The Current Status of the Disease Caused by Enterovirus 71 Infections: Epidemiology, Pathogenesis, Molecular Epidemiology, and Vaccine Development
Abstract
:1. Introduction
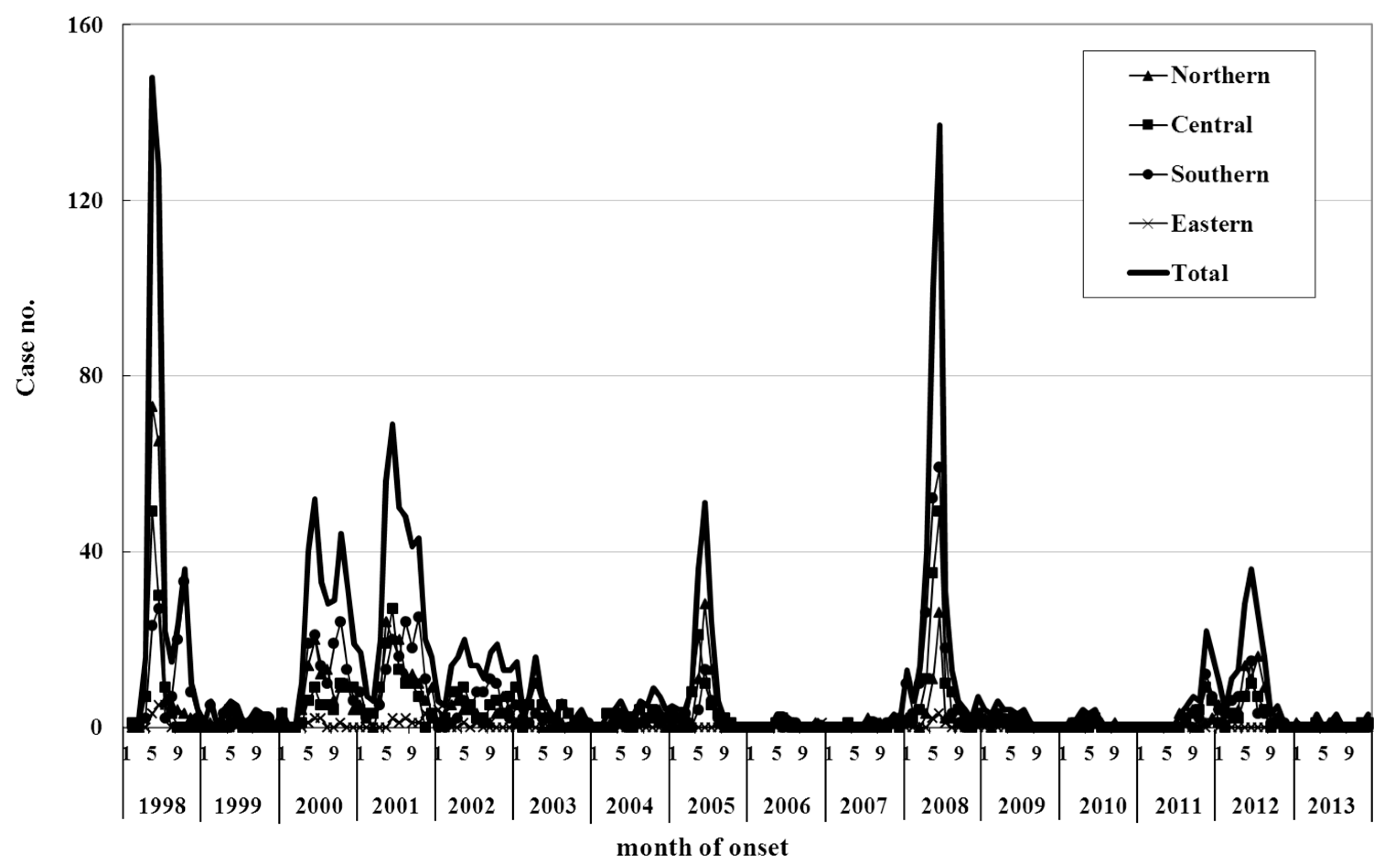
2. Epidemiology
3. Pathogenesis
4. Molecular Epidemiology
5. Vaccine Development
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Pallansch, M.A.; Cherste, M.A.; Whitton, J.L. Enterovirus: Polioviruses, coxsackieviruses, echoviruses and newer enteroviruses. In Field Virology, 6th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; pp. 490–530. [Google Scholar]
- Adams, M.J.; Lefkowitz, E.J.; King, A.M.; Harrach, B.; Harrison, R.L.; Knowles, N.J.; Kropinski, A.M.; Krupovic, M.; Kuhn, J.H.; Mushegian, A.R.; et al. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2016). Arch. Virol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.T.; Chang, H.L.; Wang, S.T.; Cheng, Y.T.; Yang, J.Y. Epidemiologic features of hand-foot-mouth disease and herpangina caused by enterovirus 71 in Taiwan, 1998–2005. Pediatrics 2007, 120, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, N.J.; Lennette, E.H.; Ho, H.H. An apparently new enterovirus isolated from patients with disease of the central nervous system. J. Infect. Dis. 1974, 129, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Alexaande, J.P., Jr.; Baden, L.; Pallansch, M.A.; Anderson, L.J. Enterovirus 71 infection and neurologic disease—United States, 1977–1991. J. Infect. Dis. 1994, 169, 905–908. [Google Scholar] [CrossRef]
- Nagy, G.; Takátsy, S.; Kukán, E.; Mihály, I.; Dömök, I. Virological diagnosis of enterovirus type 71 infections: Experiences gained during an epidemic of acute CNS disease in Hungary in 1978. Arch. Virol. 1982, 71, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Cardosa, M.J.; Krishnan, S.; Tio, P.H.; Perera, D.; Wong, S.C. Isolation of subgenus B adenovirus during a fatal outbreak of enterovirus 71-associated hand, foot, and mouth disease in Sibu, Sarawak. Lancet 1999, 354, 987–991. [Google Scholar] [CrossRef]
- Chan, K.; Goh, K.; Chong, C.; Teo, E.; Lau, G.; Ling, A. Epidemic hand, foot and mouth disease caused by human enterovirus 71, Singapore. Emerg. Infect. Dis. 2003, 9, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.G.; Parashar, U.D.; Lye, M.S.; Ong, F.G.; Zaki, S.R.; Alexander, J.P.; Ho, K.K.; Han, L.L.; Pallansch, M.A.; Suleiman, A.B.; et al. Deaths of children during an outbreak of hand, foot, and mouth disease in Sarawak, Malaysia: Clinical and pathological characteristics of the disease. Clin. Infect. Dis. 2000, 31, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.; Chen, E.R.; Hsu, K.H.; Twu, S.J.; Chen, K.T.; Tsai, S.F.; Wang, J.R.; Shih, S.R. An epidemic of enterovirus 71 infection in Taiwan. N. Engl. J. Med. 1999, 341, 929–935. [Google Scholar] [CrossRef] [PubMed]
- McMinn, P.; Stratov, I.; Nagarajan, L.; Davis, S. Neurological manifestations of enterovirus 71 infection in children during an outbreak of hand, foot, and mouth disease in Western Australia. Clin. Infect. Dis. 2001, 32, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Tu, P.V.; Thao, N.T.; Perera, D.; Huu, T.K.; Tien, N.T.; Thuong, T.C.; How, O.M.; Cardosa, M.J.; McMinn, P.C. Epidemiologic and virologic investigation of hand, foot, and mouth disease, southern Vietnam, 2005. Emerg. Infect. Dis. 2007, 13, 1733–1741. [Google Scholar] [PubMed]
- Zhang, Y.; Tan, X.J.; Wang, H.Y.; Yan, D.M.; Zhu, S.L.; Wang, D.Y.; Ji, F.; Wang, X.J.; Gao, Y.J.; Chen, L.; et al. An outbreak of hand, foot, and mouth disease associated with subgenotype C4 of human enterovirus 71 in Shandong, China. J. Clin. Virol. 2009, 44, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, H.; Shimizu, Y.; Takeuchi, Y.; Ishiko, H.; Takada, H. Outbreak of severe neurologic involvement associated with Enterovirus 71 infection. Pediatr. Neurol. 1999, 20, 17–23. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Z.; Yang, W.; Ren, J.; Tan, X.; Wang, Y.; Mao, N.; Xu, S.; Zhu, S.; Cui, A.; et al. An emerging recombinant human enterovirus 71 responsible for the 2008 outbreak of hand foot and mouth disease in Fuyang City of China. Virol. J. 2010, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Cardosa, M.J.; Perera, D.; Brown, B.A.; Cheon, D.; Chan, H.M.; Chan, K.P.; Cho, H.; McMinn, P. Molecular epidemiology of human enterovirus 71 strains and recent outbreaks in the Asia-Pacific region: Comparative analysis of the VP1 and VP4 genes. Emerg. Infect. Dis. 2003, 9, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control, Taiwan. Notifiable Communicable Diseases. Available online: http://teb.cdc.gov.tw/main_e/main.asp?kind=B05&fm=1 (accessed on 15 August 2015).
- Blomberg, J.; Lycke, E.; Ahlfors, K.; Johnsson, T.; Wolontis, S.; von Zeipel, G. New enterovirus type associated with epidemic of aseptic meningitis and-or hand, foot, and mouth disease. Lancet 1974, 2, 112. [Google Scholar] [CrossRef]
- Kennett, M.L.; Birch, C.J.; Lewis, F.A.; Yung, A.P.; Locarnini, S.A.; Gust, I.D. Enterovirus type 71 in Melbourne. Bull. World Health Organ. 1974, 51, 609–615. [Google Scholar] [PubMed]
- Hagiwara, A.; Tagaya, I.; Yoneyama, T. Epidemic of hand, foot and mouth disease associated with enterovirus 71 infection. Intervirology 1978, 9, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Tagaya, I.; Takayama, R.; Hagiwara, A. A large-scale epidemic of hand, foot and mouth disease associated with enterovirus 71 infection in Japan in 1978. Jpn. J. Med. Sci. Biol. 1981, 34, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Samuda, G.M.; Chang, W.K.; Yeug, C.Y.; Tang, P.S. Monoplegia caused by enterovirus 71: An outbreak in Hong Kong. Pediatr. Infect. Dis. J. 1987, 6, 206–208. [Google Scholar] [CrossRef] [PubMed]
- McMinn, P.; Lindsay, K.; Perera, D.; Chan, H.M.; Chan, K.P.; Cardosa, M.J. Phylogenetic analysis of enterovirus 71 strains isolated during linked epidemics in Malaysia, Singapore, and Western Australia. J. Virol. 2001, 75, 7732–7738. [Google Scholar] [CrossRef] [PubMed]
- Lum, L.C.; Wong, K.T.; Lam, S.K.; Chua, K.B.; Goh, A.Y.; Lim, W.L.; Ong, B.B.; Paul, G.; AbuBakar, S.; Lambert, M. Fatal enterovirus 71 encephalomyelitis. J. Pediatr. 1998, 133, 795–798. [Google Scholar] [CrossRef]
- Mizuta, K.; Abiko, C.; Murata, T.; Matsuzaki, Y.; Itagaki, T.; Sanjoh, K.; Sakamoto, M.; Hongo, S.; Murayama, S.; Hayasaka, K. Frequent importation of enterovirus 71 from surrounding countries into the local community of Yamagata, Japan, between 1998 and 2003. J. Clin. Microbiol. 2005, 43, 6171–6175. [Google Scholar] [CrossRef] [PubMed]
- Podin, Y.; Gias, E.L.; Ong, F.; Leong, Y.W.; Yee, S.F.; Yusof, M.A.; Perera, D.; Teo, B.; Wee, T.Y.; Yao, S.C.; et al. Sentinel surveillance for human enterovirus 71 in Sarawak, Malaysia: Lessons from the first 7 years. BMC Public Health 2006, 6, 180. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Utama, A.; Onnimala, N.; Chen, L.; Zhang, L.-B.; Ma, Y.-J.; Pongsuwanna, Y.; Miyamura, T. Molecular epidemiology of enterovirus 71 infection in the Western Pacific Region. Pediatr. Int. 2004, 46, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Ryu, W.S.; Kang, B.; Hong, J.; Hwang, S.; Kim, A.; Kim, J.; Cheon, D.S. Enterovirus 71 infection with central nervous system involvement, South Korea. Emerg. Infect. Dis. 2010, 16, 1764–1766. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.A.; Oberste, M.S.; Alexander, J.P., Jr.; Kennett, M.L.; Pallansch, M.A. Molecular epidemiology and evolution of enterovirus 71 strains isolated from 1970 to 1998. J. Virol. 1999, 73, 9969–9975. [Google Scholar] [PubMed]
- Lin, K.H.; Hwang, K.P.; Ke, G.M.; Wang, C.F.; Ke, L.Y.; Hsu, Y.T.; Tung, Y.C.; Chu, P.Y.; Chen, B.H.; Chen, H.L.; et al. Evolution of EV71 genogroup in Taiwan from 1998 to 2005: An emerging of subgenogroup C4 of EV71. J. Med. Virol. 2006, 78, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, Y.; Yang, H.; Zhu, J.; Xu, X.; Dong, J.; Zhu, Y.; Jin, Q. Genetic characteristics of human enterovirus 71 and coxsackievirus A16 circulating from 1999 to 2004 in Shenzhen, People’s Republic of China. J. Clin. Microbiol. 2005, 43, 3835–3839. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, M.; Kawasaki, Y.; Sato, M.; Honzumi, K.; Kato, A.; Hiroshima, T.; Ishiko, H.; Suzuki, H. Genetic diversity of enterovirus 71 associated with hand, foot and mouth disease epidemics in Japan from 1983 to 2003. Pediatr. Infect. Dis. J. 2006, 25, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Chow, V.T.; Chan, K.P.; Ling, A.E.; Poh, C.L. RT-PCR, nucleotide, amino acid and phylogenetic analyses of enterovirus type 71 strains from Asia. J. Virol. Methods 2000, 88, 193–204. [Google Scholar] [CrossRef]
- Wang, J.R.; Tuan, Y.C.; Tsai, H.P.; Yan, J.J.; Liu, C.C.; Su, I.J. Change of major genotype of enterovirus 71 in outbreaks of hand-foot-and-mouth disease in Taiwan between 1998 and 2000. J. Clin. Microbiol. 2002, 40, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Utama, A.; Yoshii, K.; Yoshida, H.; Yoneyama, T.; Sinniah, M.; Yusof, M.A.; Okuno, Y.; Okabe, N.; Shih, S.R.; et al. Enterovirus 71 from fatal and nonfatal cases of hand, foot and mouth disease epidemics in Malaysia, Japan and Taiwan in 1997–1998. Jpn. J. Infect. Dis. 1999, 52, 12–15. [Google Scholar] [PubMed]
- Herrero, L.J.; Lee, C.S.; Hurrebrink, R.J.; Chu, B.H.; Chua, K.B.; McMinn, P.C. Molecular epidemiology of enterovirus 71 in peninsular Malaysia, 1997–2000. Arch. Virol. 2003, 148, 1369–1385. [Google Scholar] [CrossRef] [PubMed]
- Kung, S.H.; Wang, S.F.; Huang, C.W.; Hsu, C.C.; Liu, H.F.; Yang, J.Y. Genetic and antigenic analyses of enterovirous 71 isolates in Taiwan during 1998–2005. Clin. Microbiol. Infect. 2007, 13, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.D.; Yergolkar, P.; Shankarappa, K.S. Antigenic diversity of enteroviruses associated with nonpolio acute flaccid paralysis, India, 2007–2009. Emerg. Infect. Dis. 2012, 18, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Solomon, T.; Lewthwaite, P.; Perera, D.; Cardosa, M.J.; McMinn, P.; Ooi, M.H. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect. Dis. 2010, 10, 778–790. [Google Scholar] [CrossRef]
- Bessaud, M.; Razafindratsimandresy, R.; Nougairède, A.; Joffret, M.L.; Deshpande, J.M.; Dubot-Pérès, A.; Héraud, J.M.; de Lamballerie, X.; Delpeyroux, F.; Bailly, J.L. Molecular comparison and evolutionary analyses of VP1 nucleotide sequences of new African human enterovirus 71 isolates reveal a wide genetic diversity. PLoS ONE 2014, 9, e90624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.S.; Tseng, F.C.; Wang, J.R.; Chi, C.Y.; Chong, P.; Su, I.J. Challenges to licensure of enterovirus 71 vaccines. PLoS Negl. Trop. Dis. 2012, 6, e1737. [Google Scholar] [CrossRef] [PubMed]
- Chia, M.Y.; Chiang, P.S.; Chung, W.Y.; Luo, S.T.; Lee, M.S. Epidemiology of enterovirus 71 infections in Taiwan. Pediatr. Neonatol. 2014, 55, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yeo, A.; Phoon, M.C.; Tan, E.L.; Poh, C.L.; Quak, S.H.; Chow, V.T. The largest outbreak of hand; foot and mouth disease in Singapore in 2008: The role of enterovirus 71 and coxsackievirus A strains. Int. J. Infect. Dis. 2010, 14, e1076–e1081. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.W.; Kiang, D.; Smith, D.J.; Wang, J.R. Evolution of re-emergent virus and its impact on enterovirus 71 epidemics. Exp. Biol. Med. (Maywood) 2011, 236, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.P.; Lin, T.L.; Lin, T.H.; Wu, H.S. Antigenic and genetic diversity of human enterovirus 71 from 2009 to 2012, Taiwan. PLoS ONE 2013, 8, e80942. [Google Scholar] [CrossRef] [PubMed]
- Khanh, T.H.; Sabanathan, S.; Thanh, T.T.; Thoa le, P.K.; Thuong, T.C.; Hang, V.T.; Farrar, J.; Hien, T.T.; Chau, N.V.; van Doorn, H.R. Enterovirus 71-associated hand, foot, and mouth disease, Southern Vietnam, 2011. Emerg. Infect. Dis. 2012, 18, 2002–2005. [Google Scholar] [CrossRef] [PubMed]
- AbuBakar, S.; Sam, I.C.; Yusof, J.; Lim, M.K.; Misbah, S.; MatRahim, N.; Hooi, P.S. Enterovirus 71 outbreak, Brunei. Emerg. Infect. Dis. 2009, 15, 79–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wang, J.; Guo, W.; Wang, H.; Zhu, S.; Wang, D.; Bai, R.; Li, X.; Yan, D.; Wang, H.; et al. Emergence and transmission pathways of rapidly evolving evolutionary branch C4a strains of human enterovirus 71 in the Central Plain of China. PLoS ONE 2011, 6, e27895. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.S.; Zhao, N.; Pan, H.; Wang, C.M.; Wu, B.; Zhang, H.M.; He, H.X.; Liu, D.; Amer, S.; Liu, S.L. Patterns of polymorphism and divergence in the VP1 gene of enterovirus 71 circulating in the Asia-Pacific region between 1994 and 2013. J. Virol. Methods 2013, 193, 713–728. [Google Scholar] [CrossRef] [PubMed]
- Chumakov, M.; Voroshilova, M.; Shindarov, L.; Lavrova, I.; Gracheva, L.; Koroleva, G.; Vasilenko, S.; Brodvarova, I.; Nikolova, M.; Gyurova, S.; et al. Enterovirus 71 isolated from cases of epidemic poliomyelitis-like disease in Bulgaria. Arch. Virol. 1979, 60, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Merovitz, L.; Demers, A.M.; Newby, D.; McDonald, J. Enterovirus 71 infections at a Canadian center. Pediatr. Infect. Dis. J. 2000, 19, 755–757. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Vélez, C.M.; Anderson, M.S.; Robinson, C.C.; McFarland, E.J.; Nix, W.A.; Pallansch, M.A.; Oberste, M.S.; Glodé, M.P. Outbreak of neurologic enterovirus type 71 disease: A diagnostic challenge. Clin. Infect. Dis. 2007, 45, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Ortner, B.; Huang, C.W.; Schmid, D.; Mutz, I.; Wewalka, G.; Allerberger, F.; Yang, J.Y.; Huemer, H.P. Epidemiology of enterovirus types causing neurological disease in Austria 1999–2007: Detection of clusters of echovirus 30 and enterovirus 71 and analysis of prevalent genotypes. J. Med. Virol. 2009, 81, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Bible, J.M.; Iturriza-Gomara, M.; Megson, B.; Brown, D.; Pantelidis, P.; Earl, P.; Bendig, J.; Tong, C.Y. Molecular epidemiology of human enterovirus 71 in the United Kingdom from 1998 to 2006. J. Clin. Microbiol. 2008, 46, 3192–3200. [Google Scholar] [CrossRef] [PubMed]
- Van der Sanden, S.; Koopmans, M.; Uslu, G.; Van der Avoort, H. Dutch Working Group for Clinical Virology. Epidemiology of enterovirus 71 in The Netherlands, 1963 to 2008. J. Clin. Microbiol. 2009, 47, 2826–2833. [Google Scholar] [CrossRef] [PubMed]
- Witsø, E.; Palacios, G.; Rønningen, K.S.; Cinek, O.; Janowitz, D.; Rewers, M.; Grinde, B.; Lipkin, W.I. Asymptomatic circulation of HEV71 in Norway. Virus Res. 2007, 123, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Welch, J.; Maclaran, K.; Jordan, T.; Simmonds, P. Frequency, viral loads, and serotype identification of enterovirus infections in Scottish blood donors. Transfusion 2003, 43, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Iturriza-Gomara, M.; Musoke, R.; Palakudy, T.; D’Agostino, A.; Gray, J. An epidemic of enterovirus 71 infection among HIV-1-infected orphans in Nairobi. AIDS 2004, 18, 1968–1970. [Google Scholar] [CrossRef] [PubMed]
- Schuffenecker, I.; Mirand, A.; Antona, D.; Henquell, C.; Chomel, J.J.; Archimbaud, C.; Billaud, G.; Peigue-Lafeuille, H.; Lina, B.; Bailly, J.L. Epidemiology of human enterovirus 71 infections in France, 2000–2009. J. Clin. Virol. 2011, 50, 50–56. [Google Scholar] [CrossRef] [PubMed]
- McWilliam Leitch, E.C.; Cabrerizo, M.; Cardosa, J.; Harvala, H.; Ivanova, O.E.; Koike, S.; Kroes, A.C.; Lukashev, A.; Perera, D.; Roivainen, M.; et al. The association of recombination events in the founding and emergence of subgenogroup evolutionary lineages of human enterovirus 71. J. Virol. 2012, 86, 2676–2685. [Google Scholar] [CrossRef] [PubMed]
- Hassel, C.; Mirand, A.; Lukashev, A.; TerletskaiaLadwig, E.; Farkas, A.; Schuffenecker, I.; Diedrich, S.; Huemer, H.P.; Archimbaud, C.; Peigue-Lafeuille, H.; et al. Transmission patterns of human enterovirus 71 to, from and among European countries, 2003 to 2013. Eurosurveillance 2015, 20, 30005. [Google Scholar] [CrossRef] [PubMed]
- Diedrich, S.; Weinbrecht, A.; Schreier, E. Seroprevalence and molecular epidemiology of enterovirus 71 in Germany. Arch. Virol. 2009, 154, 1139–1142. [Google Scholar] [CrossRef] [PubMed]
- Urashima, M.; Shindo, N.; Okabe, N. Seasonal models of herpangina and hand-foot-mouth disease to simulate annual fluctuations in urban warming in Tokyo. Jpn. J. Infect. Dis. 2003, 56, 48–53. [Google Scholar] [PubMed]
- Park, S.K.; Park, B.; Ki, M.; Kim, H.; Lee, K.; Jung, C.; Sohn, Y.M.; Choi, S.M.; Kim, D.K.; Lee, D.S.; et al. Transmission of seasonal outbreak of childhood enteroviral aseptic meningitis and hand-foot-mouth disease. J. Korean Med. Sci. 2010, 25, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.; Lam, T.; Wong, C.; Chuang, S.K. Is hand, foot and mouth disease associated with meteorological parameters? Epidemiol. Infect. 2010, 138, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Ooi, M.H.; Wong, S.C.; Podin, Y.; Akin, W.; del Sel, S.; Mohan, A.; Chieng, C.H.; Perera, D.; Clear, D.; Wong, D.; et al. Human enterovirus 71 disease in Sarawak, Malaysia: A prospective clinical, virological, and molecular epidemiological study. Clin. Infect. Dis. 2007, 44, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Dowell, S.F. Seasonal variation in host susceptibility and cycles of certain infectious diseases. Emerg. Infect. Dis. 2001, 7, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.Y.; Tsao, K.C.; Hsia, S.H.; Shih, S.R.; Huang, C.G.; Chan, W.K.; Hsu, K.H.; Fang, T.Y.; Huang, Y.C.; Lin, T.Y. Transmission and clinical features of enterovirus 71 infections in household contacts in Taiwan. JAMA 2004, 291, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Liao, Q.; Viboud, C.; Zhang, J.; Sun, J.; Wu, J.T.; Chang, Z.; Liu, F.; Fang, V.J.; Zheng, Y.; et al. Hand, foot, and mouth disease in China, 2008–2012: An epidemiological study. Lancet Infect. Dis. 2014, 14, 308–318. [Google Scholar] [CrossRef]
- Chang, H.L.; Chio, C.P.; Su, H.J.; Liao, C.M.; Lin, C.Y.; Shau, W.Y.; Chi, Y.C.; Cheng, Y.T.; Chou, Y.L.; Li, C.Y.; et al. The association between enterovirus 71 infections and meteorological parameters in Taiwan. PLoS ONE 2012, 7, e46845. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Sumi, A.; Toyoda, S.; Hu, Q.; Zhou, D.; Mise, K.; Zhao, J.; Kobayashi, N. Time series analysis of reported cases of hand, foot, and mouth disease from 2010 to 2013 in Wuhan, China. BMC Infect. Dis. 2015, 15, 495. [Google Scholar] [CrossRef] [PubMed]
- Gui, J.; Liu, Z.; Zhang, T.; Hua, Q.; Jiang, Z.; Chen, B.; Gu, H.; Lv, H.; Dong, C. Epidemiological characteristics and spatial-temporal clusters of hand, foot, and mouth disease in Zhejiang Province, China, 2008–2012. PLoS ONE 2015, 10, e0139109. [Google Scholar] [CrossRef] [PubMed]
- Ruan, F.; Yang, T.; Ma, H.; Jin, Y.; Song, S.; Fontaine, R.E.; Zhu, B.P. Risk factors for hand, foot, and mouth disease and herpangina and the preventative effect of hand-washing. Pediatrics 2011, 127, e898–e904. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Liu, C.C.; Chang, Y.C.; Chen, C.Y.; Wang, S.T.; Yeh, T.F. Neurologic complications in children with enterovirus 71 infection. N. Engl. J. Med. 1999, 341, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Prager, P.; Nolan, M.; Andrews, I.; Williams, G. Neurogenic pulmonary edema in enterovirus 71 encephalitis is not uniformly fatal but causes severe morbidity in survivors. Pediatr. Crit. Care Med. 2003, 4, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Dolin, R. Enterovirus 71—Emerging infections and emerging questions. N. Engl. J. Med. 1999, 341, 984–985. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.Y.; Huang, Y.C.; Yen, T.Y.; Hsia, S.H.; Hsieh, Y.C.; Li, C.C.; Chang, L.Y.; Huang, L.M. The correlation between the presence of viremia and clinical severity in patients with enterovirus 71 infection: A multi-center cohort study. BMC Infect. Dis. 2014, 14, 417. [Google Scholar] [CrossRef] [PubMed]
- Kung, C.M.; King, C.C.; Lee, C.N.; Huang, L.M.; Lee, P.I.; Kao, C.L. Differences in replication capacity between enterovirus 71 isolates obtained from patients with encephalitis and those obtained from patients with herpangina in Taiwan. J. Med. Virol. 2006, 79, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Chang, L.Y.; Hsia, S.H.; Huang, Y.C.; Chiu, C.H.; Hsueh, C.; Shih, S.R.; Liu, C.C.; Wu, M.H. The 1998 enterovirus 71 outbreak in Taiwan: Pathogenesis and management. Clin. Infect. Dis. 2002, 34, s52–s57. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.Y.; Lin, T.Y.; Hsu, K.H.; Huang, Y.C.; Lin, K.L.; Hsueh, C.; Shih, S.R.; Ning, H.C.; Hwang, M.S.; Wang, H.S.; et al. Clinical features and risk factors of pulmonary oedema after enterovirus 71-related hand, foot, and mouth disease. Lancet 1999, 354, 1682–1686. [Google Scholar] [CrossRef]
- Teoh, H.L.; Mohammad, S.S.; Britton, P.N.; Kandula, T.; Lorentzos, M.S.; Booy, R.; Jones, C.A.; Rawlinson, W.; Ramachandran, V.; Rodriguez, M.L.; et al. Clinical characteristics and functional motor outcomes of enterovirus 71 neurological disease in children. JAMA Neurol. 2016, 73, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Alvarez, M.; Bessudo, L.; Sabin, A.B. Paralytic syndromes associated with non-inflammatory cytoplasmic or nuclear neuropathology: Acute paralytic disease in Mexican children, neuropathologically distinguishable from Landry-Guillain-Barré syndrome. JAMA 1969, 207, 1481–1492. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S.; Hix, L.; Sigal, L.J.; Andino, R. Poliovirus pathogenesis in a new poliovirus receptor transgenic mouse model: Age-dependent paralysis and a mucosal route of infection. J. Gen. Virol. 2002, 83, 1707–1720. [Google Scholar] [CrossRef] [PubMed]
- Ohka, S.; Matsuda, N.; Tohyama, K.; Oda, T.; Morikawa, M.; Kuge, S.; Nomoto, A. Receptor (CD155)-dependent endocytosis of poliovirus and retrograde axonal transport of the endosome. J. Virol. 2004, 78, 7186–7198. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Liu, D.; Shen, S.; Su, Z.; Zhang, L.; Duan, Y.; Tong, F.; Liang, Y.; Wang, H.; Deng, F.; et al. Pathologic studies of fatal encephalomyelitis in children caused by enterovirus 71. Am. J. Clin. Pathol. 2016, 146, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Tyler, K.L.; McPhee, D.A.; Fields, B.N. Distinct pathways and viral spread in the host determined by reovirus S1 gene segment. Science 1986, 233, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.S.; Yao, Y.C.; Lin, S.C.; Lee, Y.P.; Wang, Y.F.; Wang, J.R.; Liu, C.C.; Lei, H.Y.; Yu, C.K. Retrograde axonal transport: A major transmission route of enterovirus 71 in mice. J. Virol. 2007, 81, 8996–9003. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.C.; Badmanathan, M.; Devi, S.; Leong, K.L.; Cardosa, M.J.; Wong, K.T. Pathologic characterization of a murine model of human enterovirus 71 encephalomyelitis. J. Neuropathol. Exp. Neurol. 2008, 67, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.T.; Munisamy, B.; Ong, K.C.; Kojima, H.; Noriyo, N.; Chua, K.B.; Ong, B.B.; Nagashima, K. The distribution of inflammation and virus in human enterovirus 71 encephalomyelitis suggests possible viral spread by neural pathways. J. Neuropahtol. Exp. Neurol. 2008, 67, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Cordey, S.; Petty, T.J.; Schibler, M.; Martinez, Y.; Gerlach, D.; van Belle, S.; Turin, L.; Zdobnov, E.; Kaiser, L.; Tapparel, C. Identification of site-specific adaptations conferring increased neural cell tropism during human enterovirus 71 infection. PLoS Pathog. 2012, 8, e1002826. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zou, Q.; Chen, L.; Zhang, H.; Wang, Y. Molecular analysis of virulent determinants of enterovirus 71. PLoS ONE 2011, 6, e26237. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.I.; Weng, K.F.; Shih, S.R. Viral and host factors that contribute to pathogenicity of enterovirus 71. Future Microbiol. 2012, 7, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Kao, S.J.; Yang, F.L.; Hsu, Y.H.; Chen, H. Mechanism of fulminant pulmonary edema caused by enterovirus 71. Clin. Infect. Dis. 2004, 38, 1784–1788. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.M.; Lei, H.Y.; Huang, K.J.; Wu, J.M.; Wang, J.R.; Yu, C.K.; Su, I.J.; Liu, C.C. Pathogenesis of enterovirus 71 brainstem encephalitis in pediatric patients: The roles of cytokines and cellular immune activation in patients with pulmonary edema. J. Infect. Dis. 2003, 188, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.D.; Yang, M.Y.; Li, C.C.; Lin, S.F.; Chong, M.C.; Wang, C.L.; Chen, R.F.; Lin, T.Y. Altered cellular but not humoral reactions in children with complicated enterovirus 71 infections in Taiwan. J. Infect. Dis. 2001, 183, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Akhmadishina, L.V.; Govorukhina, M.V.; Kovalev, E.V.; Nenadskaya, S.A.; Ivanova, O.E.; Lukashev, A.N. Enterovirus A71 Meningoencephalitis Outbreak, Rostov-on-Don, Russia, 2013. Emerg. Infect. Dis. 2015, 21, 1440–1443. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.K.; Jin, H.; Li, J.X.; Yao, X.J.; Zhou, Y.; Zhang, X.F.; Zhu, F.C. Disease burden of enterovirus 71 in rural central China: A community-based survey. Hum. Vaccines Immunother. 2015, 11, 2400–2405. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, R.H.; Fletcher, S.W.; Fletcher, G.S. Summarizing the evidence. In Clinical Epidemiology: The Essentials, 5th ed.; Wolters Kluwer Health, Inc.: New York, NY, USA, 2015; pp. 243–335. [Google Scholar]
- Huang, Y.F.; Chiu, P.C.; Chen, C.C.; Chen, Y.Y.; Hsieh, K.S.; Liu, Y.C.; Lai, P.H.; Chang, H.W. Cardiac troponin I: A reliable marker and early myocardial involvement with meningoencephalitis after fatal enterovirus 71 infection. J. Infect. 2003, 46, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Hsia, S.H.; Wu, C.T.; Cahng, J.J.; Lin, T.Y.; Chung, H.T.; Lin, K.L.; Hwang, M.S.; Chou, M.L.; Chang, L.Y. Predictors of unfavorable outcomes in enterovirus 71-related cardiopulmonary failure in children. Pediatr. Infect. Dis. J. 2005, 24, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.M.; Liu, C.C.; Tseng, H.W.; Wang, J.R.; Huang, C.C.; Chen, Y.J.; Yang, Y.J.; Lin, S.J.; Yeh, T.F. Clinical spectrum of enterovirus 71 infection in children in southern Taiwan, with an emphasis on neurological complications. Clin. Infect. Dis. 1999, 29, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Shih, S.R. Cell and tissue tropism of enterovirus 71 and other enteroviruses infections. J. Biomed. Sci. 2014, 21, 18. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, W.; Chang, H.; Tang, R.; Zhao, J.; Gan, L.; Liu, B.; Chen, J.; Wang, M. Genetic analysis of the VP1 region of enterovirus 71 reveals the emergence of genotype A in central China in 2008. Virus Genes 2010, 41, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X.; Mao, Q.Y.; Liang, Z.I.; Ji, H.; Zhu, F.C. Development of enterovirus 71 vaccine from the lab bench to phase 3 clinical trials. Expert Rev. Vaccines 2014, 13, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wu, X.; Huang, K.; Li, L.; Zheng, L.; Wan, C.; He, M.L.; Zhao, W. The variations of VP1 protein might be associated with nervous system symptoms caused by enterovirus 71 infection. BMC Infect. Dis. 2014, 14, 243. [Google Scholar] [CrossRef] [PubMed]
- Drake, J.W.; Holland, J.J. Mutation rates among RNA viruses. Proc. Natl. Acad. Sci. USA 1999, 96, 13910–13913. [Google Scholar] [CrossRef] [PubMed]
- Santti, J.; Hyypia, T.; Kinnunen, L.; Salminen, M. Evidence of recombination among enteroviruses. J. Viol. 1999, 73, 8741–8749. [Google Scholar]
- Halim, S.; Ramsingh, A.I. A point mutation in VP1 of Coxsackievirus B4 alters antigenicity. Virology 2000, 269, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Bible, J.M.; Pantelidis, P.; Chan, P.K.S.; Tong, C.Y.W. Genetic evolution of enterovirrus 71: Epidemiological and pathological implications. Rev. Med. Virol. 2007, 17, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.F.; AbuBakar, S. Phylogenetic evidence for inter-typic recombination in the emergence of human enterovirus 71 subgenotypes. BMC Microbiol. 2006, 6, 74. [Google Scholar]
- Chen, X.; Zhang, Q.; Li, J.; Cao, W.; Zhang, J.X.; Zhang, L.; Zhang, W.; Shao, Z.J.; Yan, Y. Analysis of recombination and natural selection in human eheterovirus 71. Virology 2010, 398, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Fuijimoto, T.; Chikahira, M.; Yoshida, S.; Ebira, H.; Hasegawa, A.; Totsuka, A.; Nishio, O. Outbreak of central nervous system disease associated with hand, foot, and mouth disease in Japan during the summer 2000: Detection and molecular epidemiology of enterovirus 71. Microbiol. Immunol. 2002, 46, 621–627. [Google Scholar] [CrossRef]
- Van der Sanden, S.; van Eek, J.; Martin, D.P.; van der Avoort, H.; Vennema, H.; Koopmans, M. Detection of recombination breakpoints in the genomes of human enterovirus 71 strains isolated in the Netherlands in epidemic and non-epidemic years, 1963–2010. Infect. Genet. Evol. 2011, 11, 886–894. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. A Guide to Clinical Management and Public Health Response for Hand, Foot and Mouth Disease (HFMD); World Health Organization: Geneva, Switzerland, 2011; pp. 1–62. [Google Scholar]
- Zhu, F.C.; Meng, F.Y.; Li, J.X.; Li, X.L.; Mao, Q.Y.; Tao, H.; Zhang, Y.T.; Yao, X.; Chu, K.; Chen, Q.H.; et al. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: A multicentre, randomized, double-blind, placebo-controlled, phase 3 trial. Lancet 2013, 381, 2024–2032. [Google Scholar] [CrossRef]
- Zhu, F.; Xu, W.; Xia, J.; Liang, Z.; Liu, Y.; Zhang, X.; Tan, X.; Wang, L.; Mao, Q.; Wu, J.; et al. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N. Engl. J. Med. 2014, 370, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, L.; Mo, Z.; Wang, X.; Xia, J.; Liang, Z.; Zhang, Y.; Li, Y.; Mao, Q.; Wang, J.; et al. An inactivated enterovirus 71 vaccine in healthy children. N. Engl. J. Med. 2014, 370, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Ho, M.S.; Wu, J.C.; Chen, W.J.; Huang, J.H.; Chou, S.T.; Hu, Y.C. Immunization with virus-like particles of enterovirus 71 elicits potent immune responses and protects mice against lethal challenge. Vaccine 2008, 26, 1855–1862. [Google Scholar] [CrossRef] [PubMed]
- Tung, W.S.; Bakar, S.A.; Sekawi, Z.; Rosli, R. DNA vaccine constructs against enterovirus 71 elicit immune response in mice. Genet. Vaccines Ther. 2007, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Foo, D.G.; Alonso, S.; Phoon, M.C.; Ramachandran, N.P.; Chow, V.T.; Poh, C.L. Identification of neutralizing linear epitopes from the VP1 capsid protein of Enterovirus 71 using synthetic peptides. Virus Res. 2007, 125, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Qian, Y.; Wang, S.; Serrano, J.M.; Li, W.; Huang, Z.; Lu, S. EV71: An emerging infectious disease vaccine target in the Far East? Vaccine 2010, 28, 3516–3521. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.; Hsieh, S.Y.; Liu, C.C.; Chou, A.H.; Chang, J.Y.; Wu, S.C.; Liu, S.J.; Chow, Y.H.; Su, I.J.; Klein, M. Production of EV71 vaccine candidates. Hum. Vaccines Immunother. 2012, 8, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.C.; Devi, S.; Cardosa, M.J.; Wong, K.T. Formaldehyde-inactivated whole-virus vaccine protects a murine model of enterovirus 71 encephalomyelitis against disease. J. Virol. 2010, 84, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Wang, J.; Liu, L.; Zhao, H.; Shi, H.; Zhang, Y.; Jiang, L.; Li, Q. Optimized development of a candidate strain of inactivated EV71 vaccine and analysis of its immunogenicity in rhesus monkeys. Hum. Vaccines 2010, 6, 1028–1037. [Google Scholar] [CrossRef]
- Mizuta, K.; Aoki, Y.; Suto, A.; Ootani, K.; Katsushima, N.; Itagaki, T.; Ohmi, A.; Okamoto, M.; Nishimura, H.; Matsuzaki, Y.; et al. Cross-antigenicity among EV71 strains from different genogroups isolated in Yamagata, Japan, between 1990 and 2007. Vaccine 2009, 27, 3153–3158. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Chang, L.Y. Development of enterovirus 71 vaccines. Expert Rev. Vaccines 2010, 9, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Meng, F.Y.; Mao, Q.; Li, J.X.; Wang, H.; Liang, Z.L.; Zhang, Y.T.; Gao, F.; Chen, Q.H.; Hu, Y.; et al. Clinical evaluation for batch consistency of an inactivated enterovirus 71 vaccine in a large-scale phase 3 clinical trial. Hum. Vaccines Immunother. 2014, 10, 1366–1372. [Google Scholar] [CrossRef] [PubMed]
- Reed, Z.; Cardosa, M.J. Status of research and development of vaccines for enterovirus 71. Vaccine 2016, 34, 2967–2970. [Google Scholar] [CrossRef] [PubMed]
- Chou, A.H.; Liu, C.C.; Chang, J.Y.; Jiang, R.; Hsieh, Y.C.; Tsao, A.; Wu, C.L.; Huang, J.L.; Fung, C.P.; Hsieh, S.M.; et al. Formalin-inactivated EV71 vaccine candidate induced cross-neutralizing antibody response against subgenotype B1, B4, B5 and CA4A in adult volunteers. PLoS ONE 2013, 8, e79783. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Yu, C.I.; Hu, Y.C.; Tsai, T.J.; Kuo, Y.C.; Chi, W.K.; Lin, A.N.; Chiang, B.L. Enterovirus type 71 neutralizing antibodies in the serum of macaque monkeys immunized with EV71 virus-like particles. Vaccine 2012, 30, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
| Countries | 1997 | 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Singapore | B3, B4 | B3, C1 | B3 | B4 | B4 | C1, B4 | - | - | B5 | B5 | B5 | B5 | - | - | - | - |
| Malaysia | B3, B4 | C1, B4 | - | B4, C1 | B4, C1 | - | C1, B4, B5 | B5 | B5 | B5 | - | - | - | - | - | - |
| Australia | B3 | B3 | B3, C2 | C1 | - | - | C1 | C1 | - | - | - | - | - | - | - | - |
| Japan | B3, B4, C2 | C2 | C2 | B4 | B4 | C2 | B5 | - | C4a | C4a | C2, C4a | C2 | C2 | C2 | - | - |
| Korea | - | - | - | C3 | - | - | C4b | - | - | - | - | - | C2, C4a | - | - | - |
| Taiwan | - | C2, B4 | B4 | B4 | B4 | B4 | B4 | C4 | C5 | B5 | B5 | B5 | B5 | C4 | C4 | B5 |
| China | - | - | - | - | - | - | - | - | - | - | - | C4 | C4 | C4 | C4 | - |
| Countries | 1960–1969 | 1970–1979 | 1980–1989 | 1990–1999 | 2000–2009 | 2010 | 2011 | 2012 |
|---|---|---|---|---|---|---|---|---|
| France | - | - | - | - | C1, C2, C4 | - | - | C4 |
| UK | - | - | - | C1 | C1, C2 | - | - | - |
| Germany | - | - | - | - | C1, C2 | - | - | - |
| Austria | - | - | - | - | C1, C4 | - | - | - |
| Norway | - | - | - | - | C1 | - | - | - |
| Netherlands | B0 | B1 | B2 | C1 | C1, C2 | - | - | - |
| Hungary | - | B1 | - | - | C1, C4 | - | - | - |
| Bulgaria | - | B1 | - | - | - | - | - | - |
| USA | A | B1 | B2 | C1, C2 | C2 | - | - | - |
| Canada | - | - | - | - | - | - | - | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, P.-C.; Chen, S.-C.; Chen, K.-T. The Current Status of the Disease Caused by Enterovirus 71 Infections: Epidemiology, Pathogenesis, Molecular Epidemiology, and Vaccine Development. Int. J. Environ. Res. Public Health 2016, 13, 890. https://doi.org/10.3390/ijerph13090890
Chang P-C, Chen S-C, Chen K-T. The Current Status of the Disease Caused by Enterovirus 71 Infections: Epidemiology, Pathogenesis, Molecular Epidemiology, and Vaccine Development. International Journal of Environmental Research and Public Health. 2016; 13(9):890. https://doi.org/10.3390/ijerph13090890
Chicago/Turabian StyleChang, Ping-Chin, Shou-Chien Chen, and Kow-Tong Chen. 2016. "The Current Status of the Disease Caused by Enterovirus 71 Infections: Epidemiology, Pathogenesis, Molecular Epidemiology, and Vaccine Development" International Journal of Environmental Research and Public Health 13, no. 9: 890. https://doi.org/10.3390/ijerph13090890





