Microbial Contamination and Hygiene of Fresh Cow’s Milk Produced by Smallholders in Western Zambia
Abstract
:1. Introduction
2. Experimental Section
2.1. Planning, Data Collection and Sampling
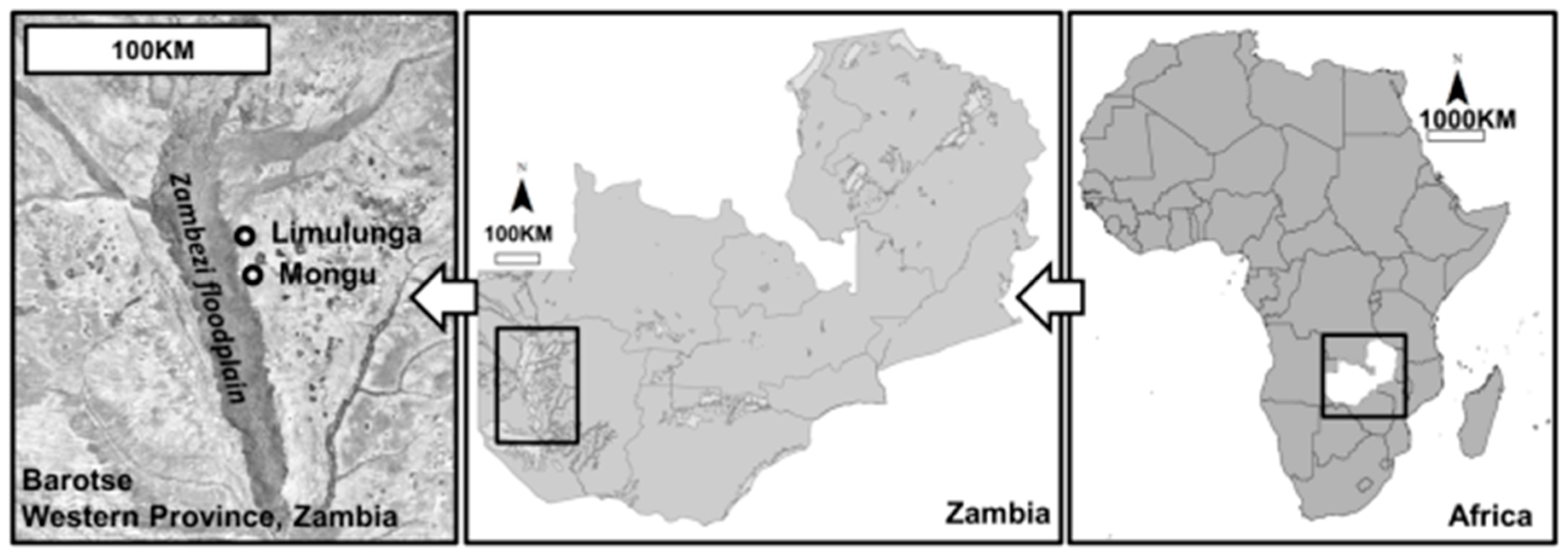
2.1.1. Scoping
2.1.2. Selection
2.1.3. Field Visits
2.2. Microbiology and Quality Assessment
2.3. Analysis
3. Results
3.1. Descriptive Analysis
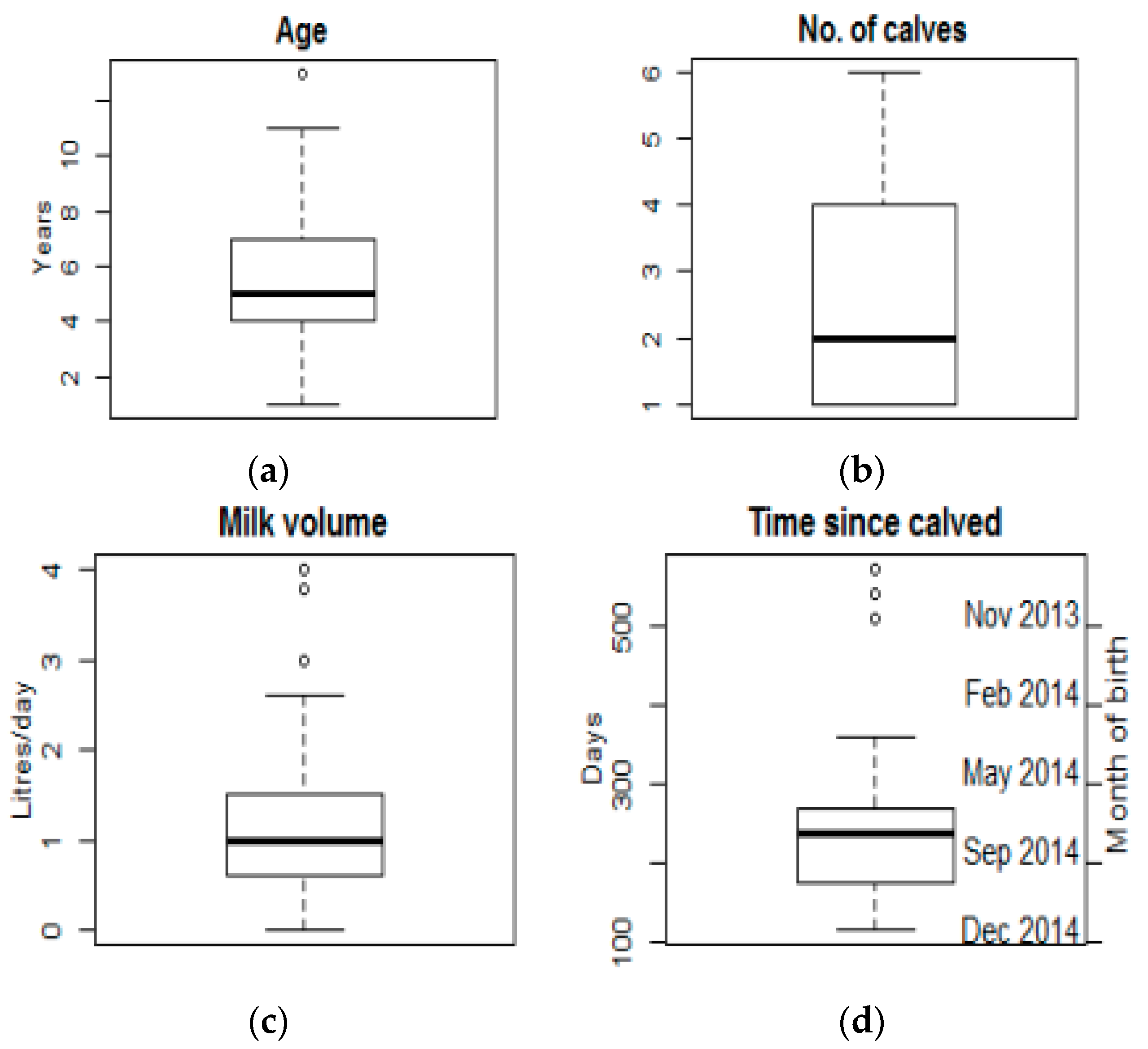
3.1.1. Herd and Cattle Characteristics
3.1.2. Husbandry and Production Characteristics
3.1.3. Milking
3.1.4. Transport to Dairy Cooperative
3.1.5. Dairy Cooperative Practices
3.1.6. Temperature
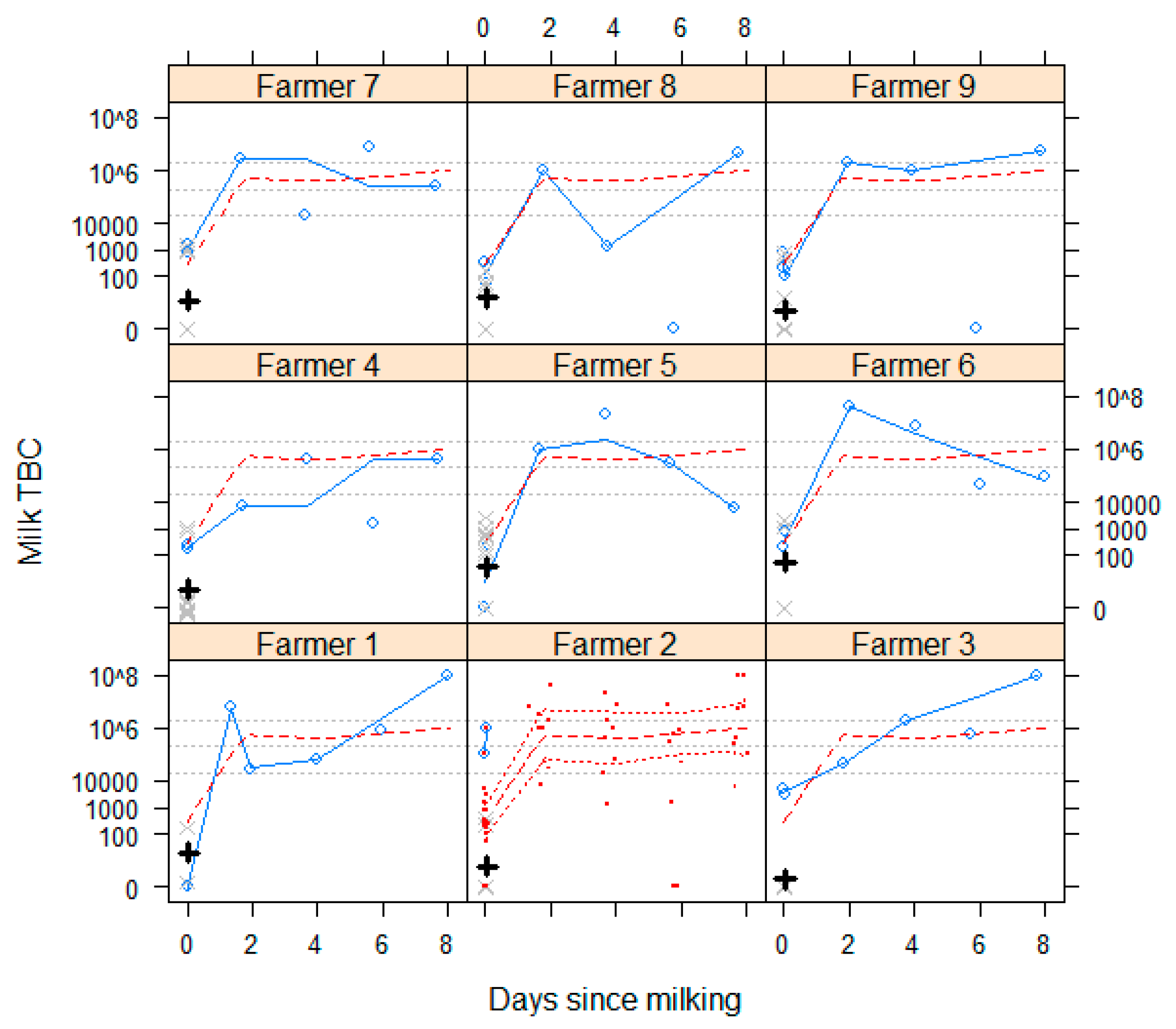
3.1.7. Milk Microbiology and Quality
3.1.8. Sour Milk
3.2. Univariable Analysis
4. Discussion
4.1. Milk Microbiology
4.2. Observed Hygienic Practice
4.3. Risk Factors
4.4. Options for Improved Milk Safety
4.4.1. Funding and Pricing
4.4.2. Refrigeration
4.4.3. Pasteurisation
4.4.4. Awareness
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baars, R.; De Jong, R.; Zwart, D. Costs and returns of the crop-cattle system in the Western Province of Zambia. Rev. Elev. Med. Vet. Pays Trop. 1996, 49, 243–251. [Google Scholar]
- Nowak, V.; Kennedy, G.; Pasqualino, M.M.; Longley, C.; Thilsted, S.H. Impact of seasonality on dietary diversity in Western Province, Zambia. Draft paper. 2016. [Google Scholar]
- Wood, A.P. Cattle and Development in Western Zambia; Pastoral Development Network Paper 28c; Overseas Development Institute: London, UK, 1989. [Google Scholar]
- Phiri, A.M. Common conditions leading to cattle carcass and offal condemnations at 3 abattoirs in the Western Province of Zambia and their zoonotic implications to consumers. J. S. Afr. Vet. Assoc. 2006, 77, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Muma, J.B.; Pandey, G.S.; Munyeme, M.; Mumba, C.; Mkandawire, E.; Chimana, H.M. Brucellosis among smallholder cattle farmers in Zambia: Public health significance. Trop. Anim. Health Prod. 2012, 44, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Roesel, K.; Grace, D. Food Safety and Informal Markets: Animal Products in Sub-Saharan Africa; Routledge: London, UK, 2014. [Google Scholar]
- Jabbar, M.A.; Baker, D.; Fadiga, M.L. Demand for Livestock Products in Developing Countries with a Focus on Quality and Safety Attributes: Evidence from Asia and Africa; ILRI Research Report 24; ILRI: Nairobi, Kenya, 2010. [Google Scholar]
- O’Connor, C.B.; Tripathi, B.R. Milk Processing Techniques—Sour Milk, RURAL Dairy Processing Training Series; International Livestock Centre for Africa: Addis Ababa, Ethiopia, 1992. [Google Scholar]
- Lund, B.M.; Baird-Parker, A.C.; Gould, G.W. The Microbiological Safety and Quality of Food; Aspen Publishers: Gaithersburg, MD, USA, 2000; Volume 1, p. 70. [Google Scholar]
- Baars, R.; Ottens, J. Grazing behaviour and diet selection of Barotse cattle on a communally grazed floodplain in west Zambia. Afr. J. Range Forage Sci. 2001, 18, 5–12. [Google Scholar] [CrossRef]
- Baars, R.; Jeanes, K. The grazing capacity of natural grasslands in the Western. Trop. Grassl. 1997, 31, 561–568. [Google Scholar]
- Van Klink, E.; Corten, J.; Kalokoni, D. Herd monitoring in traditional cattle husbandry as a tool for productivity research and livestock development. Trop. Anim. Health Prod. 1996, 28, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Van Klink, E. Aspects of Productivity of Traditionally Managed Barotse Cattle in the Western Province of Zambia. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2 November 1994. [Google Scholar]
- Knight-Jones, T.J.D.; Songe, M.M. Food Safety in Western Province, Zambia: Field Visits and Scoping Mission; Report of a Workshop and Field Trip Held at Mongu, Zambia; International Livestock Research Institute (ILRI): Nairobi, Kenya, 2015. [Google Scholar]
- Cowan, S.T.; Steel, K.J.; Barrow, G.I.; Feltham, R.K.A. Cowan and Steel’s Manual for the Identification of Medical Bacteria, 3rd ed.; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- R Core Team. R Version 3.1.2 (2014-10-31) “Pumpkin Helmet”: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Dohoo, I.R.; Meek, A.H. Somatic cell counts in bovine milk. Can. Vet. J. 1982, 23, 119–125. [Google Scholar] [PubMed]
- O’Dwyer, J. Food Assurance and Safety; Teagasc—The Agriculture and Food Development Authority: Dublin, Ireland, 2011. [Google Scholar]
- Hillerton, J.E.; Berry, E.A. Quality of the Milk Supply: European Regulations Versus Practice—NMC Annual Meeting Proceedings (1–4 February 2004). Available online: http://www.nmconline.org/articles/qualityeuro.pdf (accessed on 25 January 2016).
- European Commission. Corrigendum to Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004: Laying Down Specific Hygiene Rules for Food of Animal Origin; European Commission: Luxembourg City, Luxembourg, 2004; p. L139. [Google Scholar]
- Yambayamba, K.E.S.; Zulu, M.P. Influence of the milking environment on the microbial quality of raw milk produced by smallholder farmers in Magoye. UNZA J. Sci. Technol. 2011, 15, 37–43. [Google Scholar]
- Kunda, B.; Pandey, G.S.; Muma, J.B. Compositional and sanitary quality of raw milk produced by smallholder dairy farmers in Lusaka Province of Zambia. Livest. Res. Rural Dev. 2015, 27, 201. [Google Scholar]
- Ndungu, T.W.; Muliro, P.S.; Omwamba, M.; Oosterwijk, G.; Jansen, A. Quality control of raw milk in the smallholder collection and bulking enterprises in Nakuru and Nyandarua Counties, Kenya. Afr. J. Food Sci. 2016, 10, 70–78. [Google Scholar]
- Doyle, M.M.; Garcia, S.; Bahati, E.; Karamuzi, D.; Cullor, J.S.; Nandi, S. Microbiological analysis of raw milk in Rwanda. Afr. J. Food Sci. Technol. 2015, 6, 141–143. [Google Scholar]
- Adugna, M.; Asresie, A. A Review on microbiological quality of Ethiopian raw bovine milk. Food Sci. Qual. Man 2015, 35, 17–24. [Google Scholar]
- Belli, P.; Cantafora, A.F.A.; Stella, S.; Barbieri, S.; Crimella, C. Microbiological survey of milk and dairy products from a small scale dairy processing unit in Maroua (Cameroon). Food Control 2013, 32, 366–370. [Google Scholar] [CrossRef]
- Pandey, G.S.; Mishra, D.S.; Mule, D.; Mubita, C. Studies on sanitary quality and cell count of raw milk from dairy farms supplying milk to Dairy Produce Board in Lusaka, Zambia. Bull. Anim. Health Prod. Afr. 1996, 44, 9–13. [Google Scholar]
- Hubácková, M.; Rysánek, D. Effects of freezing milk samples on the recovery of alimentary pathogens and indicator microorganisms. Acta Vet. Brno 2007, 2007, 301–307. [Google Scholar] [CrossRef]
- Alrabadi, N.I. The effect of freezing on different bacterial counts in raw milk. Int. J. Biol. 2015, 7, 9–12. [Google Scholar] [CrossRef]
- Schukken, Y.H.; Grommers, F.J.; Smit, J.A.; Vandegeer, D.; Brand, A. Effect of freezing on bacteriologic culturing of mastitis milk samples. J. Dairy Sci. 1989, 72, 1900–1906. [Google Scholar] [CrossRef]
- Knight-Jones, T.J.D.; Mylrea, G.E.; Kahn, S. Animal production food safety: Priority pathogens for standard setting by the World Organisation for Animal Health. Rev. Sci. Tech. 2010, 29, 523–535. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs; European Commission: Luxembourg City, Luxembourg, 2005; p. 338. [Google Scholar]
- UK Government. Statutory Instrument. Food England: The Food Safety and Hygiene (England) Regulations 2013; 2013 No 2996; UK Government: London, UK, 2013.
- Holsinger, V.H.; Rajkowski, K.T.; Stabel, J.R. Milk pasteurisation and safety: A brief history and update. Rev. Sci. Tech. 1997, 16, 441–451. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Scientific Opinion on the public health risks related to the consumption of raw drinking milk. EFSA Panel on Biological Hazards (BIOHAZ). EFSA J. 2015, 13, 3940. [Google Scholar]
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; de Silva, N.R.; Gargouri, N.; et al. World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grace, D.; Omore, A.; Randolph, T.; Kang’ethe, E.; Nasinyama, G.W.; Mohammed, T. Risk assessment for E. coli O157:H7 in marketed raw and fermented milk in selected African countries. J. Food Prot. 2008, 27, 257–263. [Google Scholar]
- Makita, K.; Desissa, F.; Teklu, A.; Zewde, G.; Grace, D. Risk assessment of staphylococcal poisoning due to consumption of informally-marketed milk and home-made yoghurt in Debre Zeit, Ethiopia. Int. J. Food Microbiol. 2012, 153, 135–141. [Google Scholar] [CrossRef] [PubMed]
- FAO. Milk Production Hygiene and Udder Health—FAO Animal Production and Health Paper 78. Available online: http://www.fao.org/docrep/004/t0218e/T0218E00.htm#TOC (accessed on 26 January 2016).
- Dinges, M.M.; Orwin, P.M.; Schlievert, P.M. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 2000, 13, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Dessisa, F.; Roesel, K.; Makita, K.; Teklu, A.; Zewde, G.; Grace, D. Is Ethiopian raw milk safe for human consumption? In Food Safety and Informal Markets: Animal Products in Sub-Saharan Africa; Roesel, K., Grace, D., Eds.; Routledge: London, UK, 2014. [Google Scholar]
- Makita, K.; Fevre, E.M.; Waiswa, C.; Eisler, M.C.; Welburn, S.C. How human brucellosis incidence in urban Kampala can be reduced most efficiently? A stochastic risk assessment of informally-marketed milk. PLoS ONE 2010, 5, e14188. [Google Scholar] [CrossRef] [PubMed]
- Safapour, N.; Metcalf, R.H. Enhancement of solar water pasteurization with reflectors. Appl. Environ. Microbiol. 1999, 65, 859–861. [Google Scholar] [PubMed]
- Muma, J.B.; Samui, K.L.; Munyeme, M.; Lund, A.; Nielsen, K.; Chimana, H.; Chisenga, C.; Skjerve, E. Brucellosis in rural communities in Zambia and factors associated with increased Anti-Brucella spp. antibody presence. UNZA J. Sci. Technol. 2008, 12, 9–18. [Google Scholar]
- Munyeme, M.; Muma, J.B.; Munang’andu, H.M.; Kankya, C.; Skjerve, E.; Tryland, M. Cattle owners’ awareness of bovine tuberculosis in high and low prevalence settings of the wildlife-livestock interface areas in Zambia. BMC Vet. Res. 2010, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Kouame-Sina, S.M.; Makita, K.; Costard, S.; Grace, D.; Dadie, A.; Dje, M.; Bonfoh, B. Hazard identification and exposure assessment for bacterial risk assessment of informally marketed milk in Abidjan, Cote d’Ivoire. Food Nutr. Bull. 2012, 33, 223–234. [Google Scholar] [CrossRef] [PubMed]

| Outcome | Point of Sampling | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cow | On-Farm Pooled Herd Sample | Coop Arrival | 1–2 Days | 4 Days | 6 Days | 8 Days | Sour Milk on Day of Purchase | ||
| All Cow Samples | Within Herd Prevalence Median (min–max) | ||||||||
| Bacterial growth | 26/86 (30%) | 29% (0%–57%) | 8/9 (89%) | 8/9 (89%) | 9/9 (100%) | 8/8 (100%) | 6/6 (100%) | 8/8 (100%) | 8/9 (89%) |
| Staphylococcus aureus * | 19/86 (22%) | 22% (0%–50%) | 6/9 (67%) | 7/9 (78%) | 8/9 (89%) | 7/8 (88%) | 6/6 (100%) | 8/8 (100%) | 2/9 (22%) |
| Escherichia. coli | 11/86 (13%) | 0% (0%–50%) | 6/9 (67%) | 8/9 (89%) | 9/9 (100%) | 8/8 (100%) | 6/6 (100%) | 8/8 (100%) | 4/9 (44%) |
| Bacillus spp. | 7/86 (8%) | 7% (0%–25%) | 0/9 (0%) | 0/9 (0%) | 3/9 (33%) | 3/8 (38%) | 1/6 (17%) | 6/8 (75%) | 5/9 (56%) |
| Staphylococcus spp. | 11/86 (13%) | 11% (0%–50%) | 2/9 (22%) | 6/9 (67%) | 8/9 (89%) | 8/8 (100%) | 6/6 (100%) | 7/8 (88%) | 2/9 (22%) |
| Streptococcus spp. | 2/86 (2%) | 0% (0%–15%) | 0/9 (0%) | 0/9 (0%) | 1/9 (11%) | 0/8 (0%) | 0/6 (100%) | 0/8 (0%) | 0/9 (0%) |
| TBC > 1000 | 4/86 (5%) | 0% (0%–50%) | 3/9 (33%) | 2/9 (22%) | 9/9 (100%) | 8/8 (100%) | 6/6 (100%) | 8/8 (100%) | 3/9 (33%) |
| Geometric mean | 6 | 7 (1–39) | 269 | 425 | 630,000 | 345,000 | 213,000 | 1,416,000 | 491 |
| Coliform count > 0 | 0/86 | All negative | 0/9 (0%) | 0/9 (0%) | 1/9 (11%) | 1/8 (12%) | 0/6 (0%) | 3/8 (37%) | 0/9 (0%) |
| Geometric mean | - | - | - | - | 2 | 3 | - | 20 | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knight-Jones, T.J.D.; Hang’ombe, M.B.; Songe, M.M.; Sinkala, Y.; Grace, D. Microbial Contamination and Hygiene of Fresh Cow’s Milk Produced by Smallholders in Western Zambia. Int. J. Environ. Res. Public Health 2016, 13, 737. https://doi.org/10.3390/ijerph13070737
Knight-Jones TJD, Hang’ombe MB, Songe MM, Sinkala Y, Grace D. Microbial Contamination and Hygiene of Fresh Cow’s Milk Produced by Smallholders in Western Zambia. International Journal of Environmental Research and Public Health. 2016; 13(7):737. https://doi.org/10.3390/ijerph13070737
Chicago/Turabian StyleKnight-Jones, Theodore J.D., M. Bernard Hang’ombe, Mwansa M. Songe, Yona Sinkala, and Delia Grace. 2016. "Microbial Contamination and Hygiene of Fresh Cow’s Milk Produced by Smallholders in Western Zambia" International Journal of Environmental Research and Public Health 13, no. 7: 737. https://doi.org/10.3390/ijerph13070737






