In Situ, High-Resolution Profiles of Labile Metals in Sediments of Lake Taihu
Abstract
:1. Introduction
2. Material and Methods
2.1. Description of Sampling Sites
2.2. DGT Preparation and Field Deployment
2.3. Sediment Sampling
2.4. Sample Analysis
2.5. Data Processing and Statistical Analysis
3. Results and Discussion
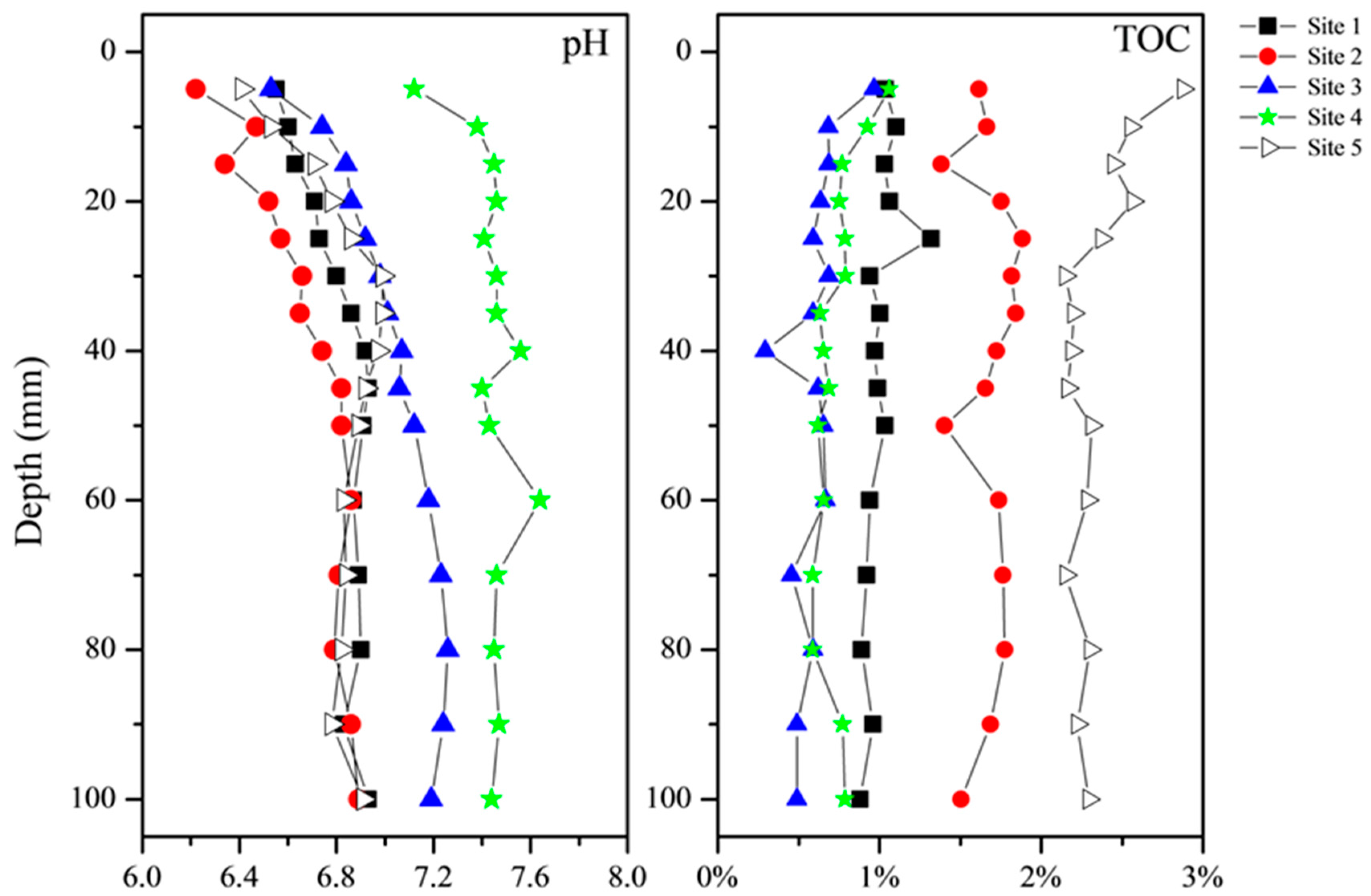
3.1. Sediment pH and TOC
3.2. Total Concentrations of Metals
3.3. Fractionation of Metals
3.4. Vertical FDGT Profiles of Metals
3.5. Overall Lability and Spatial Heterogeneity of Metals in Sediments
3.6. Relative Mobility of Metals in Sediments
3.7. Mechanisms for Fe, Mn and Co Remobilization
3.8. Mechanisms for Other Metal Remobilization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Roulier, J.L.; Belaud, S.; Coquery, M. Comparison of dynamic mobilization of Co, Cd and Pb in sediments using DGT and metal mobility assessed by sequential extraction. Chemosphere 2010, 79, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Das Gupta, A. Implication of environmental flows in river basin management. Phys. Chem. Earth 2008, 33, 298–303. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Y.; Shen, Z.; Niu, J.; Tang, Z. Distribution and speciation of heavy metals in sediments from the mainstream, tributaries, and lakes of the Yangtze River catchment of Wuhan, China. J. Hazard. Mater. 2009, 166, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Zhang, Y.; Hu, X.; Meng, W. Distribution and bioaccumulation of heavy metals in aquatic organisms of different trophic levels and potential health risk assessment from Taihu lake, China. Ecotoxicol. Environ. Saf. 2012, 81, 55–64. [Google Scholar]
- Weng, H.; Zhu, Y.; Qin, Y.; Chen, J.; Chen, X. Accumulation discrepancy of heavy metal and organic pollutants in three near-shore depositional environments, southeastern China. J. Asian Earth Sci. 2008, 31, 522–532. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Tran, H.; Wang, D.; Zhu, Y. Heavy metal contamination and risk assessment in water, paddy soil, and rice around an electroplating plant. Environ. Sci. Pollut. Res. 2011, 18, 1623–1632. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yinglan, A.; Jiang, H.; Fu, Q.; Zheng, B. Modeling the source contribution of heavy metals in surficial sediment and analysis of their historical changes in the vertical sediments of a drinking water reservoir. J. Hydrol. 2015, 520, 37–51. [Google Scholar] [CrossRef]
- Gao, Y.; Leermakers, M.; Gabelle, C.; Divis, P.; Billon, G.; Ouddane, B.; Fischer, J.C.; Wartel, M.; Baeyens, W. High-resolution profiles of trace metals in the pore waters of riverine sediment assessed by DET and DGT. Sci. Total Environ. 2006, 362, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Lesven, L.; Gillan, D.; Sabbe, K.; Billon, G.; De Galan, S.; Elskens, M.; Baeyens, W.; Leermakers, M. Geochemical behavior of trace elements in sub-tidal marine sediments of the Belgian coast. Mar. Chem. 2009, 117, 88–96. [Google Scholar] [CrossRef]
- Qin, B.; Xu, P.; Wu, Q.; Luo, L.; Zhang, Y. Environmental issues of Lake Taihu, China. Hydrobiologia 2007, 581, 3–14. [Google Scholar] [CrossRef]
- Yuan, H.; Shen, J.; Liu, E.; Wang, J.; Meng, X. Assessment of nutrients and heavy metals enrichment in surface sediments from Taihu Lake, a eutrophic shallow lake in China. Environ. Geochem. Health 2011, 33, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Duan, H.; Gu, X.; Zhang, S. Detecting aquatic vegetation changes in Taihu Lake, China using multi-temporal satellite imagery. Sensors 2008, 8, 3988–4005. [Google Scholar] [CrossRef]
- Yu, T.; Zhang, Y.; Wu, F.; Meng, W. Six-decade change in water chemistry of large freshwater Lake Taihu, China. Environ. Sci. Technol. 2013, 47, 9093–9101. [Google Scholar]
- Liu, E.; Birch, G.F.; Shen, J.; Yuan, H.; Zhang, E.; Cao, Y. Comprehensive evaluation of heavy metal contamination in surface and core sediments of Taihu Lake, the third largest freshwater lake in China. Environ. Earth Sci. 2012, 67, 39–51. [Google Scholar] [CrossRef]
- Yi, Y.; Yang, Z.; Zhang, S. Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Environ. Pollut. 2011, 159, 2575–2585. [Google Scholar] [CrossRef] [PubMed]
- Suresh, G.; Sutharsan, P.; Ramasamy, V.; Venkatachalapathy, R. Assessment of spatial distribution and potential ecological risk of the heavy metals in relation to granulometric contents of Veeranam lake sediments, India. Ecotoxicol. Environ. Saf. 2012, 84, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Bastami, K.D.; Bagheri, H.; Kheirabadi, V.; Zaferani, G.G.; Teymori, M.B.; Hamzehpoor, A.; Soltani, F.; Haghparast, S.; Harami, S.R.M.; Ghorghani, N.F.; et al. Distribution and ecological risk assessment of heavy metals in surface sediments along southeast coast of the Caspian Sea. Mar. Pollut. Bull. 2014, 81, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Bo, L.; Wang, D.; Li, T.; Li, Y.; Zhang, G.; Wang, C.; Zhang, S. Accumulation and risk assessment of heavy metals in water, sediments, and aquatic organisms in rural rivers in the Taihu Lake region, China. Environ. Sci. Pollut. Res. 2015, 22, 6721–6731. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, M.; Dreesen, F.; De Paepe, J.; Blust, R.; Bervoets, L. Do acid volatile sulfides (AVS) influence the accumulation of sediment-bound metals to benthic invertebrates under natural field conditions? Environ. Sci. Technol. 2009, 43, 4510–4516. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Cai, Y.; Duan, H.; Gao, J.; Fan, C. Use of DGT and conventional methods to predict sediment metal bioavailability to a field inhabitant freshwater snail (Bellamya aeruginosa) from Chinese eutrophic lakes. J. Hazard. Mater. 2014, 264, 184–194. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, M.; Blust, R.; Bervoets, L. The relation between Acid Volatile Sulfides (AVS) and metal accumulation in aquatic invertebrates: Implications of feeding behavior and ecology. Environ. Pollut. 2010, 158, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Peng, Y.; Yue, D.; Yin, Q.; Xiao, L. Assessment of heavy metal enrichment, bioavailability, and controlling factors in sediments of Taihu Lake, China. Soil Sediment Contam. 2015, 24, 262–275. [Google Scholar] [CrossRef]
- Camusso, M.; Gasparella, A. Measuring bioavailable trace metals from freshwater sediments by diffusive gradients in thin films (DGT) in monitoring procedures for quality assessment. Ann. Chim. 2006, 96, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Williams, P.N.; Luo, J.; Ma, H.; Wang, X. Sediment metal bioavailability in Lake Taihu, China: Evaluation of sequential extraction, DGT, and PBET techniques. Environ. Sci. Pollut. Res. 2015, 22, 12919–12928. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, C.; Wang, Z.; Cao, Z. Fractionation of heavy metals in surface sediments of Taihu Lake, East China. Environ. Geochem. Health 2004, 26, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Pueyo, M.; Mateu, J.; Rigol, A.; Vidal, M.; López-Sánchez, J.F.; Rauret, G. Use of the modified BCR three-step sequential extraction procedure for the study of trace element dynamics in contaminated soils. Environ. Pollut. 2008, 152, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Fan, C. Dynamics of reactive sulfide and its control on metal bioavailability and toxicity in metal-polluted sediments from Lake Taihu, China. Arch. Environ. Contam. Toxicol. 2011, 60, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Chomchoei, R.; Shiowatana, J.; Pongsakul, P. Continuous-flow system for reduction of metal readsorption during sequential extraction of soil. Anal. Chim. Acta 2002, 472, 147–159. [Google Scholar] [CrossRef]
- Davlson, W.; Zhang, H. In situspeciation measurements of trace components in natural waters using thin-film gels. Nature 1994, 367, 546–548. [Google Scholar] [CrossRef]
- Zhang, C.; Ding, S.; Xu, D.; Tang, Y.; Wong, M.H. Bioavailability assessment of phosphorus and metals in soils and sediments: A review of diffusive gradients in thin films (DGT). Environ. Monit. Assess. 2014, 186, 7367–7378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Davison, W.; Miller, S.; Tych, W. In situ high resolution measurements of fluxes of Ni, Cu, Fe, and Mn and concentrations of Zn and Cd in porewaters by DGT. Geochim. Cosmochim. Acta 1995, 59, 4181–4192. [Google Scholar] [CrossRef]
- Fones, G.R.; Davison, W.; Hamilton-Taylor, J. The fine-scale remobilization of metals in the surface sediment of the North-East Atlantic. Cont. Shelf Res. 2004, 24, 1485–1504. [Google Scholar] [CrossRef]
- Van Leeuwen, H.P.; Town, R.M.; Buffle, J.; Cleven, R.; Davison, W.; Puy, J.; van Riemsdijk, W.H.; Sigg, L. Dynamic speciation analysis and bioavailability of metals in aquatic systems. Environ. Sci. Technol. 2005, 39, 8545–8556. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Davison, W.; Mortimer, R.J.G.; Krom, M.D.; Hayes, P.J.; Davies, I.M. Localised remobilization of metals in a marine sediment. Sci. Total Environ. 2002, 296, 175–187. [Google Scholar] [CrossRef]
- Huo, S.; Zhang, J.; Yeager, K.M.; Xi, B.; Qin, Y.; He, Z.; Wu, F. Mobility and sulfidization of heavy metals in sediments of a shallow eutrophic lake, Lake Taihu, China. J. Environ. Sci. 2015, 31, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Santner, J.; Larsen, M.; Kreuzeder, A.; Glud, R.N. Two decades of chemical imaging of solutes in sediments and soils—A review. Anal. Chim. Acta 2015, 878, 9–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehto, N.J.; Davison, W.; Zhang, H. The use of ultra-thin diffusive gradients in thin-films (DGT) devices for the analysis of trace metal dynamics in soils and sediments: A measurement and modelling approach. Environ. Chem. 2012, 9, 415–423. [Google Scholar] [CrossRef]
- Zhang, H.; Davison, W. Performance characteristics of diffusion gradients in thin films for the in situ measurement of trace metals in aqueous solution. Anal. Chem. 1995, 67, 3391–3400. [Google Scholar] [CrossRef]
- Ding, S.; Han, C.; Wang, Y.; Yao, L.; Wang, Y.; Xu, D.; Sun, Q.; Williams, P.N.; Zhang, C. In situ, high-resolution imaging of labile phosphorus in sediments of a large eutrophic lake. Water Res. 2015, 74, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Wang, Y.; Xu, D.; Zhu, C.; Zhang, C. Gel-based coloration technique for the submillimeter-scale imaging of labile phosphorus in sediments and soils with diffusive gradients in thin films. Environ. Sci. Technol. 2013, 47, 7821–7829. [Google Scholar] [CrossRef] [PubMed]
- Lu, R. Analytical Methods for Soil and Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 1999. [Google Scholar]
- Kartal, Ş.; Aydın, Z.; Tokalıoğlu, Ş. Fractionation of metals in street sediment samples by using the BCR sequential extraction procedure and multivariate statistical elucidation of the data. J. Hazard. Mater. 2006, 132, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Harper, M.P.; Davison, W.; Tych, W. DIFS—A modelling and simulation tool for DGT induced trace metal remobilisation in sediments and soils. Environ. Model. Softw. 2000, 15, 55–66. [Google Scholar] [CrossRef]
- Tankere-Muller, S.; Zhang, H.; Davison, W.; Finke, N.; Larsen, O.; Stahl, H.; Glud, R.N. Fine scale remobilisation of Fe, Mn, Co, Ni, Cu and Cd in contaminated marine sediment. Mar. Chem. 2007, 106, 192–207. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Li, B.; Feng, C.; Chen, Y.; Shen, Z. Distribution and speciation of heavy metals in surface sediments from the Yangtze estuary and coastal areas. Environ. Earth Sci. 2012, 69, 1537–1547. [Google Scholar] [CrossRef]
- Yao, S.; Xue, B. Nutrients and heavy metals in multi-cores from Zhushan Bay at Taihu Lake, the largest shallow lake in the Yangtze Delta, China. Quat. Int. 2010, 226, 23–28. [Google Scholar]
- Guevara-Riba, A.; Sahuquillo, A.; Rubio, R.; Rauret, G. Assessment of metal mobility in dredged harbour sediments from Barcelona, Spain. Sci. Total Environ. 2004, 32, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Plach, J.M.; Warren, L.A. Differentiating natural organic matter roles in freshwater floc and bed sediment lead dynamics. Chem. Geol. 2012, 304, 97–105. [Google Scholar] [CrossRef]
- Ernstberger, H.; Zhang, H.; Tye, A.; Young, S.; Davison, W. Desorption kinetics of Cd, Zn, and Ni measured in soils by DGT. Environ. Sci. Technol. 2005, 39, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.L.; Yverneau, H.; Cremazy, A.; Jarolimek, C.V.; Price, H.L.; Jolley, D.F. DGT-induced copper flux predicts bioaccumulation and toxicity to bivalves in sediments with varying properties. Environ. Sci. Technol. 2012, 46, 9038–9046. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.; Bolam, T.; Kroger, S.; Mason, C.; Birchenough, S.; Silburn, B.; Sivyer, D.; Mayes, A.G.; Fones, G.R. The application of passive sampler (DGT) technology for improved understanding of metal behaviour at a marine disposal site. E3S Web Conf. 2013, 1, 02002. [Google Scholar] [CrossRef]
- Mortimer, C.H. The exchange of dissolved substances between mud and water in lakes. J. Ecol. 1941, 29, 280–329. [Google Scholar] [CrossRef]
- Leermakers, M.; Gao, Y.; Gabelle, C.; Lojen, S.; Ouddane, B.; Wartel, M.; Baeyens, W. Determination of high resolution pore water profiles of trace metals in sediments of the Rupel River (Belgium) using DET (diffusive equilibrium in thin films) and DGT (diffusive gradients in thin films) techniques. Water Air Soil Pollut. 2005, 166, 265–286. [Google Scholar] [CrossRef]
- Poulton, S.W.; Canfield, D.E. Development of a sequential extraction procedure for iron: Implications for iron partitioning in continentally derived particulates. Chem. Geol. 2005, 214, 209–221. [Google Scholar] [CrossRef]
- Wang, X.; Liu, F.; Tan, W.; Li, W.; Feng, X.; Sparks, D.L. Characteristics of phosphate adsorption-desorption onto ferrihydrite: Comparison with well-crystalline Fe (hydr) oxides. Soil Sci. 2013, 178, 1–11. [Google Scholar] [CrossRef]
- Lovley, D.R.; Goodwin, S. Hydrogen concentrations as an indicator of the predominant terminal electron accepting reaction in aquatic sediments. Geochim. Cosmochim. Acta 1988, 52, 2993–3003. [Google Scholar] [CrossRef]
- Thamdrup, B.; Fossing, H.; Jørgensen, B.B. Manganese, iron and sulfur cycling in a coastal marine sediment, Aarhus Bay, Denmark. Geochim. Cosmochim. Acta 1994, 58, 5115–5129. [Google Scholar] [CrossRef]
- Shuttleworth, S.M.; Davison, W.; Hamilton-Taylor, J. Two-dimensional and fine structure in the concentrations of iron and manganese in sediment pore-waters. Environ. Sci. Technol. 1999, 33, 4169–4175. [Google Scholar] [CrossRef]
- Moffett, J.W.; Ho, J. Oxidation of cobalt and manganese in seawater via a common microbially catalyzed pathway. Geochim. Cosmochim. Acta 1996, 60, 3415–3424. [Google Scholar] [CrossRef]
- Fones, G.R.; Davison, W.; Holby, O.; Jorgensen, B.B.; Thamdrup, B. High-resolution metal gradients measured by in situ DGT/DET deployment in Black Sea sediments using an autonomous benthic lander. Limnol. Oceanogr. 2001, 46, 982–988. [Google Scholar] [CrossRef]
- Stockdale, A.; Davison, W.; Zhang, H.; Hamilton-Taylor, J. The association of cobalt with iron and manganese (oxyhydr) oxides in marine sediment. Aquat. Geochem. 2010, 16, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Hamilton-Taylor, J.; Smith, E.J.; Davison, W.; Zhang, H. A novel DGT-sediment trap device for the in situ measurement of element remobilization from settling particles in water columns and its application to trace metal release from Mn and Fe oxides. Limnol. Oceanogr. 1999, 44, 1772–1780. [Google Scholar] [CrossRef]
- Lienemann, C.P.; Taillefert, M.; Perret, D.; Gaillard, J.F. Association of cobalt andmanganese in aquatic systems: Chemical and microscopic evidence. Geochim. Cosmochim. Acta 1997, 61, 1437–1446. [Google Scholar] [CrossRef]
- Taillefert, M.; MacGregor, B.J.; Gaillard, J.F.; Lienemann, C.P.; Perret, D.; Stahl, D.A. Evidence for a dynamic cycle between Mn and Co in the water column of a stratified lake. Environ. Sci. Technol. 2002, 36, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Manceau, A.; Drits, V.; Silvester, E.; Bartoli, C.; Lanson, B. Structural mechanism of Co2+ oxidation by the phyllomanganate buserite. Am. Mineral. 1997, 82, 1150–1175. [Google Scholar] [CrossRef]
- Naylor, C.; Davison, W.; Motelica-Heino, M.; Van den Berg, G.A.; Van der Heijdt, L.M. Potential kinetic availability of metals in sulphidic freshwater sediments. Sci. Total Environ. 2006, 357, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.B.; Fan, C.X.; Ding, S.M.; Zhang, L.; Li, B. Acid volatile sulfides and Simultaneously extracted metals in metal polluted area of Taihu Lake, China. Bull. Environ. Contam. Toxicol. 2008, 80, 351–355. [Google Scholar] [CrossRef] [PubMed]
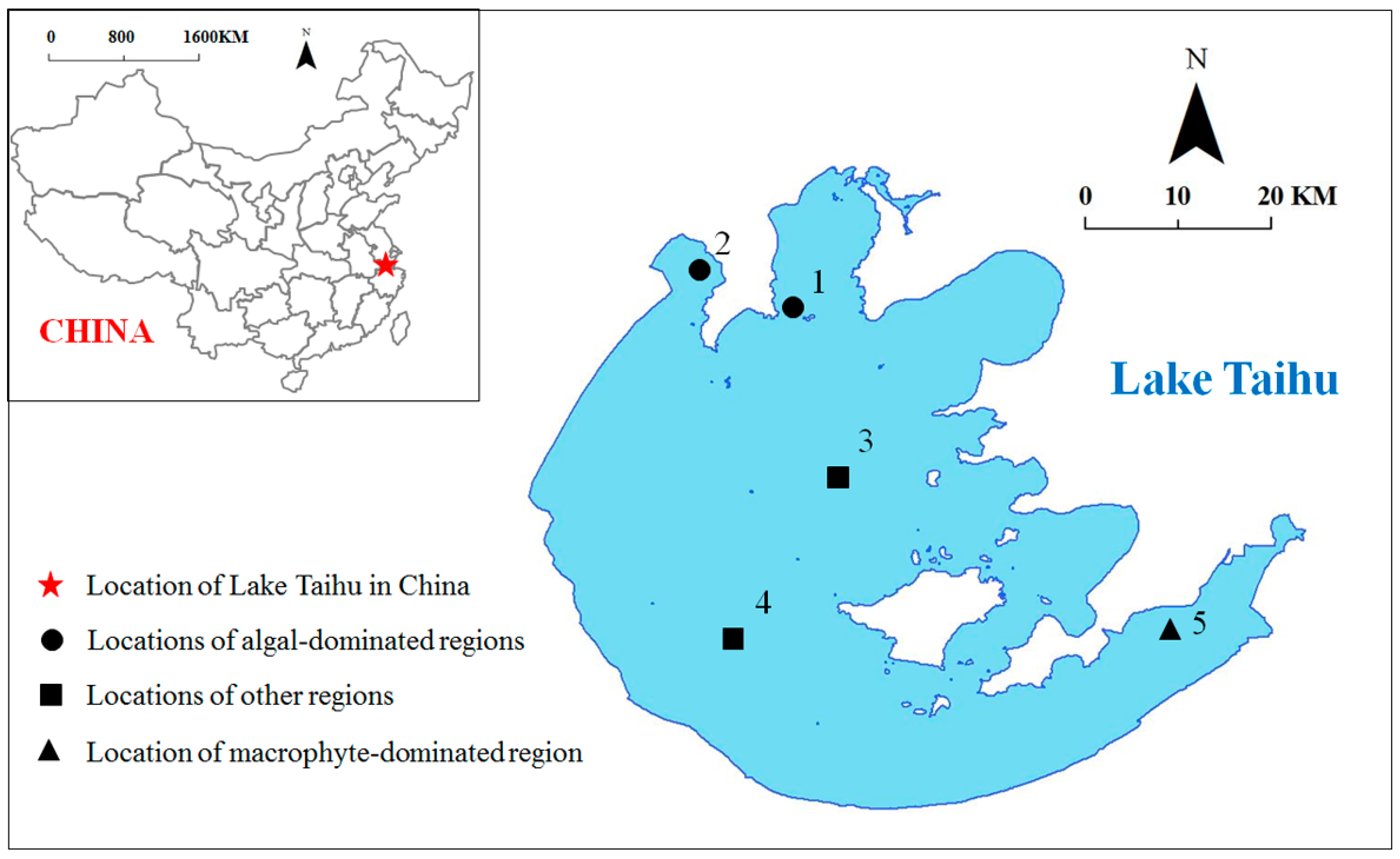





| Site | Longitude | Latitude | Description |
|---|---|---|---|
| 1 | 120°8′45.24″ | 31°24′24.48″ | In Meliang Bay; algae-dominated |
| 2 | 120°2′42.12″ | 31°27′0.04″ | In Zhushan Bay; algae-dominated |
| 3 | 120°10′48.72″ | 31°14′17.99″ | In the central part of the lake |
| 4 | 120°4′36.12″ | 31°5′24.36″ | In the west part of the lake |
| 5 | 120°30′47.88″ | 31°5′21.88″ | In East Taihu; macrophyte-dominated |
| Metal | Sampling Sites | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Fe × 103 | 2.00 | 2.38 | 1.78 | 1.77 | 1.46 |
| 1.03–4.56 | 1.52–3.04 | 1.40–2.19 | 1.37–2.00 | 1.18–1.60 | |
| Mn × 103 | 0.62 | 1.57 | 0.37 | 0.71 | 0.42 |
| 0.48–0.75 | 1.32–1.74 | 0.31–0.48 | 0.52–1.09 | 0.36–0.48 | |
| Zn | 101 | 350 | 75.1 | 84.2 | 105 |
| 89.2–116 | 232–412 | 61.8–94.6 | 72.5–98.3 | 97.4–118 | |
| Ni | 33.3 | 90.4 | 26.4 | 29.7 | 27.0 |
| 27.2–39.9 | 62.0–126 | 22.9–31.1 | 23.1–45.3 | 21.0–38.1 | |
| Cu | 24.1 | 101 | 16.3 | 17.4 | 17.1 |
| 20.1–27.6 | 72.7–113 | 12.5–19.8 | 13.8–21.1 | 15.8–20.9 | |
| Pb | 34.4 | 44.8 | 27.3 | 27.4 | 36.5 |
| 28.2–41.3 | 39.0–53.7 | 20.5–33.5 | 24.1–29.4 | 26.6–44.0 | |
| Co | 10.73 | 14.02 | 9.15 | 9.25 | 7.50 |
| 9.14–12.2 | 10.6–15.7 | 7.57–10.9 | 7.46–11.7 | 6.12–9.31 | |
| Cd | 0.48 | 0.68 | 0.39 | 0.45 | 0.43 |
| 0.39–0.55 | 0.53–0.79 | 0.30–0.43 | 0.35–0.51 | 0.30–0.56 | |
| Site | 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | RSD | Mean | RSD | Mean | RSD | Mean | RSD | Mean | RSD | |
| Fe × 10 | 7.51 | 65.5 | 1.55 | 95.0 | 1.28 | 93.0 | 1.16 | 154.1 | 14.61 | 96.5 |
| Mn × 10 | 8.60 | 50.3 | 5.31 | 76.2 | 4.48 | 59.2 | 4.80 | 90.0 | 5.98 | 89.3 |
| Zn | 5.05 | 11.1 | 8.63 | 20.3 | 3.34 | 24.0 | 3.46 | 11.8 | 3.32 | 38.5 |
| Ni × 10−1 | 5.82 | 19.1 | 16.73 | 20.8 | 4.23 | 12.5 | 4.86 | 11.4 | 4.16 | 26.4 |
| Cu × 10−1 | 1.41 | 15.0 | 4.21 | 19.2 | 1.22 | 5.2 | 1.73 | 21.2 | 0.68 | 64.6 |
| Pb × 10−2 | 2.40 | 15.2 | 4.77 | 18.4 | 2.19 | 14.9 | 3.60 | 14.5 | 2.02 | 29.1 |
| Co × 10−2 | 2.63 | 42.9 | 4.85 | 56.0 | 2.54 | 51.8 | 1.84 | 59.3 | 1.19 | 36.3 |
| Cd × 10−1 | 2.62 | 5.6 | 3.33 | 6.2 | 1.78 | 22.8 | 3.72 | 5.5 | 3.49 | 6.8 |
| Elements | Sampling Sites | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Fe | 0.03 | <0.01 | <0.01 | <0.01 | 0.09 |
| Mn | 1.21 | 0.42 | 1.05 | 0.64 | 1.66 |
| Zn | 0.62 | 0.56 | 0.61 | 0.66 | 1.20 |
| Ni | 0.15 | 0.24 | 0.24 | 0.36 | 0.49 |
| Cu | 0.10 | 0.06 | 0.11 | 0.15 | 0.08 |
| Pb | 0.01 | 0.02 | 0.01 | 0.02 | 0.02 |
| Co | 0.05 | 0.07 | 0.05 | 0.04 | 0.08 |
| Cd | 5.00 | 3.31 | 4.09 | 5.43 | 9.77 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Gong, M.; Li, Y.; Xu, L.; Wang, Y.; Jing, R.; Ding, S.; Zhang, C. In Situ, High-Resolution Profiles of Labile Metals in Sediments of Lake Taihu. Int. J. Environ. Res. Public Health 2016, 13, 884. https://doi.org/10.3390/ijerph13090884
Wang D, Gong M, Li Y, Xu L, Wang Y, Jing R, Ding S, Zhang C. In Situ, High-Resolution Profiles of Labile Metals in Sediments of Lake Taihu. International Journal of Environmental Research and Public Health. 2016; 13(9):884. https://doi.org/10.3390/ijerph13090884
Chicago/Turabian StyleWang, Dan, Mengdan Gong, Yangyang Li, Lv Xu, Yan Wang, Rui Jing, Shiming Ding, and Chaosheng Zhang. 2016. "In Situ, High-Resolution Profiles of Labile Metals in Sediments of Lake Taihu" International Journal of Environmental Research and Public Health 13, no. 9: 884. https://doi.org/10.3390/ijerph13090884






