbonisation- and HCl-dried | 1092 | 1.13 | 10 | 7 | Phenol | - | [4] | |
| AWWTPS | Not carbonised | H2SO4 (1:1) ZnCl2 (1:1) | 650 650 | 0.5 0.083 | 40 5 | NA | Dried/HCl | 216 472 | 0.09 0.10 | Phenol | 24.8 88.16 | [51] | ||||||
| PMS | 700 | 1 | 15 °C | N2 | ZnCl2 (2:1) | 80 | 8 | Na | Dried/HCl-dried | 316.32 | 0.4357 | 6.124 | phenol | 15.58 | [29] | |||
| PMS | 560 | 0.41 | 20 °C | N2 | ZnCl2 (0.9:1) | 80 | 6 | Dried/HCl-dried | 907.20 | 0.42 | 3.13 | 4.6 | Phenol 4-Nonyl phenol 2-chloro phenol | 370 296 325 | [31] | |||
| DS | 600 | 1 | - | - | ZnCl2 (40%) | RT | 24 | Na | HCl | 195 | 0.06 | 0.14 | 3.5 | phenol | 45.12 | [28] | ||
| DS | 500 | 1 | 20 °C | N2 | 0–2 M citric acid and 0.5 M ZnCl2 | RT | 24 | Dried/carbonisation-HCL-dried | 792.4 | 4-chloro phenol Phenol | 372.94 189.16 | [61] | ||||||
| AWWTPS | 625 | 0.5 | 40 °C | N2 | H2SO4 (1:1) | Na | 48 | NA | NA | Dried/carbonisation-HCL-dried | 390 | NA | 0.12/0.5 0.12 | Phenol (Indigo carmine + phenol) | 42.04/29.46 10.2 | [59] | ||
| DWWTPS | 650 | 1 | 10 °C | NA | 3 M H2SO4 (1:1) | Na | 48 | Dried/carbonised-HCl-Dried | 166.20 | Na | Na | 5.5 | 2-chlor phenol | 47.98 | [30] | |||
| WBS | H2SO4 | 700 | 0.5 | NA | NA | Dried/carbonised | 253 | 0.08 | Na | Na | Phenol | 10 | [24] | |||||
| AGCWS | Not carbonised | KOH (1:1) | 450/750 | 0.5 | 10 | N2 | Dried/HCl | 131/950 | <0.01/0.40 | 0.12/0.23 | 4-chloro phenol | 140.8/170.6 | [9] | |||||
| KOH (3:1) | 450/750 | 0.5 | 10 | N2 | 262/1832 | 0.01/0.75 | 0.16/0.36 | 146.54/265.08 | ||||||||||
| DMADS | Not carbonised | K2CO3 (1:1) | 800 | 1 | 18–20 | N2 | Dried/washed with water Dried/washed with HCL (5%) | 421.8 863.8 | 8.2 5.2 | Phenol | Oxidation 87.1% (5 g/L) 93.2% | [53] | ||||||
| DMADS | Not carbonised | K2CO3 (1:1) | 800 | 1 | 18-20 | N2 | Dried/washed with water | 421.8 | 8.2 | Phenol o-Cresol o-chlorophenol p-nitrophenol | Oxidation 87.1% (5 g/L) 0.88 0.83 0.06 | [54] | ||||||
| WWTPS | 1000 | 1 | 5 °C | N2, 50 mL/min | NaOH (1:1) | 500/600/800 | 2 | 5 | N2 | Carbonised-HCl/washed-dried | 319/346/307 | 0.438/0.465/0.403 | 17.2/12.3/14 | NA | phenol | -/96.15/- | [44] | |
| Type of Sludge | Drying Conditions | Diameter (mm) | Adsorbate | Temperature (°C) | pH | Model Used | Uptake Capacity (mg/g) | Ref |
|---|---|---|---|---|---|---|---|---|
| PMS | 60/24 h | 0.006 mm | o-Chlorophenol | 25 | 1 | Langmuir | 281.1 | [92] |
| p-Chlorophenol | 25 | 1 | 287.2 | |||||
| DAS | - | Phenol | 25 | 1.0 | Langmuir/Freundlich | 91.0 | [94] | |
| AAS | 60/24 h | - | Phenol | 1.0 | Langmuir/Freundlich | 180.9 | [95] | |
| WWTPS | HNO3 washed and rinse with 0.1 NaCl | Phenol | NA | 7 | None | 0.06 | [96] | |
| AWWTP | 60/24 h | NA | Phenol | 40 | 8 | Freundlich | 42.7 | [97] |
| AGS | Dried | NA | 4-Chlorophenol | 25 | 3.6 | Langmuir/Freundlich | 7.77 | [90] |
| AAS | 60/24 h | 0.775 | Phenol | 30 | NA | Langmuir | 90.5421 | [98] |
| Binary(phenol + Pb) | 30 | Langmuir | 30.7843 | |||||
| SSTP 5% (w/v) | 60/24 h | <0.1 mm | Phenol | NA | 6–8 | None | 17.3 from 100 ppm phenol | [99] |
| AS (50%) MR | NA | 1 | Phenol | NA | 1.0 | Breakthrough curves | 9.0 | [100] |
| ASB | 105/6 h | NA | Nonylphenol | 22 | NA | Freundlich | 90% removal from 4.15 mg/L | [101] |
| Type of Sludge | Activation Method | SBET | Phenolic Compound | Langmuir | Freundlich | Ref | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Qm (mg/g) | A (L/mg) | R2 | KF | n | R2 | |||||
| DASS | KOH (3:1) | 1832 | 4-Chlorophenol | 265.8 | 0.0156 | 0.994 | [9] | |||
| CO2 (800) | 94 | 4-Chlorophenol | 301 | 0.0014 | 0.972 | |||||
| Air (400) | 91 | 4-Chlorophenol | 22.96 | 0.00169 | 0.965 | |||||
| AWWTPS | H2SO4 (1:1) | 390 | Phenol | 42.04 | 0.02 | 0.969 | 6.33 | 3.51 | 0.9748 | [59] |
| WWTPS | NaOH (1:1 | 346 | Phenol | 96.154 | 0.128 | 0.979 | 18.065 | 2.48 | 0.989 | [44] |
| AWWTPS | ZnCL2 (40%) | 195.28 | Phenol | 18.3 | 0.114 | - | [120] | |||
| 2-Chlorophenol | 51.8 | 0.118 | - | |||||||
| 4-Chlorophenol | 58.1 | 0.129 | - | |||||||
| 2,4-Dichlorophenol | 137.0 | 0.162 | - | |||||||
| DWWTPS | 3 M H2SO4 (1:1) | 166.20 | 2-Chlorophenol | 47.977 | 0.485 | 0.918 | 18 | 4.18 | 0.977 | [30] |
| DWWTPS | 3 M H2SO4 (1:1) | 162.2 | Phenol | 26.16 | 0.109 | 0.927 | 6.059 | 3.02 | 0.996 | [60] |
| DRAWS | 838-steam | 265 | Phenol | 244.4 | 0.0007 | 0.972 | 0.009 | 0.469 | 0.992 | [27] |
| p-Chlorophenol | 216.2 | 0.00967 | 0.990 | 0.004 | 0.144 | 0.879 | ||||
| p-Nitrophenol | 235.5 | 0.00095 | 0.559 | 0.841 | 1.354 | 0.925 | ||||
| p-Hydroxybenzoic acid | 150.4 | 0.00095 | 0.175 | 0.031 | 0.741 | 0.889 | ||||
| PMS | ZnCL2 (0.9:1) | 907.20 | Phenol | 370.4 | 0.008 | 0.988 | 9.897 | 1.688 | 0.957 | [31] |
| 4-Nitrophenol | 296.1 | 0.0631 | 0.993 | 53.75 | 2.935 | 0.960 | ||||
| 2-Chlorophenol | 325.1 | 0.0249 | 0.994 | 26.58 | 2.144 | 0.944 | ||||
| CFS | N2 (750) | 44 | 4-Chlorophenol | 37.88 | 0.004 | 0.992 | 0.0012 | 2.027 | 0.968 | [39] |
| PMS | ZnCL2 (1:3.5) | 1092 | Phenol | None | None | None | 0.44 | 1.149 | NA | [4] |
| DUSS | ZnCL2 (40%) | 195 | Phenol(W) | 45.12 | 38.8 | 0.665 | 0.044 | 1.26 | 0.978 | [28] |
| Phenol(C) | 49.25 | 0.402 | 0.675 | 0.143 | 1.40 | 0.667 | ||||
| PMS | ZnCL2 (2:1) | 316.32 | Phenol | 15.585 | 1.0185 | 0.962 | 7.3781 | 3.534 | 0.996 | [29] |
| 44.4 (LF) | 0.013 (LF) | 0.998 (LF) | ||||||||
| VLS | 600 N2 | 59 | Phenol | 170 | 0.0022 | 0.975 | 4.9 | 1.29 | 0.961 | [25] |
| 1000 N2 | 96 | 182 | 0.0051 | 0.988 | 0.2 | 0.617 | 0.936 | |||
| LS | 600 N2 | 33 | Phenol | 161 | 0.0032 | 0.862 | 0.6 | 0.75 | 0.888 | [25] |
| 800 N2 | 62 | 185 | 0.0034 | 0.897 | 0.5 | 0.74 | 0.826 | |||
| POES | 800(Air) | NA | Phenol | 12.078 | 0.069 | 0.957 | 2.048 | 2.79 | 0.999 | [26] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu’azu, N.D.; Jarrah, N.; Zubair, M.; Alagha, O. Removal of Phenolic Compounds from Water Using Sewage Sludge-Based Activated Carbon Adsorption: A Review. Int. J. Environ. Res. Public Health 2017, 14, 1094. https://doi.org/10.3390/ijerph14101094
Mu’azu ND, Jarrah N, Zubair M, Alagha O. Removal of Phenolic Compounds from Water Using Sewage Sludge-Based Activated Carbon Adsorption: A Review. International Journal of Environmental Research and Public Health. 2017; 14(10):1094. https://doi.org/10.3390/ijerph14101094
Chicago/Turabian StyleMu’azu, Nuhu Dalhat, Nabeel Jarrah, Mukarram Zubair, and Omar Alagha. 2017. "Removal of Phenolic Compounds from Water Using Sewage Sludge-Based Activated Carbon Adsorption: A Review" International Journal of Environmental Research and Public Health 14, no. 10: 1094. https://doi.org/10.3390/ijerph14101094