Identifying Ethical Issues in Mental Health Research with Minors Adolescents: Results of a Delphi Study
Abstract
:1. Introduction
- to generate an epidemiological database on the general health status of European adolescents in the age group between 14 and 16 from 10 European countries (based on the collection of assessment data including demographic information, psychopathology, lifestyles, values and risk-behaviors)
- to evaluate three types of school-based interventions in comparison to a minimal intervention control group on mental literacy; these interventions included gatekeeper training (QPR), training adolescents in mental health promotion (Awareness) which is now named Youth Aware of Mental Health (YAM), and professional screening of adolescents for mental health problems and risk behaviors (ProfScreen).
- What needs for ethical guidance do research experts articulate for conducting safe, knowledge-based and fruitful MHR with children and adolescents?
- What specific ethical issues should define MHR, others than those mentioned by the research ethics codes and guidelines?
2. Key Concepts—Capacity to Consent, Emancipated/Mature Minors, and Vulnerability
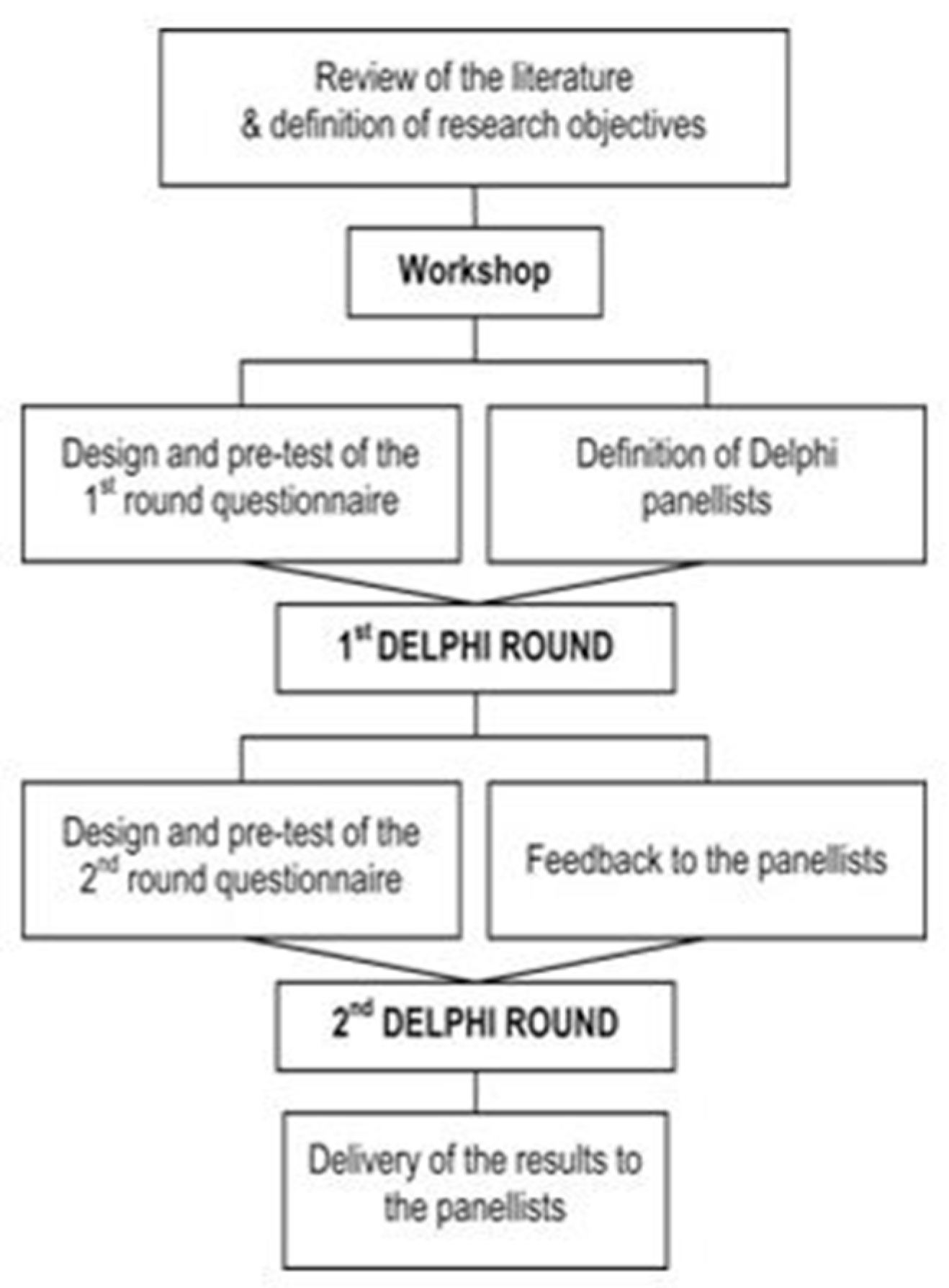
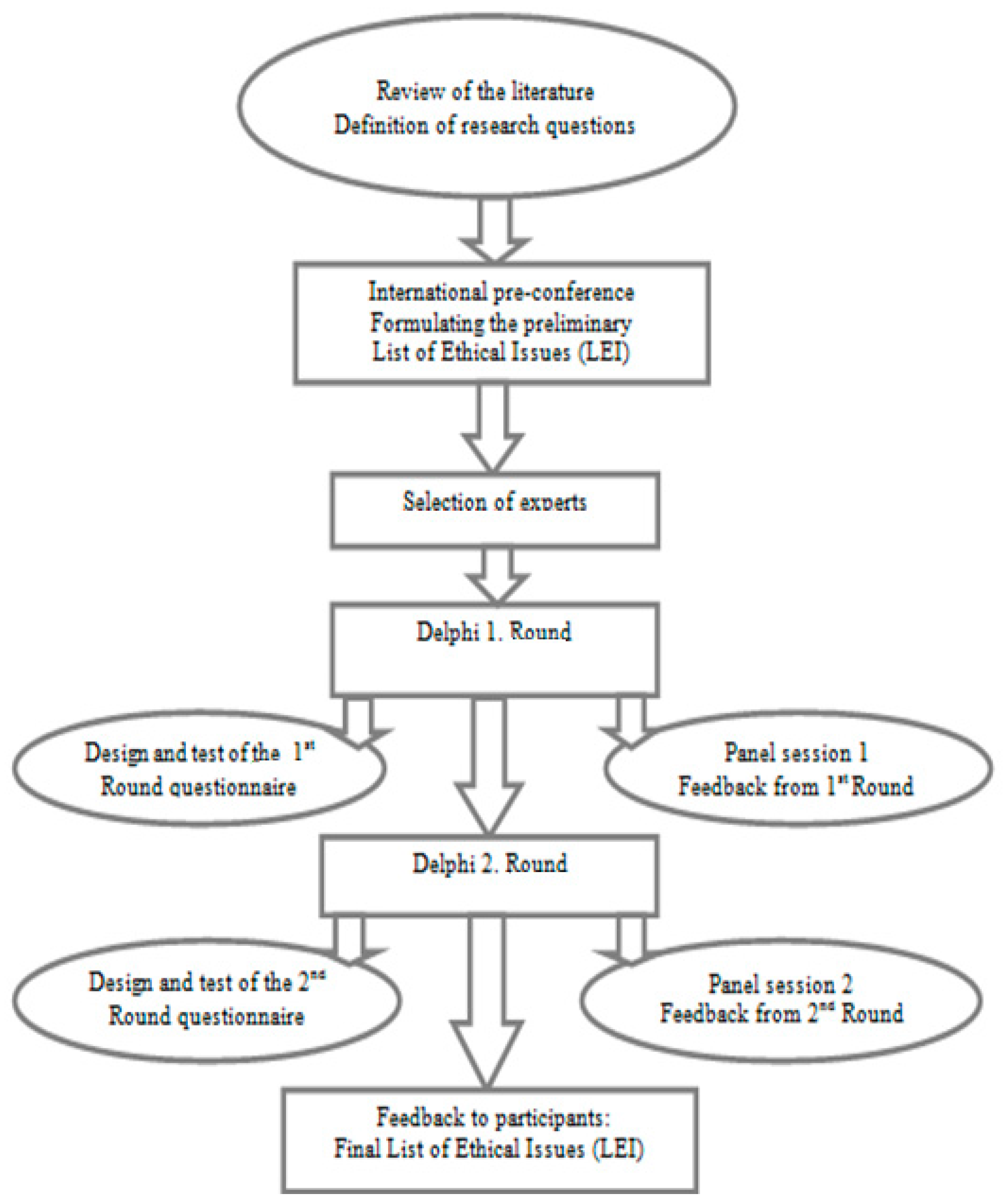
3. Materials and Methods
- Pre-conference—formulating the preliminary LEI
- Selection of Experts—sending invitation letters for participation
- 1st. Delphi Round—Design, distribution, completion of data collection and analysis of the first on-line questionnaire
- Panel session 1
- Feedback to participants—communicating the new LEI and summary of the results from the 1. Round
- 2nd. Delphi Round—Design and distribution of the second on-line questionnaire
- Panel session 2
- Feedback to participants from the 2. Round
- Final Feedback—Providing final LEI to participants
3.1. Pre-conference—List of Ethical Issues (LEI)
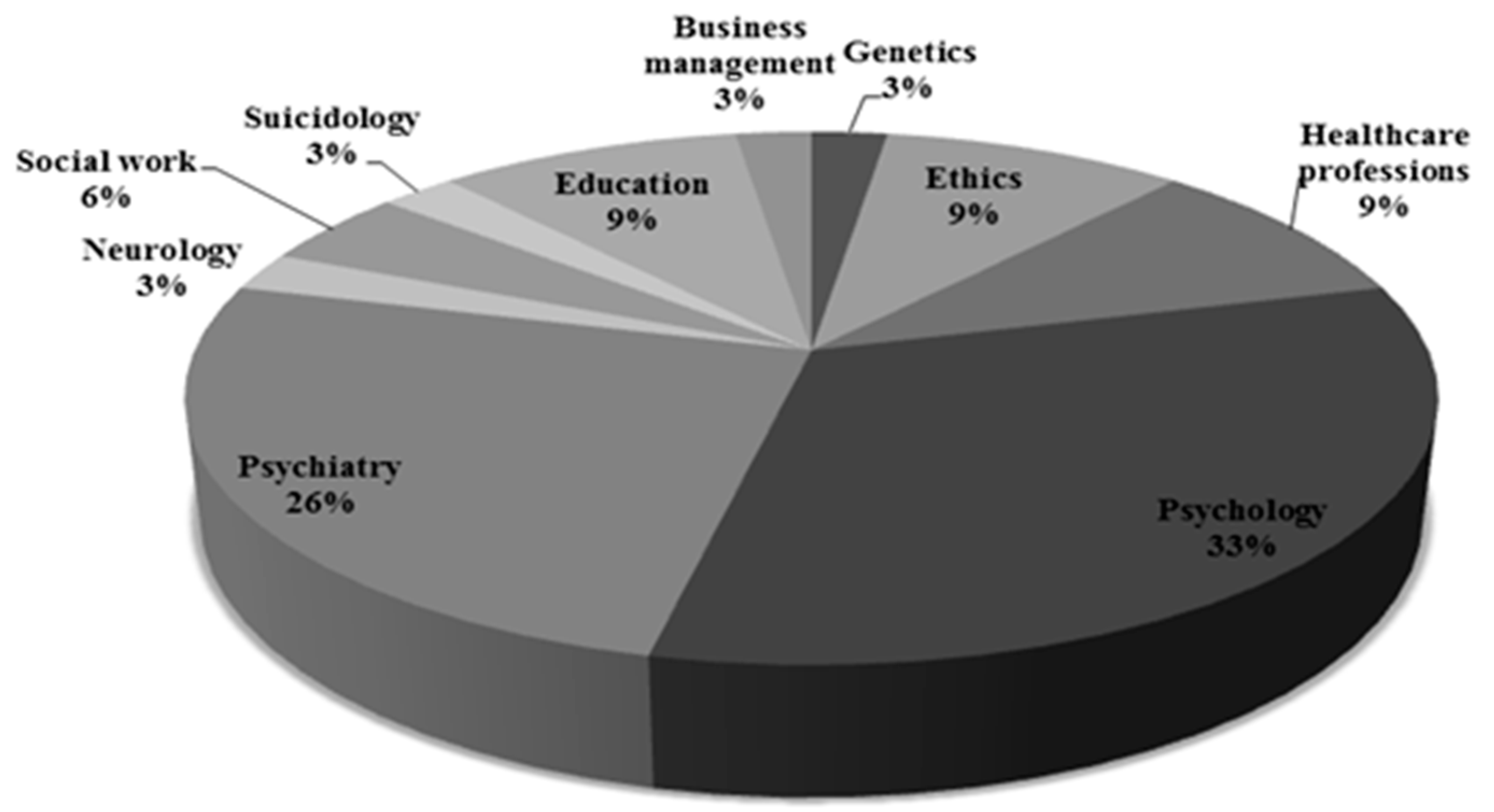
3.2. Selection of Experts—Using the SEYLE Study as a Paradigm Project
3.3. First Delphi Round
3.4. Panel Session 1 and Feedback to Participants
3.5. Second Delphi Round
3.6. Panel Session 2 and Feedback to Participants from the 2. Round
3.7. Final Feedback
4. Results
4.1. Ethical Issues Encountered in Research with Minors
4.2. The Circumstances under which Parental Consent Might Be Waived
- benefit for the minor (e.g., if parents withhold their consent to a study with potential major benefit for the minor and if supported by the decision of an institutional review board (IRB));
- minimal risk/no harm (i.e., “if no harm is possible, e.g., questionnaire studies”; “if the research entails only very little risk and burden”; “if the research is more of a routine, with no significant risk or additional unusual tasks required from the participants”; “the research does not involve any procedure being performed which would not be part of routine case management”);
- the nature or consequences of the research (i.e., “in certain sensitive topics if agreed by IRB”; “if the research involves intimate issues like sexual orientation—homosexuality, or the relationship between the minor and his/her parents”; “if the consequences do not affect the parents”);
- parents’ status (e.g., “if parents are not involved in the education of the child at present”; “if parents are not available“; “if parents lack competence for consenting“; “antisocial/problematic parents”);
- minor’s status (i.e., “always, if the minor is deemed to be competent (from the age of 14))”;
- special circumstances (“life-death situation”).
4.3. Minor’s Dissent
4.4. Obligation to Disclose Sensitive Information
- quantitative (how much information): i.e., “the overall results of the study”; “basic information on the scientific question(s) of the study”; “strictly the information necessary for taking the right measures in case of high risk of harm”, or “no results”;
- qualitative (what kind of information): i.e., “if the minor shows behavior that puts himself/herself or others at serious risk (planned crimes)”; “if the child is reporting significant risk behaviors and health problems”; “suicidal ideation and behavior, pathological gambling, drug abuse, promiscuity”; “risk for suicide, severe alcohol or in case of life-threatening behavior (self-cutting, suicide attempt, bulimia, anorexia)”; “information about possibility of the need of extra-support (extra-care): medical or psychological”.
4.5. Breaching Confidentiality Agreement with a Minor Deemed Competent to Consent
- “if the participant is at risk of suicide (e.g., suicidal ideation or attempts)”;
- “if the physical or mental health of the minor is in serious danger (suicidal, self-damaging behavior, severe mental disorder, drug abuse, sexual abuse)”;
- low or non-compliance to a proposed intervention if the data showed a self-harm risk (e.g., s/he does not come to the therapy sessions);
- child abuse or pressing need of medical care.
4.6. Handling of Critical Data
4.7. Preventing Minors to Withdraw from the MHR Studies
5. Discussion
5.1. Competence to Give Consent
5.2. Limits of Confidentiality
5.3. Risk of Harm
6. Strengths and Limitations
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ASD | Autism Spectrum Disorder |
| BPS | British Psychological Society |
| CIOMS | Council of International Organizations of Medical Sciences |
| CFR | Code of Federal Regulation |
| IRB | Institutional Review Board |
| MHR | Mental Health Research |
| RCT | Randomized Controlled Trial |
| WHO | World Health Organization |
Appendix
| CATEGORIES | ITEMS of EACH CATEGORY | Consensus for Acceptation (≤70%) | Consensus for Rejection (≤30%) | Ambiguos Needs (<70% and >30%) |
|---|---|---|---|---|
| Assent | Minor’s assent must be obtained always in addition with parental consent | 80.64% | ||
| Assent should contain general information about the purpose of the study and address the information in a language appropriate to the minor’s cognitive development | 96.77% | |||
| Minor should be able to understand what is proposed in the study and that he/she has the opportunity to refuse | 100% | |||
| Minor consent | Mature minors can give consent for participating in research if they are deemed to be competent | 87% | ||
| The competence requested for the validity of a minor’s consent in research is related to the minor’s cognitive development to understand the information about the nature and purpose of the study, to weigh the information and foresee the consequences, and to arrive at a decision in question | 90.32% | |||
| Emancipated minors who are pregnant, parents themselves, in military service or self-supported can give consent for participating in research | 80.64% | |||
| The competent minor should show understanding regarding the nature and purpose of the study and consequences of his participation (potential risks, anticipated benefits, and limits of confidentiality) | 100% | |||
| The minor should be able to give reasons why he/she wants to participate (in order to justify a reasonable and free participation) | 83.87% | |||
| The minor must give consent voluntarily | 96.74% | |||
| Circumstances under which the consent of a competent minor might suffice alone | If the minor is over 16 and the research involves no more than minimal risk or if the risk of harm is potentially low (e.g., questionnaire studies), with IRB approval | 83.87% | ||
| If parents withhold their consent to a study with potential major benefit for the minor and if supported by the decision of an Institutional Review Board | 61.29% | |||
| If the minor is competent to make the decision and if the consequences do not affect the parents or if the issue is not of a child protection | 77.41% | |||
| If parents are not involved in the education of the child at present or if they are not available | 51.61% | |||
| In research involving minors under 16, it is hardly acceptable to take the minor's consent as sufficient alone | 64.51% | |||
| The minor’s consent cannot be accepted as sufficient alone for research projects. It can be accepted only in practice, for treatment | 16.12% | |||
| Nature of information that should be reported in case of a minor enrolled in a risk-taking behavior study | If there are any major and acute risks of harm to self (suicidal ideation and behavior), pathological gambling, drug abuse, promiscuity | 87.09% | ||
| If the minor shows behavior that puts others at serious risk (e.g., planned crime) | 93.54% | |||
| Obligation to disclose | Information should be provided to the parents regarding the overall results of the study in the respective school or group of their child | 61.29% | ||
| If the child is reporting significant risk behaviors, then direct permission should be sought from the parents and child for a further clinical interview to confirm the issues | 83.87% | |||
| If information collected for the research makes researchers believe that medical care is needed for the minor, parents/guardians should be advised to seek medical care | 90.32% | |||
| Breaching confidentiality with a minor deemed competent to give consent | If there is a high risk of harmful behavior to self (suicidal thoughts and ideation, sexual abuse), if the physical or mental health of the minor is in serious danger (severe mental disorder, nocuous drug abuse), if his/her behavior is an acute threat to others | 96.77% | ||
| If data show a risk of self-harm and if the child is not compliant to a proposed intervention (e.g., he/she does not come to the therapy sessions) | 70.96% | |||
| Information that might be disclosed is if the minor is in need for medical care | 74.19% | |||
| Handling of critical data (e.g. self-damaging behavior) if minor decides to withdraw from the study | Is depending on the minor’s decision | 32.25% | ||
| Withdrawing does not allow using data | 54.83% | |||
| It should not be used to calculate any results. All data from the withdrawers should be separated from the active data to analyze the active dropouts using the approach “intention-to-treat“ | 64.51% | |||
| The data should be used anonymously if scientifically possible, but otherwise destroyed | 61.29% | |||
| It should be deleted if the participant wants and data is retractable | 80.64% | |||
| The critical data should be irreversibly deleted | 35.48% | |||
| Data must be archived and can be used | 41.93% | |||
| Managing confidential information regarding critical data with minor who reaches the legal age of consent (while being enrolled in the study) | The management of confidentiality regarding critical data should be part and regulated in consent and assent, prior to the onset of the research. | 87.09% | ||
| In studies investigating current suicidal behavior, the results must be immediately checked and clinicians have to be informed right away. Additionally, the minors and their parents should receive contact addresses via the study information about the appropriate help centers, where they can call if any psychological problems or uncertainties concerning health status might arise in the process or as a consequence of the process of the testing | 87.09% | |||
| If there is evidence or indication for critical behavior that matches the specified circumstances for breaching confidentiality the parents should be informed | 70.96% | |||
| Once the minors are over 18, the information is now theirs and their permission would need to be sought before it was released to a third party | 87.96% | |||
| Waiving of parental consent - procedures recommended in a situation in which a competent minor who can give consent, refuses that the parents be asked to give their consent | An independent expert should be involved to answer the question if the minor is competent to give or refuse consent | 45.16% | ||
| If the research entails only minimal risks, the adolescents (over age of 14) should be enrolled without parental consent; if risks are higher, it can only be done after the parents consented. Otherwise the minor should not be recruited | 45.16% | |||
| The minor should be able to justify his/her wish not to inform the parents. If there are still good reasons (i.e., parental violence or drug abuse) after discussion and he/she fulfills all criteria for competent consent, he/she should be allowed to participate | 74.19% | |||
| The minor should be included in the study if the parents are not affected by the consequences | 32.25% | |||
| If the study has major implications for the child’s health or life (e.g., self-damage behaviors identification) the involvement of the parents should be included in the consent form as a possibility/necessity under certain conditions. If the child does not want to involve the parents, he/she could be asked about another adult (relative) he/she trusts to (with contact data) | 61.29% |
References
- WHO. Preventing Suicide: A Global Imperative. 2014. Available online: http://www.who.int/mental_health/suicide-prevention/world_report_2014/en/ (accessed on 17 August 2015).
- Hern, M.; Miller, M.; Sommers, M.; Dyehouse, J. Sensitive topics and adolescents: making research about risk behaviors happen. Issues Compr. Pediatr. Nurs. 1998, 21, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Dickson-Swift, V.; James, E.; Liamputtong, P. Undertaking Sensitive Research in the Health and Social Sciences: Managing Boundaries, Emotions and Risks; Cambridge University Press: Cambridge, UK, 2008; pp. 1–9. [Google Scholar]
- Pasternak, R.H.; Geller, G.; Parrish, C.; Cheng, T.L. Adolescent and parent perceptions on youth participation in risk behavior research. Arch. Pediatr. Adolesc. Med. 2006, 160, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Hiriscau, I.E.; Stingelin-Giles, N.; Reiter-Theil, S. Ethical issues in research with minors. Orientation by codes and many open questions. Rev. Romana Bioet. 2013, 11, 74–83. [Google Scholar]
- Ethics Codes in Medicine. In Foundations and Achievements of Codification Since 1947; Tröhler, U.; Reiter-Theil, S.; Herych, E. (Eds.) Ashgate: Aldershot, UK, 1998.
- Solyom, A.E.; Moreno, J.D. Protection of children and adolescents in psychiatric research: An unfinished business. Hec. Forum. 2005, 17, 210–226. [Google Scholar] [PubMed]
- International Ethical Guidelines for Biomedical Research Involving Human Subjects, Prepared by the Council of International Organizations of Medical Sciences (CIOMS) in Collaboration with the World Health Organization (WHO). Geneva 2002. Available online: http://www.cioms.ch/publications/layout_guide2002.pdf (accessed on 4 February 2014).
- Code of Human Research Ethics, British Psychological Society, 2010. Available online: http://www.bps.org.uk/sites/default/files/documents/code_of_human_research_ethics.pdf (accessed on 4 February 2014).
- WMA Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects, 1964. Available online: http://www.wma.net/en/30publications/10policies/b3/index.html (accessed on 4 February 2014).
- Ganzini, L.; Ladislav, V.; Nelson, W.A.; Fox, A. Ten myths about decision-making capacity. J. Am. Med. Dir. Assoc. 2004, 5, 263–267. [Google Scholar] [CrossRef]
- Koch, H.G.; Reiter-Theil, S.; Helmchen, H. Informed consent in psychiatry. European perspectives of ethics, law and clinical practice. J. Med. Ethics 1999, 25, 428–429. [Google Scholar]
- Hickey, K. Minor’s rights in medical decision-making. JONA’S Healthc. Law Ethics Regul. 2007, 9, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Gillick v West Norfolk and Wisbech Area Health Authority (1985) UKHL 7, (1986) 1 FLR 229, (1986) AC 112. Available online: http://www.bailii.org/ (accessed on 14 December 2015).
- Code of Federal Regulations, 2009. Title 45 Public Welfare. Department of Health and Human Services Part 46, Protection of Human Subjects. Available online: http://www.hhs.gov/ohrp/policy/ohrpregulations.pdf (accessed on 4 February 2014).
- Grisso, T.; Appelbaum, P.S. The Assessment of Decision-Making Capacity: A Guide for Physicians and Other Health Professionals; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Helgesson, G. Children, longitudinal studies, and informed consent. Med. Health Care Philos 2005, 8, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; Stewart, A. Ethical issues in child and adolescent psychiatry. J. Med. Ethics 1987, 13, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.E.; Williams, B.J.; Knowles, A. Breaching confidentiality with adolescent clients: A survey of Australian psychologists about the considerations that influence their decisions. Psychiatry Psychol. Law 2011. [Google Scholar] [CrossRef]
- Lothen-Kline, C.; Howard, D.E.; Hamburger, E.K.; Worrell, K.D.; Boekeloo, B.O. Truth and consequences: ethics, confidentiality, and disclosure in adolescent longitudinal prevention research. J. Adolesc. Health 2003, 33, 385–394. [Google Scholar] [CrossRef]
- Hiriscau, E.I.; Stingelin-Giles, N.; Stadler, C.; Schmeck, K.; Reiter-Theil, S. A right to confidentiality or a duty to disclose? Ethical guidance for conducting prevention research with children and adolescents. Eur. Child Adolesc. Psychiatr. 2014, 23, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Lehrman, N.S.; Sharav, V.H. Ethical problems in psychiatric research. J. Ment. Health Adm. 1997, 24, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.E. Turn-of-the-century ethical issues in child psychiatric research. Curr. Psychiatry Rep. 2001, 3, 109–114. [Google Scholar] [CrossRef] [PubMed]
- The Delphi Method: Techniques and Applications; Linstone, H.A.; Turoff, M. (Eds.) Addison-Wesley: Reading, MA, USA, 1975; pp. 3–12.
- Rao, J.K.; Anderson, L.A.; Sukumar, B.; Beauchesne, D.A.; Stein, T.; Frankel, R.M. Engaging communication experts in a Delphi process to identify patient behaviors that could enhance communication in medical encounters. BMC Health Serv. Res. 2010. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.R. Malleable Delphi: Delphi research technique, its evolution, and business applications. Int. Rev. Bus. Res. Papers IRBRP 2010, 6, 235–243. [Google Scholar]
- Makkonen, M.; Patari, S.; Jantunen, A.; Viljainen, S. Competition in the European electricity markets—Outcomes of a Delphi study. Energy Policy 2012, 44, 431–440. [Google Scholar] [CrossRef]
- Wasserman, D.; Carli, V.; Wasserman, C.; Apter, A.; Balazs, J.; Bobes, J.; Bracale, R.; Brunner, R.; Bursztein-Lipsicas, C.; Corcoran, P.; et al. Saving and Empowering Young Lives in Europe (SEYLE): A randomized controlled trial. BMC Public Health 2010. [Google Scholar] [CrossRef] [PubMed]
- Carli, V.; Hadlaczky, G.; Wasserman, C.; Stingelin-Giles, N.; Reiter-Theil, S.; Wasserman, D. Maintaining confidentiality in prospective studies: Anonymous repeated measurements via email (ARME) procedure. J. Med. Ethics 2012, 38, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Fink, A.; Kosecoff, J.; Chassin, M.; Brook, R.H. Consensus methods: Characteristics and guidelines for use. Am. J. Public Health 1984, 74, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-C.; Sandford, B.A. The Delphi technique: Making sense of consensus. Pract. Assess. Res. Eval. 2007, 12, 1–8. [Google Scholar]
- Powell, C. The Delphi technique: Myths and realities. J. Adv. Nurs. 2003, 41, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Reiter-Theil, S. What does empirical research contribute to medical ethics? Camb. Q. Healthc. Ethics 2012, 21, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.; Pierscionek, K.B. Children, Gilick competency and consent for involvement in research. J. Med. Ethics 2007, 33, 659–662. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.L.; Larcher, V.; Kurz, R. Informed consent/assent in children. Statement of the Ethics Working Group of the Confederation of European Specialists in Paediatrics (CESP). Eur. J. Pediatr. 2003, 162, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M. Competence and consent to treatment in children and adolescents. Adv. Psychiatr. Treat. 2001, 7, 150–159. [Google Scholar] [CrossRef]
- McCarthy, A.M.; Richman, L.C.; Hoffman, R.P.; Rubenstein, L. Psychological screening of children for participation in nontherapeutic invasive research. Arch. Pediatr. Adolesc. Med. 2001, 155, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Alderson, P. Competent children? Minors’ consent to health care treatment and research. Soc. Sci. Med. 2007, 65, 2272–2283. [Google Scholar] [CrossRef] [PubMed]
- Hiriscau, E.I.; Reiter-Theil, S. Competence. In Encyclopedia of Global Bioethics; Springer: Berlin, Germany, 2015. [Google Scholar]
- Morrow, V.; Richards, M. The ethics of social research with children: An overview. Child. Soc. 1996, 10, 90–105. [Google Scholar] [CrossRef]
- Canadian Institutes of Health Research. (1998; with 2000, 2002, 2005 amendments). Tri-Council Policy Statement. In Ethical Conduct for Research Involving Humans; Canadian Institute of Health Research: Ottawa, ON, Canada, 1998. [Google Scholar]
- Valentine, G.; Butler, R.; Skelton, T. The ethical and methodological complexities of doing research with “vulnerable” young people. Ethics Place Environ. 2001, 4, 119–125. [Google Scholar] [CrossRef]
- Langhinrichsen-Rohling, J.; Arata, C.; O’Brien, N.; Bowers, D.; Klibert, J. Sensitive research with adolescents: Just how upsetting are self-report surveys anyway? Violence Vict. 2006, 21, 425–444. [Google Scholar] [CrossRef] [PubMed]
- Royal College of Psychiatrists’ Working Party. Guidelines for Researchers and for Research Ethics Committee on Psychiatric Research Involving Human Participants; Royal College of Psychiatrists’ Working Party: London, UK, 2000. [Google Scholar]
- Larcher, V.; Hutchinson, A. How should paediatricians assess Gillick competence? Arch. Dis. Child. 2010, 95, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Gould, M.S.; Marrocco, F.A.; Kleinman, M.; Thomas, J.G.; Mostkoff, K.; Cote, J.; Davies, M. Evaluating iatrogenic risk of youth suicide screening programs: A randomized controlled trial. JAMA 2005, 293, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, D.; Hoven, C.W.; Wasserman, C.; Wall, M.; Eisenberg, R.; Hadlaczky, G.; Kelleher, I.; Sarchiapone, M.; Apter, A.; Balazs, J.; et al. School-based suicide prevention programmes: The SEYLE cluster-randomised, controlled trial. Lancet 2015, 385, 1536–1544. [Google Scholar] [CrossRef]
- Peñta, J.B.; Caine, E.D. Screening as an approach for adolescent suicide prevention. Suicide Life Threat. Behav. 2006, 36, 614–637. [Google Scholar] [CrossRef] [PubMed]
- Rudd, M.D.; Mandrusiak, M.; Joiner, T.E., Jr.; Berman, A.L.; Van Orden, K.A.; Hollar, D. The emotional impact and ease of recall of warning signs for suicide: A controlled study. Suicide Life Threat. Behav. 2006, 36, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.E. Determining what could/should be: The Delphi technique and its application. In Proceedings of the 2006 Annual Meeting of the Mid-Western Educational Research Association, Columbus, OH, USA, 26 October 2006.
|
| New item |
| What special considerations need to be taken into account when obtaining consent in cases with separated parents or children/adolescents in foster care or if parents and minors disagree about the participation in research? |
| Item 3: What level of detailed research findings should be provided to parents regarding their offspring? |
| Item 5: Should the participants be informed prior to the research that confidentiality might be breached in certain circumstances and to whom the information provided might be disclosed (parents, social workers, other authorities? |
|
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hiriscau, E.I.; Stingelin-Giles, N.; Wasserman, D.; Reiter-Theil, S. Identifying Ethical Issues in Mental Health Research with Minors Adolescents: Results of a Delphi Study. Int. J. Environ. Res. Public Health 2016, 13, 489. https://doi.org/10.3390/ijerph13050489
Hiriscau EI, Stingelin-Giles N, Wasserman D, Reiter-Theil S. Identifying Ethical Issues in Mental Health Research with Minors Adolescents: Results of a Delphi Study. International Journal of Environmental Research and Public Health. 2016; 13(5):489. https://doi.org/10.3390/ijerph13050489
Chicago/Turabian StyleHiriscau, Elisabeta Ioana, Nicola Stingelin-Giles, Danuta Wasserman, and Stella Reiter-Theil. 2016. "Identifying Ethical Issues in Mental Health Research with Minors Adolescents: Results of a Delphi Study" International Journal of Environmental Research and Public Health 13, no. 5: 489. https://doi.org/10.3390/ijerph13050489





