Effect of Nano-Al2O3 on the Toxicity and Oxidative Stress of Copper towards Scenedesmus obliquus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Test Medium
2.3. Preparation of Exposure Solution and Its Characterization
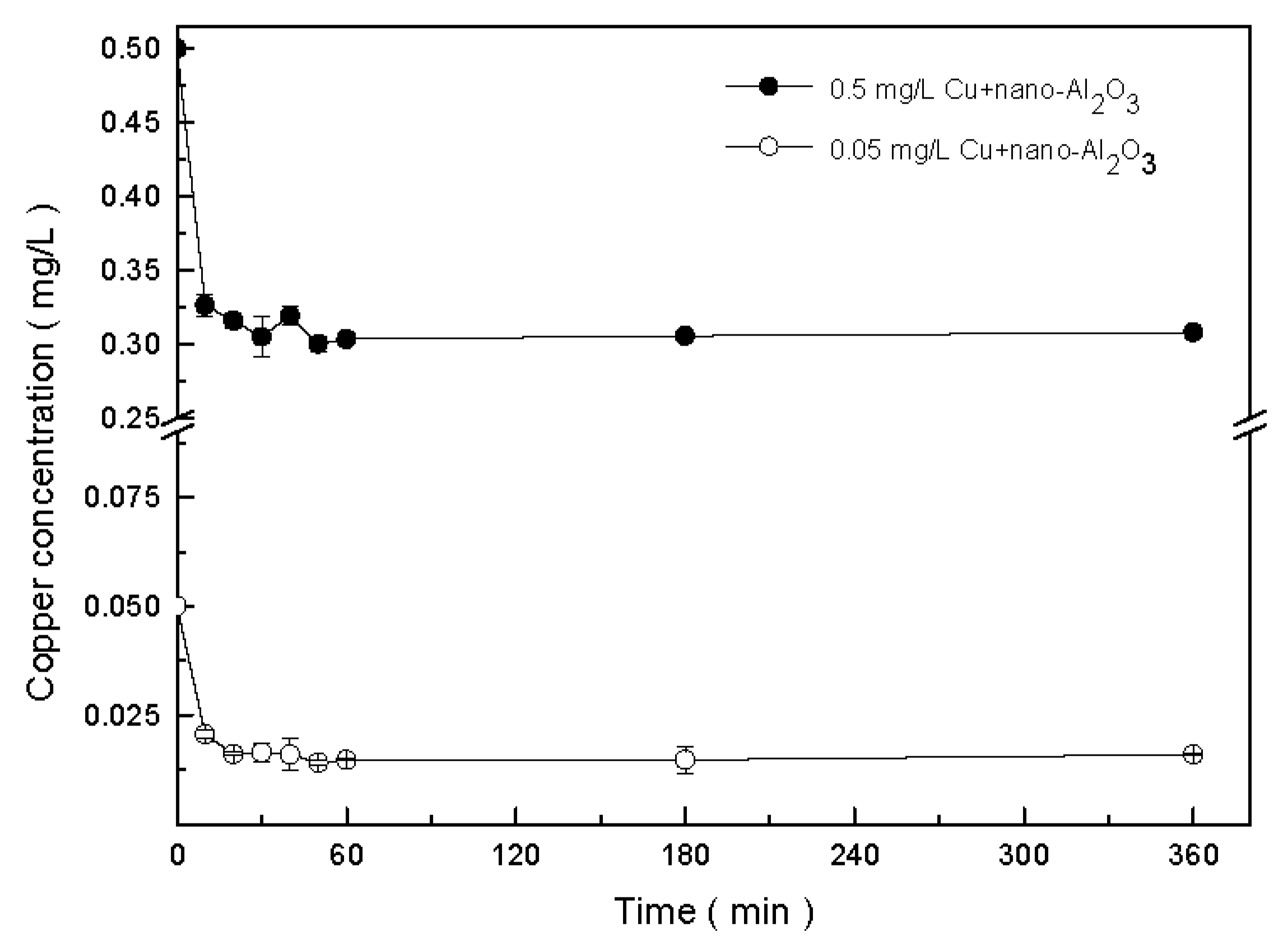
2.4. Adsorption of Copper onto Nano-Al2O3
2.5. Toxicity Test
- % Iy: growth inhibition;
- YC: mean value of cell number in the control group; and
- YT: value of cell number for the treatment replicate.
2.6. Statistical Analysis
3. Results and Discussion
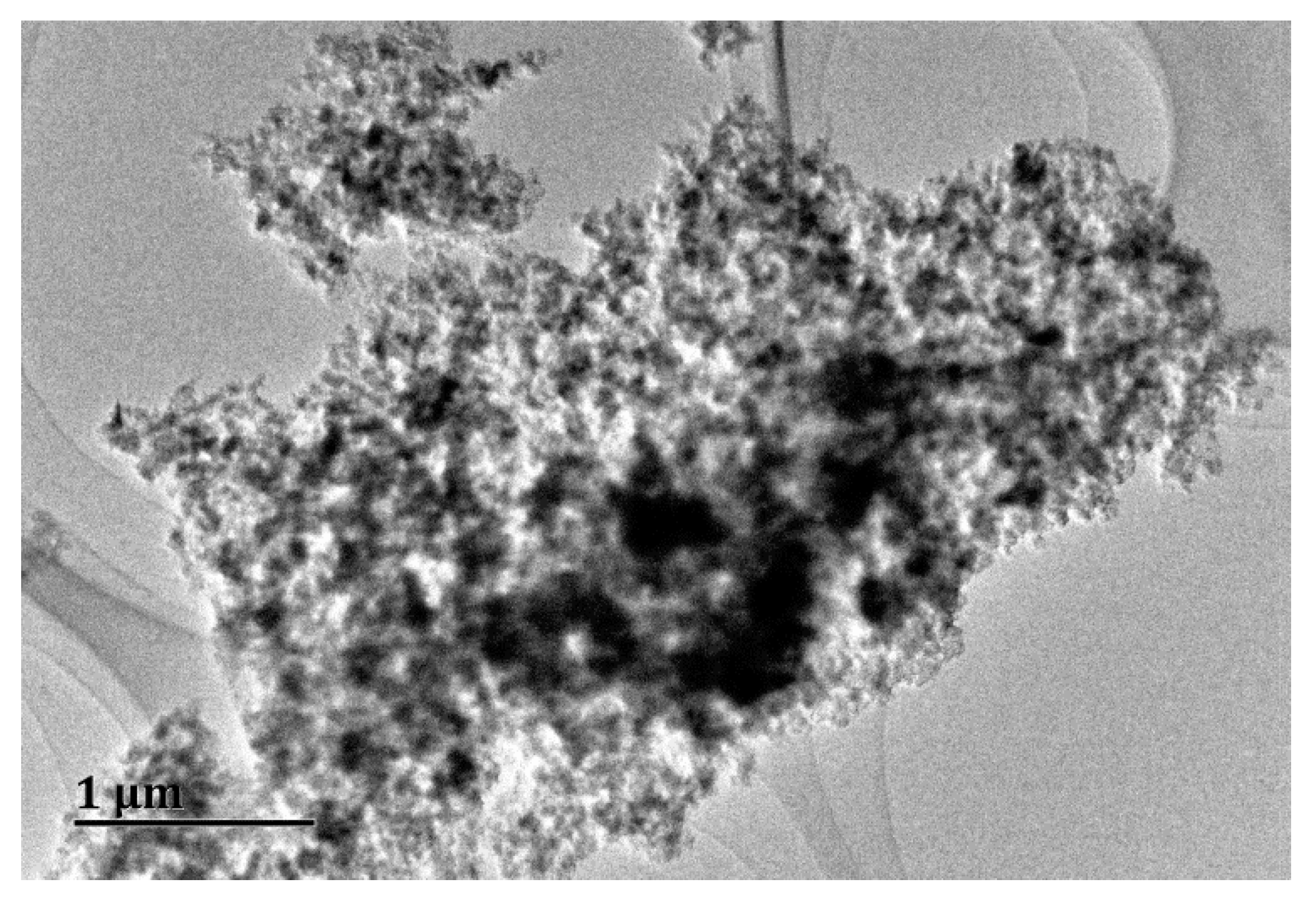
3.1. Interaction of Copper and Nano-Al2O3
3.2. Influence of Nano-Al2O3 on the Toxicity of Copper
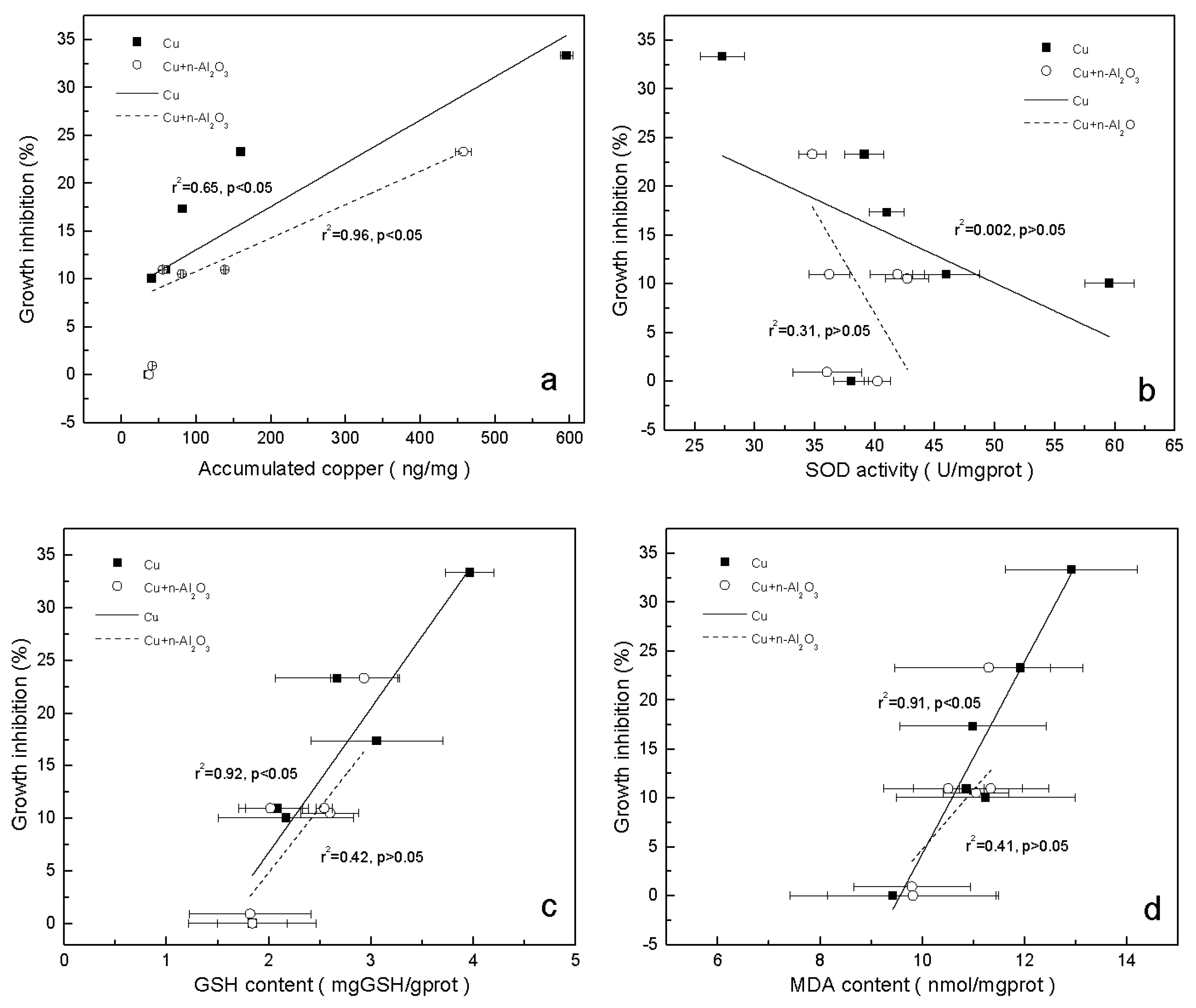
3.3. Accumulation of Copper in S. obliquus
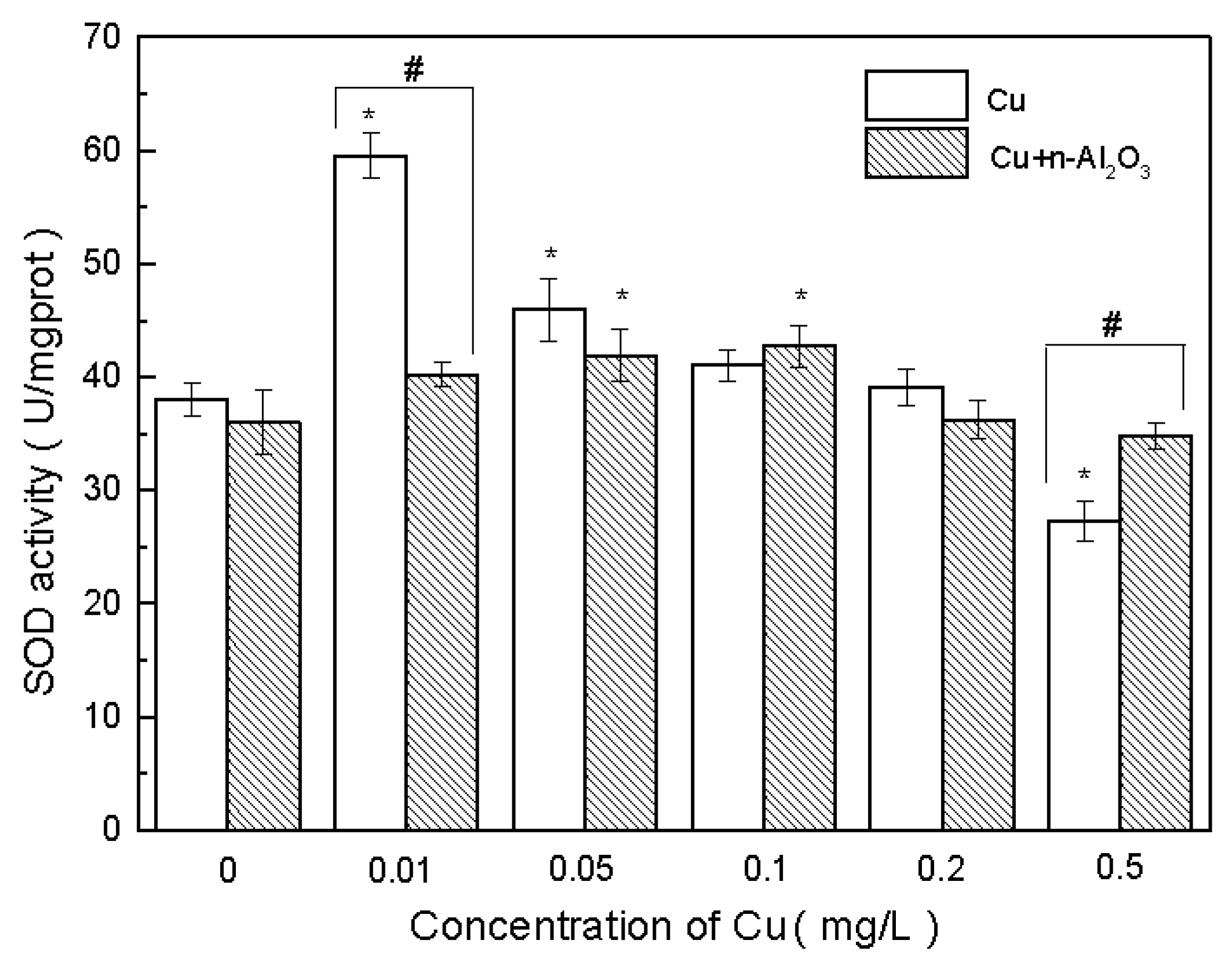
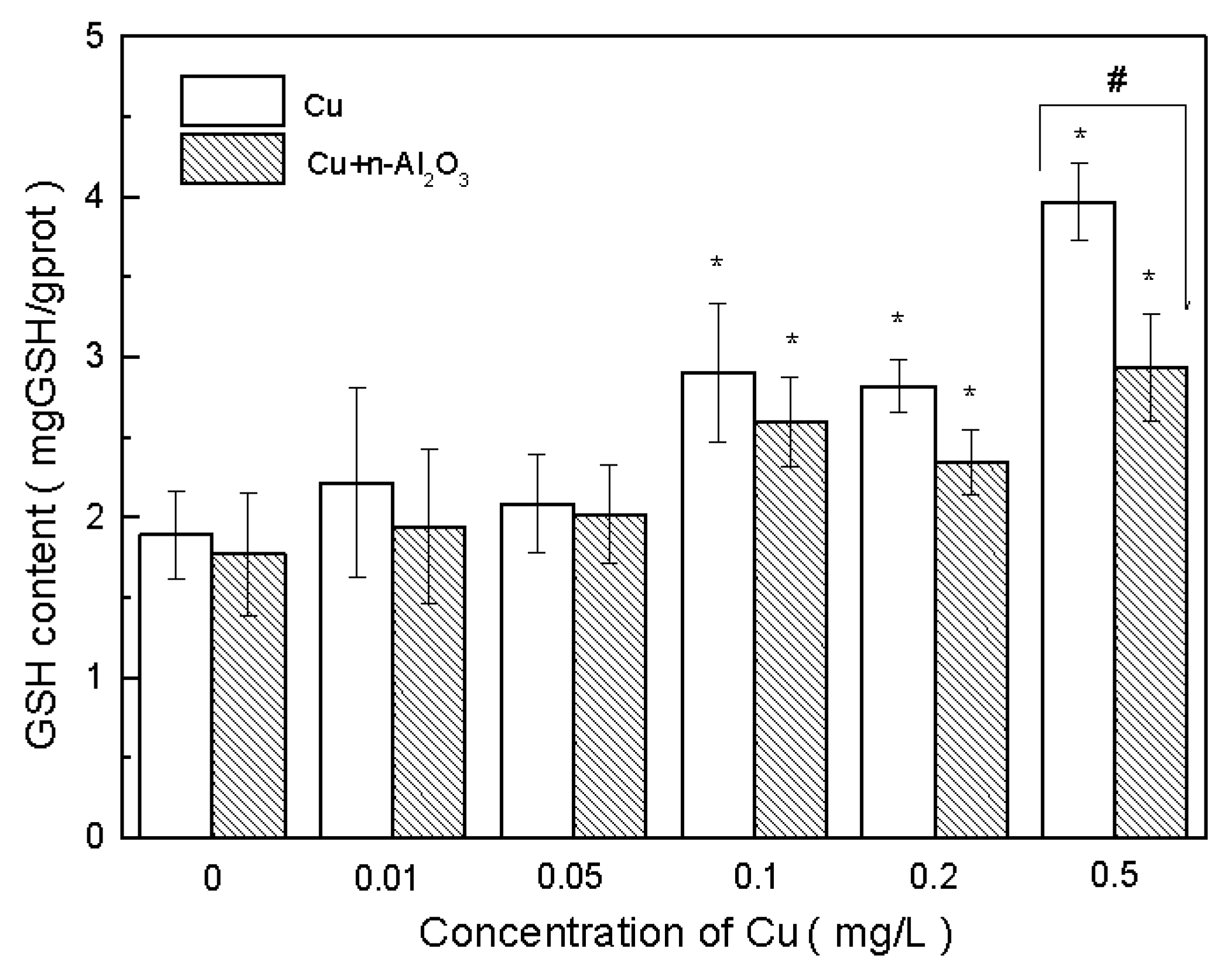
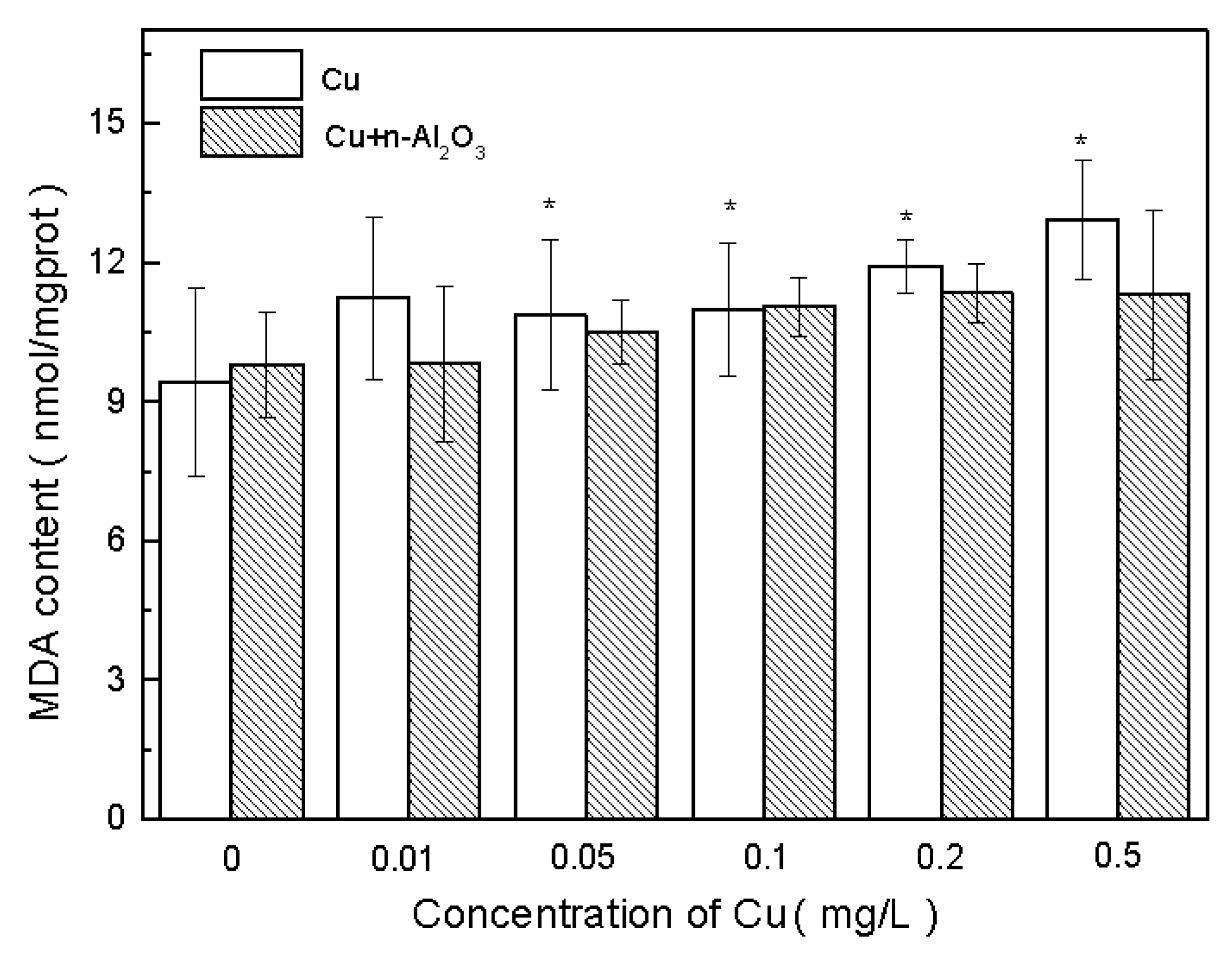
3.4. Oxidative Stress and Antioxidant Defense
3.5. Mechanism of Reduced Copper Toxicity by Nano-Al2O3
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xu, H.; Xiao, F.; Wang, D.; Ye, C. Survey of treatment process in water treatment plant and the characteristics of flocs formed by two new coagulants. Colloids Surf. A 2014, 456, 211–221. [Google Scholar] [CrossRef]
- Karwowska, E.; Mrozowicz, M.; Zawada, A.; Za˛ bkowski, T.; Ziemkowska, W.; Kunicki, A.; Olszyna, A. Impact of Al2O3 nanopowders characterised by various physicochemical properties on growth of green alga Scenedesmus quadricauda. Adv. Appl. Ceram. 2012, 111, 142–148. [Google Scholar] [CrossRef]
- Yuan, G. Environmental nanomaterials: Occurrence, syntheses, characterization, health effect, and potential applications. J. Environ. Sci. Health A 2004, 39, 2545–2548. [Google Scholar] [CrossRef]
- Wang, H.; Wick, R.L.; Xing, B. Toxicity of nanoparticulate and bulk ZnO, Al2O3 and TiO2 to the nematode caenorhabditis elegans. Environ. Pollut. 2009, 157, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Załęska-Radziwiłł, M.; Doskocz, N. DNA changes in Pseudomonas putida induced by aluminum oxide nanoparticles using RAPD analysis. Desalination Water Treat. 2016, 57, 1573–1581. [Google Scholar] [CrossRef]
- Ma, S.; Zhou, K.; Yang, K.; Lin, D. Heteroagglomeration of oxide nanoparticles with algal cells: Effects of particle type, ionic strength and pH. Environ. Sci. Technol. 2014, 49, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, I.M.; Pakrashi, S.; Chandrasekaran, N.; Mukherjee, A. Studies on toxicity of aluminum oxide (Al2O3) nanoparticles to microalgae species: Scenedesmus sp. and Chlorella sp. J. Nanopart. Res. 2011, 13, 3287–3299. [Google Scholar] [CrossRef]
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.B.; Gunten, U.V.; Wehrli, B. Global water pollution and human health. Annu. Rev. Environ. Resour. 2010, 35, 109–136. [Google Scholar] [CrossRef]
- Gottschalk, F.; Sonderer, T.; Scholz, R.W.; Nowack, B. Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions. Environ. Sci. Technol. 2009, 43, 9216–9222. [Google Scholar] [CrossRef] [PubMed]
- Von, M.N.; Slaveykova, V.I. Oxidative stress induced by inorganic nanoparticles in bacteria and aquatic microalgae—State of the art and knowledge gaps. Nanotoxicology 2014, 8, 605–630. [Google Scholar]
- Zhao, G.; Wu, X.; Tan, X.; Wang, X. Sorption of heavy metal ions from aqueous solutions: A review. Open Colloid Sci. J. 2010, 4, 19–31. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Liu, Y.; Xu, J.; Han, Y.; Xu, X. Preparation, performances of PVDF/ZnO hybrid membranes and their applications in the removal of copper ions. Appl. Surf. Sci. 2014, 316, 333–340. [Google Scholar] [CrossRef]
- Ghaemi, N. A new approach to copper ion removal from water by polymeric nanocomposite membrane embedded with γ-alumina nanoparticles. Appl. Surf. Sci. 2016, 364, 221–228. [Google Scholar] [CrossRef]
- Yang, W.-W.; Miao, A.-J.; Yang, L.-Y. Cd2+ toxicity to a green alga Chlamydomonas reinhardtii as influenced by its adsorption on TiO2 engineered nanoparticles. PLoS ONE 2012, 7, e32300. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, S.; Qiao, J.; Wang, H.; Li, L. Synergistic effects of nano-sized titanium dioxide and zinc on the photosynthetic capacity and survival of Anabaena sp. Int. J. Mol. Sci. 2013, 14, 14395–14407. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, N.; Von der Kammer, F.; Hofmann, T.; Baalousha, M.; Ottofuelling, S.; Baun, A. Algal testing of titanium dioxide nanoparticles—Testing considerations, inhibitory effects and modification of cadmium bioavailability. Toxicology 2010, 269, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.W.; Wang, Y.; Huang, B.; Wang, N.X.; Wei, Z.B.; Luo, J.; Miao, A.J.; Yang, L.Y. TiO2 nanoparticles act as a carrier of Cd bioaccumulation in the ciliate tetrahymena thermophila. Environ. Sci. Technol. 2014, 48, 7568–7575. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeldt, R.R.; Seitz, F.; Schulz, R.; Bundschuh, M. Heavy metal uptake and toxicity in the presence of titanium dioxide nanoparticles: A factorial approach using Daphnia magna. Environ. Sci. Technol. 2014, 48, 6965–6972. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeldt, R.R.; Seitz, F.; Senn, L.; Schilde, C.; Schulz, R.; Bundschuh, M. Nanosized titanium dioxide reduces copper toxicity—The role of organic material and the crystalline phase. Environ. Sci. Technol. 2015, 49, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeldt, R.R.; Seitz, F.; Zubrod, J.P.; Feckler, A.; Merkel, T.; Lüderwald, S.; Bundschuh, R.; Schulz, R.; Bundschuh, M. Does the presence of titanium dioxide nanoparticles reduce copper toxicity? A factorial approach with the benthic amphipod gammarus fossarum. Aquat. Toxicol. 2015, 165, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Dalai, S.; Pakrashi, S.; Bhuvaneshwari, M.; Iswarya, V.; Chandrasekaran, N.; Mukherjee, A. Toxic effect of Cr(VI) in presence of n-TiO2 and n-Al2O3 particles towards freshwater microalgae. Aquat. Toxicol. 2014, 146, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, J.; Forthaus, B.E.; Wang, J. Synergistic toxic effect of nano-Al2O3 and As(V) on Ceriodaphnia dubia. Environ. Pollut. 2011, 159, 3003–3008. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, J.; Klejdus, B.; Hedbavny, J.; Bačkor, M. Effect of copper and salicylic acid on phenolic metabolites and free amino acids in Scenedesmus quadricauda (chlorophyceae). Plant Sci. 2010, 178, 307–311. [Google Scholar] [CrossRef]
- Lombardi, A.T.; Hidalgo, T.M.R.; Vieira, A.A.H.; Sartori, A.L. Toxicity of ionic copper to the freshwater microalga Scenedesmus acuminatus (chlorophyceae, chlorococcales). Phycologia 2007, 46, 74–78. [Google Scholar] [CrossRef]
- Yan, H.; Pan, G. Toxicity and bioaccumulation of copper in three green microalgal species. Chemosphere 2002, 49, 471–476. [Google Scholar] [CrossRef]
- Baszynski, T.; Tukendorf, A.; Ruszkowska, M.; Skorzynska, E.; Maksymieci, W. Characteristics of the photosynthetic apparatus of copper non-tolerant spinach exposed to excess copper. J. Plant Physiol. 1988, 132, 708–713. [Google Scholar] [CrossRef]
- Li, M.; Hu, C.; Zhu, Q.; Chen, L.; Kong, Z.; Liu, Z. Copper and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in the microalga Pavlova viridis (prymnesiophyceae). Chemosphere 2006, 62, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, B.; Mehta, S.; Amar, A.; Gaur, J. Oxidative stress in Scenedesmus sp. During short-and long-term exposure to Cu2+ and Zn2+. Chemosphere 2006, 62, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Karuppiah, M.; Gupta, G. Chronological changes in toxicity of and heavy metals in sediments of two Chesapeake Bay tributaries. J. Hazard. Mater. 1998, 59, 159–166. [Google Scholar] [CrossRef]
- Šerbula, S.; Stanković, V.; Živković, D.; Kamberović, Ž.; Gorgievski, M.; Kalinović, T. Characteristics of wastewater streams within the bor copper mine and their influence on pollution of the Timok River, Serbia. Mine Water Environ. 2016, 1–6. [Google Scholar] [CrossRef]
- Peng, C.; Liu, Y.; Bi, J.; Xu, H.; Ahmed, A.S. Recovery of copper and water from copper-electroplating wastewater by the combination process of electrolysis and electrodialysis. J. Hazard. Mater. 2011, 189, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-W.; Li, Y.; Miao, A.-J.; Yang, L.-Y. Cd2+ toxicity as affected by bare TiO2 nanoparticles and their bulk counterpart. Ecotoxicol. Environ. Saf. 2012, 85, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, X.; Zhang, Z.; Chen, Y.; Crittenden, J.C. Influence of titanium dioxide nanoparticles on speciation and bioavailability of arsenite. Environ. Pollut. 2008, 157, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Poursani, A.S.; Nilchi, A.; Hassani, A.H.; Shariat, M.; Nouri, J. A novel method for synthesis of nano-γ-Al2O3: Study of adsorption behavior of chromium, nickel, cadmium and lead ions. Int. J. Environ. Sci. Technol. 2015, 12, 2003–2014. [Google Scholar] [CrossRef]
- General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. Standard Methods for Chemicals-Algae Growth Inhibition/T 21805–2008; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2008.
- OECD. OECD guidelines for the testing of chemicals. Methods Mol. Biol. 2013, 947, 37–56. [Google Scholar]
- Paoletti, F.; Mocali, A. Determination of superoxide dismutase activity by purely chemical system based on NAD(P)H oxidation. Meth. Enzymol. 1989, 186, 209–220. [Google Scholar]
- Anderson, M.E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985, 113, 548. [Google Scholar] [PubMed]
- Shaw, J.; Large, A.; Donkin, P.; Evans, S.; Staff, F.; Livingstone, D.; Chipman, J.; Peters, L. Seasonal variation in cytochrome P450 immunopositive protein levels, lipid peroxidation and genetic toxicity in digestive gland of the mussel Mytilus edulis. Aquat. Toxicol. 2004, 67, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fan, W.; Xue, F.; Wang, X.; Li, X.; Guo, L. Chronic effects of six micro/nano-Cu2O crystals with different structures and shapes on Daphnia magna. Environ. Pollut. 2015, 203, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Fang, X.; Somasundaran, P.; Chandran, K. Short-term effects of TiO2, CeO2, and Zno nanoparticles on metabolic activities and gene expression of Nitrosomonas europaea. Chemosphere 2015, 128, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Aruoja, V.; Dubourguier, H.C.; Kasemets, K.; Kahru, A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae pseudokirchneriella subcapitata. Sci. Total Environ. 2009, 407, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, S.; Jalali, M.; Afkhami, A. Heavy metals removal from aqueous solutions using TiO2, MgO, and Al2O3 nanoparticles. Chem. Eng. Commun. 2013, 200, 448–470. [Google Scholar] [CrossRef]
- Wang, X.S.; Zhu, L.; Lu, H.J. Surface chemical properties and adsorption of Cu(II) on nanoscale magnetite in aqueous solutions. Desalination 2011, 276, 154–160. [Google Scholar] [CrossRef]
- Kumar, S.; Rawat, N.; Kar, A.; Tomar, B.; Manchanda, V. Effect of humic acid on sorption of technetium by alumina. J. Hazard. Mater. 2011, 192, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Yin, K.; Yu, X. Effect of natural aquatic colloids on Cu(II) and Pb(II) adsorption by Al2O3 nanoparticles. Chem. Eng. J. 2013, 225, 464–473. [Google Scholar] [CrossRef]
- Brady, D.; Letebele, B.; Duncan, J.; Rose, P. Bioaccumulation of metals by scenedesmus, selenastrum and chlorella algae. Water SA 1994, 20, 213. [Google Scholar]
- Mcbride, M.B.; Baveye, P. Diffuse double-layer models, long-range forces, and ordering in clay colloids. Soilence Soc. Amer. J. 2002, 66, 1207–1217. [Google Scholar] [CrossRef]
- French, R.A.; Jacobson, A.R.; Kim, B.; Isley, S.L.; Penn, R.L.; Baveye, P.C. Influence of ionic strength, pH, and cation valence on aggregation kinetics of titanium dioxide nanoparticles. Environ. Sci. Technol. 2009, 43, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.D.; Zevi, Y.; Kou, X.M.; Xiao, J.; Wang, X.J.; Jin, Y. Effect of dissolved organic matter on the stability of magnetite nanoparticles under different pH and ionic strength conditions. Sci. Total Enviro. 2010, 408, 3477–3489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, L.; Zhang, D.; Lu, S.; Yu, X. Impact of environmental conditions on the adsorption behavior of radionuclide 63 Ni(II) on γ-Al2O3. Colloids Surf. A Physicochem Eng. Asp. 2011, 380, 16–24. [Google Scholar] [CrossRef]
- Velzeboer, I.; Hendriks, A.J.; Ragas, A.M.; Van De Meent, D. Nanomaterials in the environment aquatic ecotoxicity tests of some nanomaterials. Environ. Sci. Technol. 2008, 27, 1942–1947. [Google Scholar]
- Zhang, W.; Xiong, B.; Chen, L.; Lin, K.; Cui, X.; Bi, H.; Guo, M.; Wang, W. Toxicity assessment of Chlorella vulgaris and Chlorella protothecoides following exposure to Pb(II). Environ. Toxicol. Pharmacol. 2013, 36, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.; Sigaud-kutner, T.; Leitao, M.A.; Okamoto, O.K.; Morse, D.; Colepicolo, P. Heavy metal-induced oxidative stress in algae. J. Phycol. 2003, 39, 1008–1018. [Google Scholar] [CrossRef]
- Yang, H.; Huang, Z.-Y.; Li, J.; Hu, Y. Mt-like proteins: Potential bio-indicators of chlorella vulgaris for zinc contamination in water environment. Ecol. Indic. 2014, 45, 103–109. [Google Scholar] [CrossRef]
- Sabatini, S.E.; Rocchetta, I.; Nahabedian, D.E.; Luquet, C.M.; Eppis, M.R.; Bianchi, L.; Ríos de Molina, C. Oxidative stress and histological alterations produced by dietary copper in the fresh water bivalve Diplodon chilensis. Comp. Biochem. Physiol. Pt. C 2011, 154, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Chen, L.; Lu, N.; Zhao, Y.; Yuan, X. Toxic effects of enrofloxacin on Scenedesmus obliquus. Front. Environ. Sci. Eng. 2012, 6, 107–116. [Google Scholar] [CrossRef]
- Sabatini, S.E.; Juárez, Á.B.; Eppis, M.R.; Bianchi, L.; Luquet, C.M.; de Molina, M.D.C.R. Oxidative stress and antioxidant defenses in two green microalgae exposed to copper. Ecotoxicol. Environ. Saf. 2009, 72, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Long, Z.; Lin, D. Toxicity of oxide nanoparticles to the green algae Chlorella sp. Chem. Eng. J. 2011, 170, 525–530. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Pokora, W.; Baścik-Remisiewicz, A.; Tukaj, S.; Kalinowska, R.; Pawlik-Skowrońska, B.; Dziadziuszko, M.; Tukaj, Z. Adaptation strategies of two closely related Desmodesmus armatus (green alga) strains contained different amounts of cadmium: A study with light-induced synchronized cultures of algae. J. Plant Physiol. 2014, 171, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhu, N.; Lavoie, M.; Wang, J.; Qian, H.; Fu, Z. Copper toxicity to phaeodactylum tricornutum: A survey of the sensitivity of various toxicity endpoints at the physiological, biochemical, molecular and structural levels. Biol. Met. 2014, 27, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Stoiber, T.L.; Shafer, M.M.; Armstrong, D.E. Induction of reactive oxygen species in Chlamydomonas reinhardtii in response to contrasting trace metal exposures. Environ. Toxicol. 2013, 28, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Nagalakshmi, N.; Prasad, M.N.V. Copper-induced oxidative stress in Scenedesmus bijugatus: Protective role of free radical scavengers. Bull. Environ. Contam. Toxicol. 1998, 61, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Butler, K.S.; Casey, B.J.; Garborcauskas, G.V.; Dair, B.J.; Elespuru, R.K. Assessment of titanium dioxide nanoparticle effects in bacteria: Association, uptake, mutagenicity, co-mutagenicity and DNA repair inhibition. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 768, 14–22. [Google Scholar] [CrossRef] [PubMed]
| Component | Concentration (mg/L) | Component | Concentration (mg/L) |
|---|---|---|---|
| NaNO3 | 1500 | K2HPO4 | 40 |
| CaCl2·2H2O | 36 | MgSO4·7H2O | 75 |
| C6H10FeNO8 | 6 | Na2CO3 | 20 |
| C6H8O7 | 6 |
| Concentration of Nano-Al2O3 (mg/L) | Concentration of Copper (mg/L) | |||||
|---|---|---|---|---|---|---|
| 0 | 0 | 0.01 | 0.05 | 0.1 | 0.2 | 0.5 |
| 1.0 | 0 | 0.01 | 0.05 | 0.1 | 0.2 | 0.5 |
| Concentration of Nano-Al2O3 (mg/L) | Concentration of Copper (mg/L) | Time (h) | MHD (nm) | PdI | Zeta Potential (mV) |
|---|---|---|---|---|---|
| 1.0 | 0 | 0 | 439.3 ± 4.9 | 0.223 − 0.332 | −20.5 ± 0.7 |
| 24 | 540.4 ± 22.4 | 0.247 − 0.270 | −17.7 ± 0.5 | ||
| 48 | 1648.3 ± 122.3 | 0.180 − 0.222 | −19.9 ± 1.2 | ||
| 72 | 1860.0 ± 32.7 | 0.267 − 0.338 | −20.6 ± 1.8 | ||
| 0.05 | 0 | 327.1 ± 7.5 | 0.224 − 0.255 | −19.8 ± 1.1 | |
| 24 | 501.3 ± 6.8 | 0.226 − 0.245 | −18.6 ± 1.2 | ||
| 48 | 1478.2 ± 120.6 | 0.322 − 0.376 | −17.7 ± 0.7 | ||
| 72 | 1720.1 ± 74.9 | 0.326 − 0.331 | −20.8 ± 2.2 | ||
| 0.5 | 0 | 336.5 ± 4.1 | 0.210 − 0.270 | −20.1 ± 2.3 | |
| 24 | 338.9 ± 4.5 | 0.275 − 0.336 | −19.4 ± 1.8 | ||
| 48 | 1530.3 ± 161.7 | 0.241 − 0.250 | −18.8 ± 0.7 | ||
| 72 | 1781.4 ± 97.5 | 0.242 − 0.367 | −20.6 ± 1.4 |
| Concentration of Nano-Al2O3 (mg/L) | Concentration of Copper (mg/L) | 24 (h) | 48 (h) | 72 (h) | Accumulated Copper (ng/mg) |
|---|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 0 | 35.47 ± 0.37 |
| 0.01 | −13.67 | −0.96 | 10.05 | 40.83 ± 0.42 | |
| 0.05 | −4.02 | 0.97 | 10.96 | 59.54 ± 0.01 | |
| 0.1 | −6.23 | 7.23 | 17.35 | 81.67 ± 1.33 | |
| 0.2 | −1.61 | 13.98 | 23.29 | 159.83 ± 2.02 | |
| 0.5 | −2.41 | 23.63 | 33.33 | 596.04 ± 8.36 | |
| 1.0 | 0 | −16.88 | −0.48 | 0.91 | 40.21 ± 0.21 |
| 0.01 | −16.87 | −2.89 | 0.01 | 37.42 ± 0.01 | |
| 0.05 | −9.64 | 4.34 | 10.95 | 55.68 ± 1.39 | |
| 0.1 | −7.48 | 5.15 | 10.50 | 81.29 ± 2.16 | |
| 0.2 | −9.64 | 5.79 | 10.96 | 138.62 ± 1.58 | |
| 0.5 | −6.43 | 6.27 | 23.29 | 458.21 ± 11.09 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhou, S.; Fan, W. Effect of Nano-Al2O3 on the Toxicity and Oxidative Stress of Copper towards Scenedesmus obliquus. Int. J. Environ. Res. Public Health 2016, 13, 575. https://doi.org/10.3390/ijerph13060575
Li X, Zhou S, Fan W. Effect of Nano-Al2O3 on the Toxicity and Oxidative Stress of Copper towards Scenedesmus obliquus. International Journal of Environmental Research and Public Health. 2016; 13(6):575. https://doi.org/10.3390/ijerph13060575
Chicago/Turabian StyleLi, Xiaomin, Suyang Zhou, and Wenhong Fan. 2016. "Effect of Nano-Al2O3 on the Toxicity and Oxidative Stress of Copper towards Scenedesmus obliquus" International Journal of Environmental Research and Public Health 13, no. 6: 575. https://doi.org/10.3390/ijerph13060575





