Predicting the Cytotoxic Potency of Cigarette Smoke by Assessing the Thioredoxin Reductase Inhibitory Capacity of Cigarette Smoke Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Lines
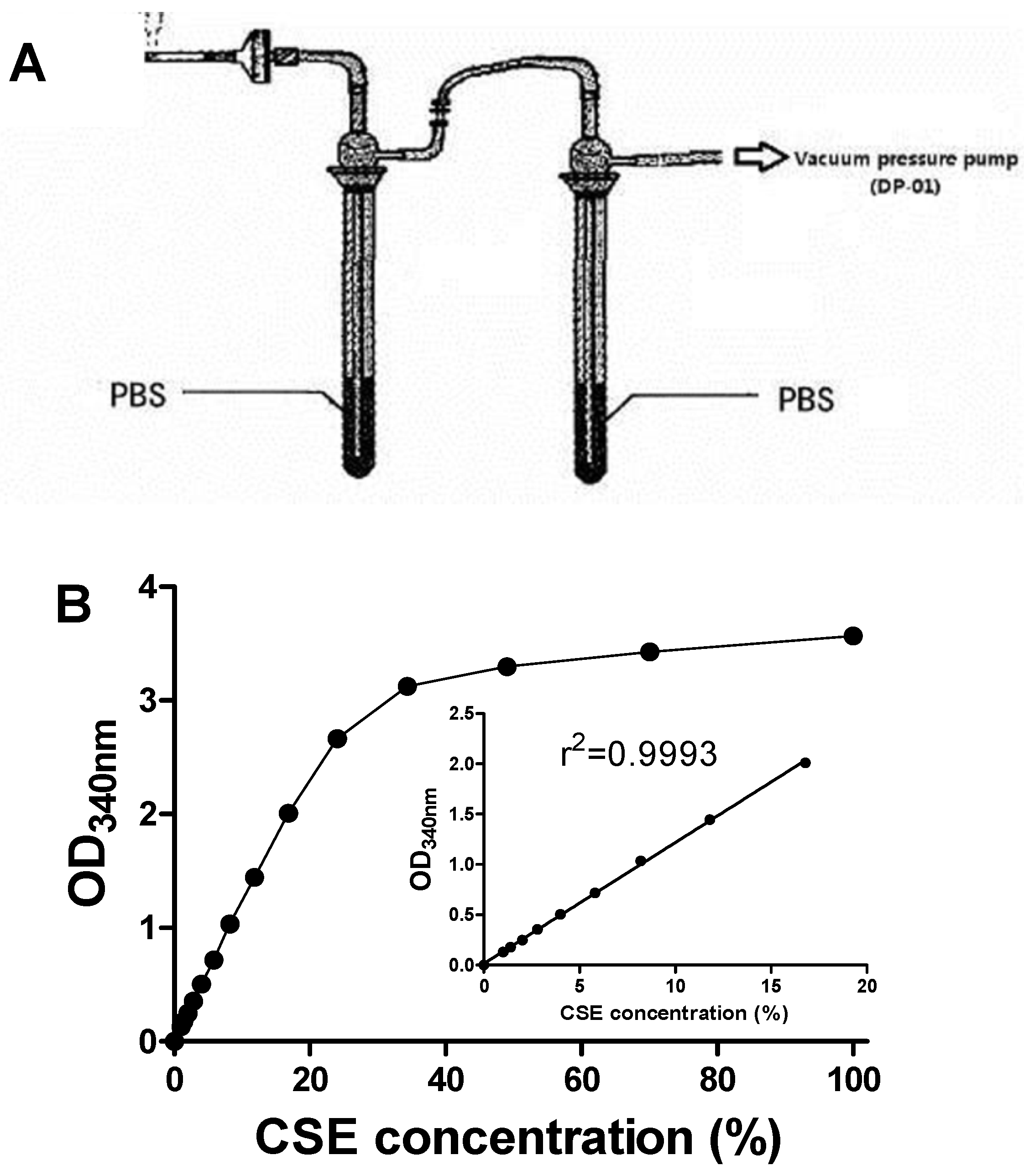
2.3. Preparation of CSE
2.4. Cell Culture and Viability Assay
2.5. Preparation of Cell Lysate
2.6. Separation of Alkylated Proteins
2.7. Western Blotting
2.8. Preparation of Mouse-Liver Homogenate
2.9. TrxR Inhibitory Capacity of CSE
2.10. Assays of Other Enzymatic Activities
2.11. Statistical Analyses
3. Results
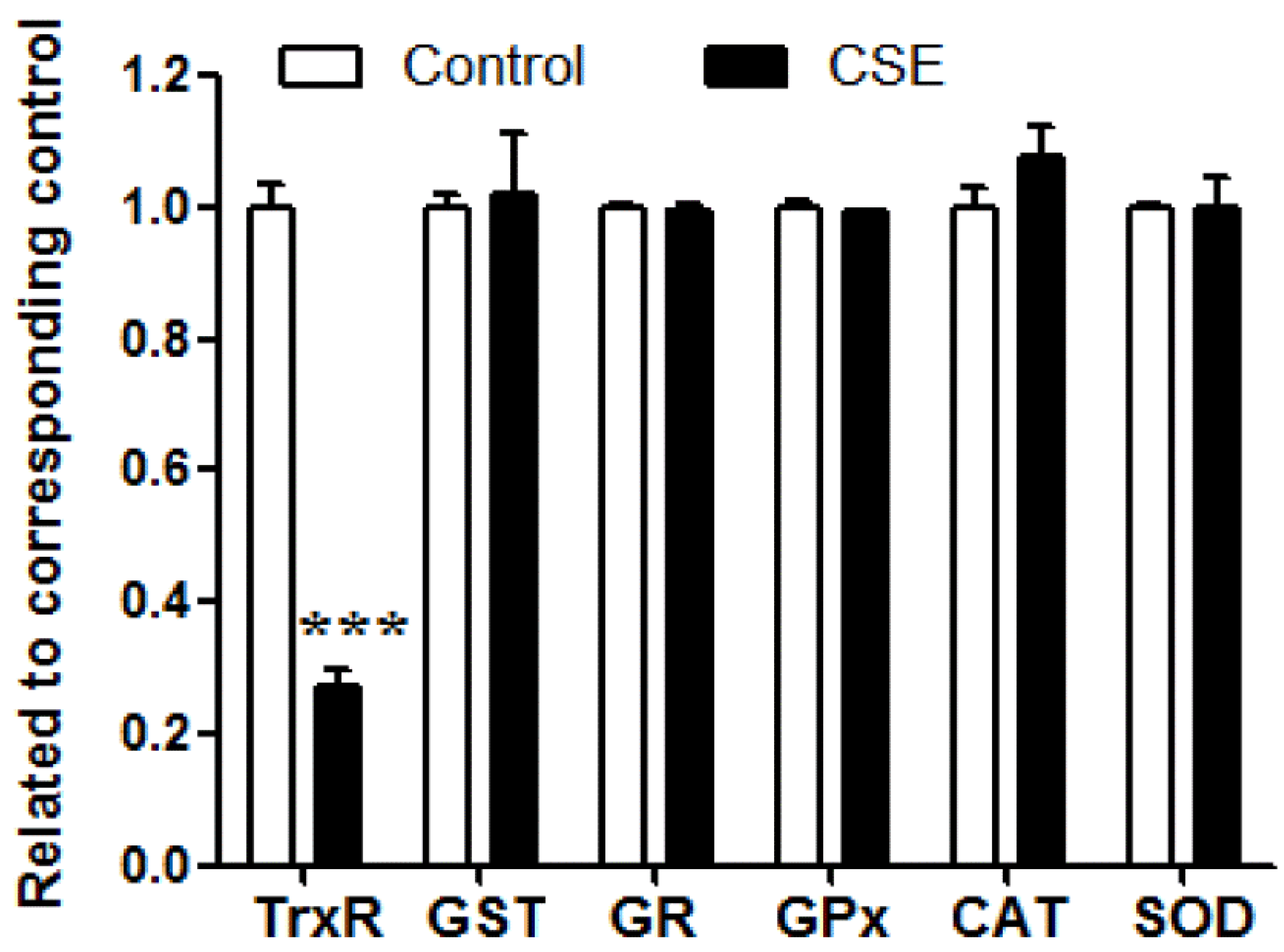
3.1. Influence of CSE on the Activities of Antioxidant Enzymes
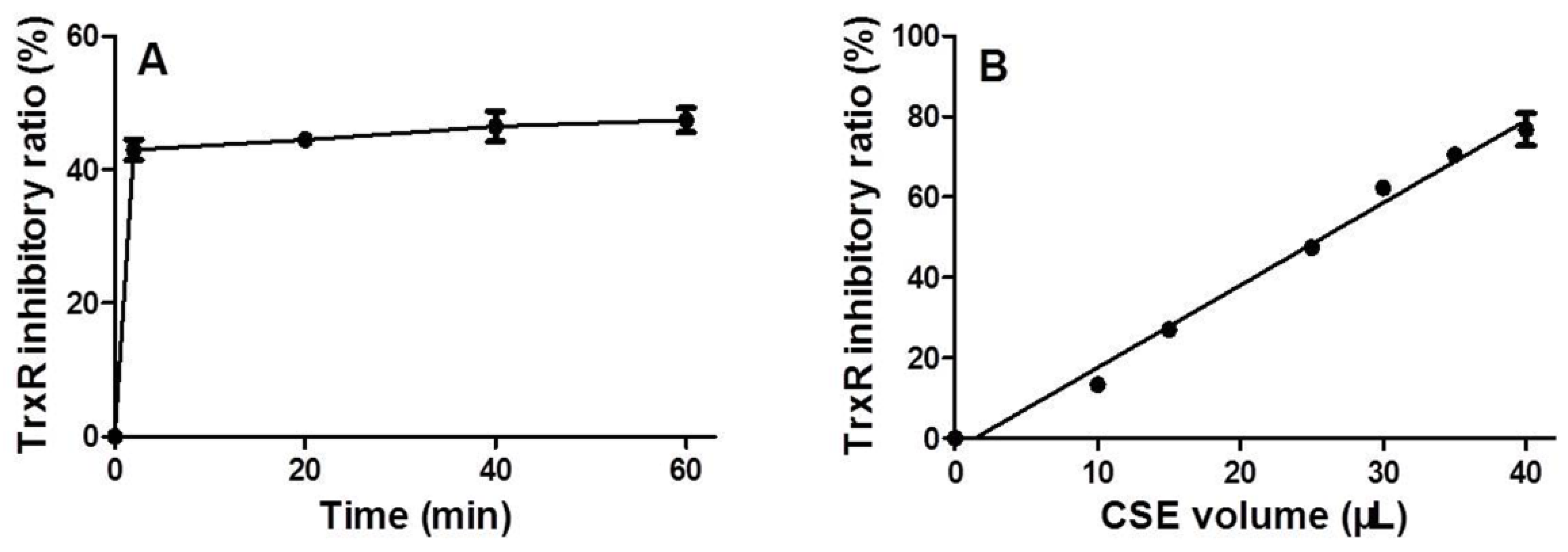
3.2. Time and Dose Effects of CSE on Trxr Activity
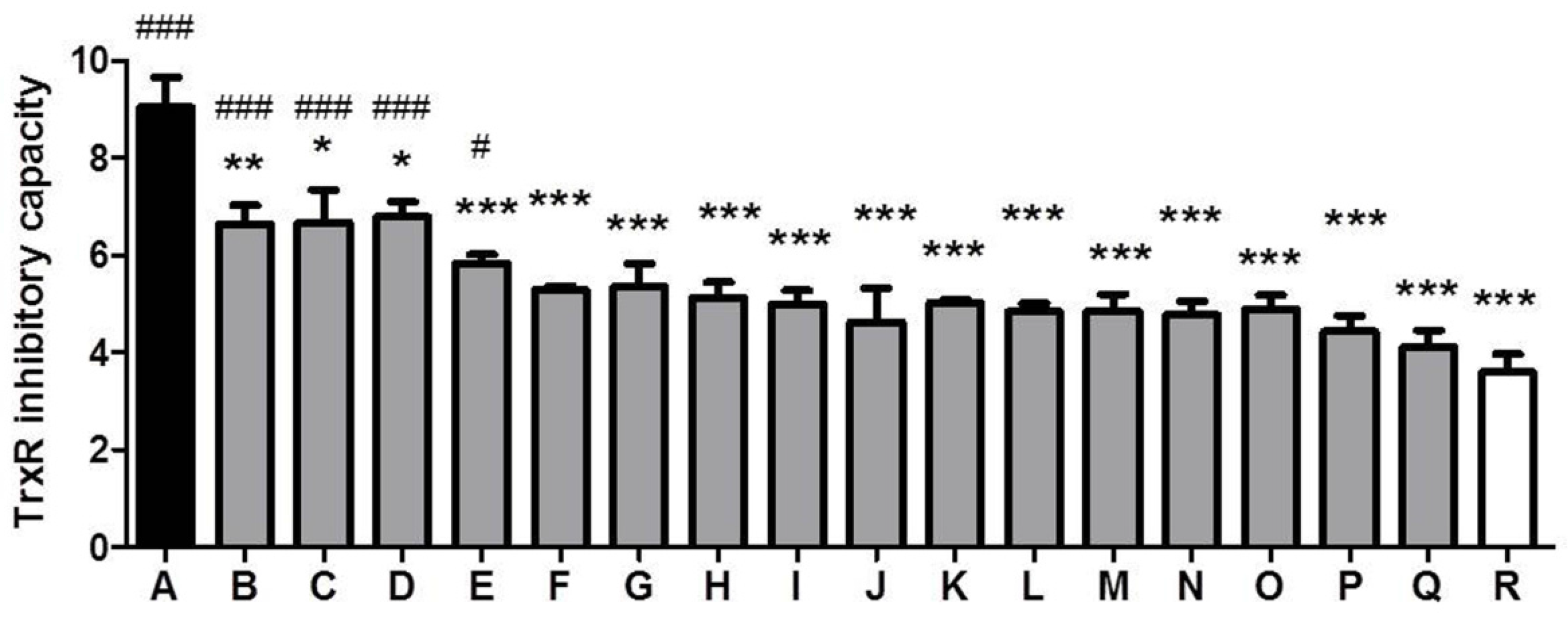
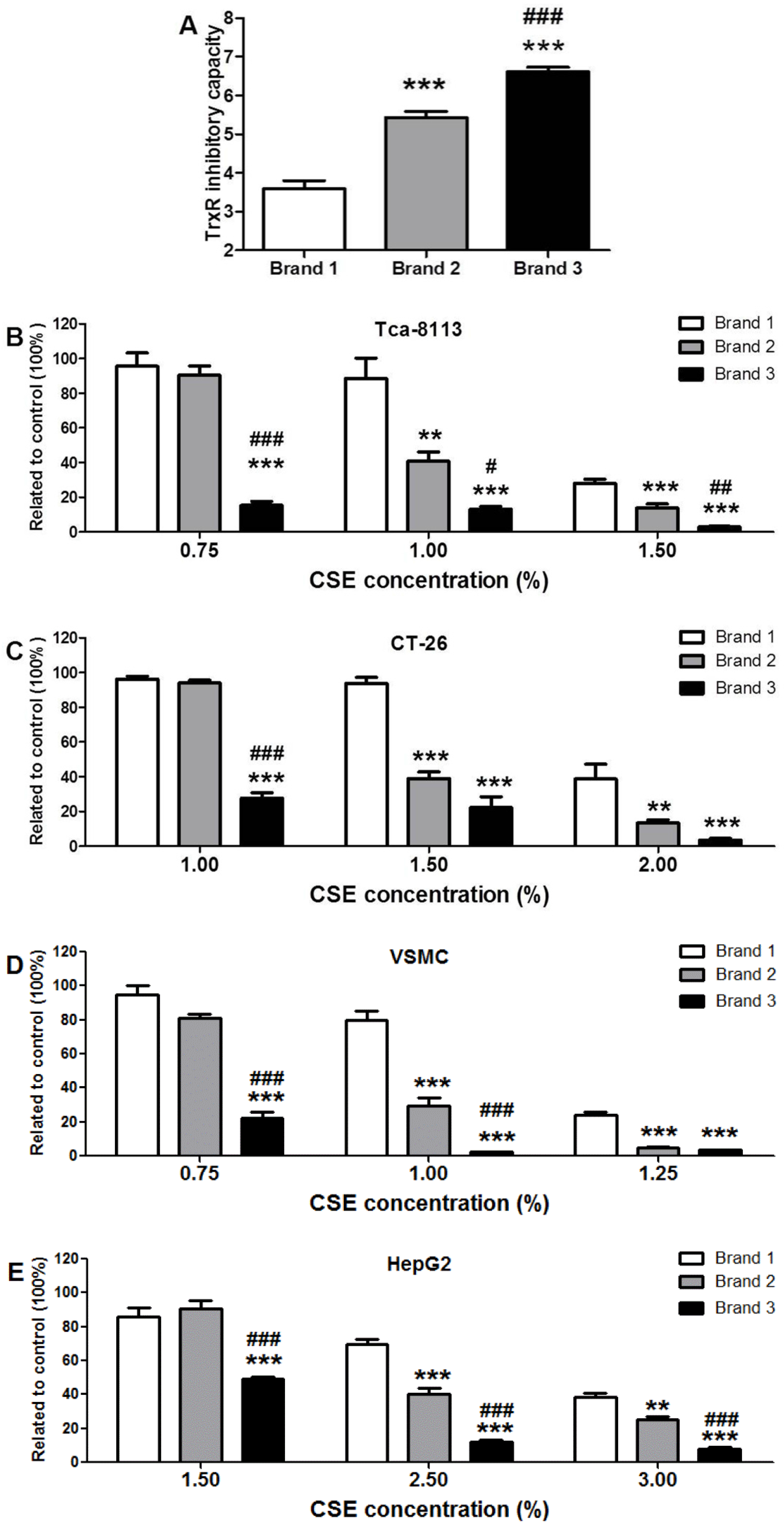
3.3. Comparison of TrxR Inhibitory Capacity of CSEs Prepared from Different Cigarette Brands
3.4. Cytotoxic Potency of CSEs with Different TrxR Inhibitory Capacities
3.5. CSE Inhibits Intracellular TrxR Activity and Causes Crosslinking and Alkylation of Intracellular TrxR1
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CSE | cigarette smoke extract |
| TrxR | thioredoxin reductase |
| Sec | selenocysteine |
| PREPs | potentially reduced exposure products |
| IC50 | half maximal inhibitory concentration |
| Trx | thioredoxin |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| DTNB | 5,5-dithiobis (2-nitrobenzotic acid) |
| GR | glutathione reductase |
| DMSO | dimethylsulfoxide |
| MTT | 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide |
| Tca8113 | human squamous carcinoma cell line |
| CT26 | murine colon carcinoma cell line |
| HepG2 | human liver hepatocellular carcinoma cell line |
| VSMC | RAT-vascular smooth muscle cell |
| PBS | potassium phosphate buffer |
| NaCNBH4 | sodium cyanoborohydride |
| GPx | glutathione peroxidase |
| SOD | superoxide dismutase |
| CAT | catalase |
| GST | glutathione S-transferase |
| CDNB | 1-chloro-2,4-dinitrobenzene |
| SecTRAPs | Sec thioredoxin reductase-derived apoptotic proteins |
References
- Bartecchi, C.E.; MacKenzie, T.D.; Schrier, R.W. The human costs of tobacco use. N. Engl. J. Med. 1994, 330, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Bondurant, S.; Wallace, R.; Shetty, P.; Stratton, K. Clearing the Smoke: Assessing the Science Base for Tobacco Harm Reduction; National Academies Press: Washington, DC, USA, 2001; pp. 1–500. [Google Scholar]
- Perfetti, T.A.; Rodgman, A. The complexity of tobacco and tobacco smoke. Contrib. Tob. Res. 2001, 24, 215–232. [Google Scholar] [CrossRef]
- Fowles, J.; Dybing, E. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tob. Control 2003, 12, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.S.; Zhang, L.; Jain, R.B.; Jain, N.; Wang, R.Y.; Ashley, D.L.; Watson, C.H. Levels of tobacco-specific nitrosamines and polycyclic aromatic hydrocarbons in mainstream smoke from different tobacco varieties. Cancer Epidem. Biomark. Prev. 2008, 17, 3366–3371. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, C.; Gigante, D.; Nunziata, A. A review of in vitro methods to assess the biological activity of tobacco smoke with the aim of reducing the toxicity of smoke. Toxicol. In Vitro 2003, 17, 587–594. [Google Scholar] [CrossRef]
- Arnér, E.S. Focus on mammalian thioredoxin reductases—Important selenoproteins with versatile functions. Biochim. Biophys. Acta 2009, 1790, 495–526. [Google Scholar] [CrossRef] [PubMed]
- Arnér, E.S. Selenoproteins-what unique properties can arise with selenocysteine in place of cysteine? Exp. Cell Res. 2010, 316, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Zhang, L.; Song, Y.; Wang, B.; Zhang, B.; Cui, X.; Hu, G.; Liu, Y.; Wu, J.; Fang, J. Small molecule inhibitors of mammalian thioredoxin reductase. Free Radic. Biol. Med. 2012, 52, 257–165. [Google Scholar] [CrossRef] [PubMed]
- Saccoccia, F.; Angelucci, F.; Boumis, G.; Carotti, D.; Desiato, G.; Miele, A.E.; Bellelli, A. Thioredoxin reductase and its inhibitors. Curr. Protein Pept. Sci. 2014, 15, 621–646. [Google Scholar] [CrossRef] [PubMed]
- Cebula, M.; Schmidt, E.E.; Arnér, E.S. TrxR1 as a potent regulator of the Nrf2-Keap1 response system. Antioxid. Redox Signal. 2015, 23, 823–853. [Google Scholar] [CrossRef] [PubMed]
- Pryor, W.A.; Hales, B.J.; Premovic, P.I.; Church, D.F. The radicals in cigarette tar: Their nature and suggested physiological implications. Science 1983, 220, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Pryor, W.A.; Stone, K.; Zang, L.Y.; Bermúdez, E. Fractionation of aqueous cigarette tar extracts: Fractions that contain the tar radical cause DNA damage. Chem. Res. Toxicol. 1998, 11, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Randall, M.J.; Hristova, M.; Van der Vliet, A. Protein alkylation by the α,β-unsaturated aldehyde acrolein. A reversible mechanism of electrophile signaling? FEBS. Lett. 2013, 587, 3808–3814. [Google Scholar] [CrossRef] [PubMed]
- Colombo, G.; Aldini, G.; Orioli, M.; Giustarini, D.; Gornati, R.; Rossi, R.; Colombo, R.; Carini, M.; Milzani, A.; Dalle-Donne, I. Water-soluble alpha,beta-unsaturated aldehydes of cigarette smoke induce carbonylation of human serum albumin. Antioxid. Redox Signal. 2010, 12, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Bazzini, C.; Rossetti, V.; Civello, D.A.; Sassone, F.; Vezzoli, V.; Persani, L.; Tiberio, L.; Lanata, L.; Bagnasco, M.; Paulmichl, M.; et al. Short- and long-term effects of cigarette smoke exposure on glutathione homeostasis in human bronchial epithelial cells. Cell Physiol. Biochem. 2013, 32, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Baglole, C.J.; Sime, P.J.; Phipps, R.P. Cigarette smoke-induced expression of heme oxygenase-1 in human lung fibroblasts is regulated by intracellular glutathione. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 295, L624–L636. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Levander, O.A. High-throughput 96-well microplate assays for determining specific activities of glutathione peroxidase and thioredoxin reductase. Methods Enzymol. 2002, 347, 113–121. [Google Scholar] [PubMed]
- Sun, K.; Eriksson, S.E.; Tan, Y.; Zhang, L.; Arnér, E.S.; Zhang, J. Serum thioredoxin reductase levels increase in response to chemically induced acute liver injury. Biochim. Biophys. Acta 2014, 1840, 2105–2111. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, I.; Mannervik, B. Glutathione reductase. Methods Enzymol. 1985, 113, 484–490. [Google Scholar] [PubMed]
- Sun, Y.; Oberley, L.W.; Li, Y. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [PubMed]
- Claiborne, A. Catalase activity. In CRC Handbook of Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 283–284. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [PubMed]
- Muller, T.; Haussmann, H.J.; Schepers, G. Evidence for peroxynitrite as an oxidative stress-inducing compound of aqueous cigarette smoke fractions. Carcinogenesis 1997, 18, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Matsuno, S.; Kagota, S.; Haginaka, J.; Kunitomo, M. Peroxynitrite- mediated oxidative modification of low-density lipoprotein by aqueous extracts of cigarette smoke and the preventive effect of fluvastatin. Atherosclerosis 2004, 172, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Fujiwara, N.; Koh, Y.H.; Miyamoto, Y.; Suzuki, K.; Honke, K.; Taniguchi, N. Induction of thioredoxin reductase gene expression by peroxynitrite in human umbilical vein endothelial cells. Biol. Chem. 2002, 383, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, J.; Wang, X.; Liu, X.; Zuo, L.; Bai, K.; Shang, J.; Ma, L.; Liu, T.; Wang, L.; et al. Thioredoxin reductase was nitrated in the aging heart after myocardial ischemia/reperfusion. Rejuvenation Res. 2013, 16, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Pappas, R.S.; Fresquez, M.R.; Martone, N.; Watson, C.H. Toxic metal concentrations in mainstream smoke from cigarettes available in the USA. J. Anal. Toxicol. 2014, 38, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.C.; Stephens, W.E.; Finch, A.A.; Geraki, K. Controls on the valence species of arsenic in tobacco smoke: XANES investigation with implications for health and regulation. Environ. Sci. Technol. 2014, 48, 3449–3456. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.M.; Myers, C.R. The effects of hexavalent chromium on thioredoxin reductase and peroxiredoxins in human bronchial epithelial cells. Free Radic. Biol. Med. 2009, 47, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.M.; Chew, E.H.; Hashemy, S.I.; Lu, J.; Holmgren, A. Inhibition of the human thioredoxin system. A molecular mechanism of mercury toxicity. J. Biol. Chem. 2008, 283, 11913–11923. [Google Scholar] [CrossRef] [PubMed]
- Mura, P.; Camalli, M.; Bindoli, A.; Sorrentino, F.; Casini, A.; Gabbiani, C.; Corsini, M.; Zanello, P.; Rigobello, M.P.; Messori, L. Activity of rat cytosolic thioredoxin reductase is strongly decreased by trans-[bis(2-amino-5-methylthiazole)tetrachlororuthenate(III)]: First report of relevant thioredoxin reductase inhibition for a ruthenium compound. J. Med. Chem. 2007, 50, 5871–5874. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Chew, E.H.; Holmgren, A. Targeting thioredoxin reductase is a basis for cancer therapy by arsenic trioxide. Proc. Natl. Acad. Sci. USA 2007, 104, 12288–12293. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, K.; Shibamoto, T. Determination of toxic carbonyl compounds in cigarette smoke. Environ. Toxicol. 2006, 21, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Staimer, N.; Nguyen, T.B.; Nizkorodov, S.A.; Delfino, R.J. Glutathione peroxidase inhibitory assay for electrophilic pollutants in diesel exhaust and tobacco smoke. Anal. Bioanal. Chem. 2012, 403, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Colombo, G.; Dalle-Donne, I.; Orioli, M.; Giustarini, D.; Rossi, R.; Clerici, M.; Regazzoni, L.; Aldini, G.; Milzani, A.; Butterfield, D.A.; et al. Oxidative damage in human gingival fibroblasts exposed to cigarette smoke. Free Radic. Biol. Med. 2012, 52, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R. The Evolving Versatility of Selenium in Biology. Antioxid. Redox Signal. 2015, 23, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Noya, Y.; Seki, K.; Asano, H.; Mai, Y.; Horinouchi, T.; Higashi, T.; Terada, K.; Hatate, C.; Hoshi, A.; Nepal, P.; et al. Identification of stable cytotoxic factors in the gas phase extract of cigarette smoke and pharmacological characterization of their cytotoxicity. Toxicology 2013, 314, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Holmgren, A. Selenoproteins. J. Biol. Chem. 2009, 284, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Randall, M.J.; Spiess, P.C.; Hristova, M.; Hondal, R.J.; van der Vliet, A. Acrolein- induced activation of mitogen-activated protein kinase signaling is mediated by alkylation of thioredoxin reductase and thioredoxin 1. Redox Biol. 2013, 1, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Jan, Y.H.; Heck, D.E.; Malaviya, R.; Casillas, R.P.; Laskin, D.L.; Laskin, J.D. Cross-linking of thioredoxin reductase by the sulfur mustard analogue mechlorethamine(methylbis(2-chloroethyl)amine) in human lung epithelial cells and rat lung: Selective inhibition of disulfide reduction but not redox cycling. Chem. Res. Toxicol. 2014, 27, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Eriksson, S.E.; Cebula, M.; Sandalova, T.; Hedström, E.; Pader, I.; Cheng, Q.; Myers, C.R.; Antholine, W.E.; Nagy, P.; et al. The conserved Trp114 residue of thioredoxin reductase 1 has a redox sensor-like function triggering oligomerization and crosslinking upon oxidative stress related to cell death. Cell Death Dis. 2015, 6. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Ning, M.; Xu, Y.; Wang, C.; Zhao, G.; Cao, Q.; Zhang, J. Predicting the Cytotoxic Potency of Cigarette Smoke by Assessing the Thioredoxin Reductase Inhibitory Capacity of Cigarette Smoke Extract. Int. J. Environ. Res. Public Health 2016, 13, 348. https://doi.org/10.3390/ijerph13030348
Zhang L, Ning M, Xu Y, Wang C, Zhao G, Cao Q, Zhang J. Predicting the Cytotoxic Potency of Cigarette Smoke by Assessing the Thioredoxin Reductase Inhibitory Capacity of Cigarette Smoke Extract. International Journal of Environmental Research and Public Health. 2016; 13(3):348. https://doi.org/10.3390/ijerph13030348
Chicago/Turabian StyleZhang, Longjie, Min Ning, Yingbo Xu, Chenghui Wang, Guangshan Zhao, Qingqing Cao, and Jinsong Zhang. 2016. "Predicting the Cytotoxic Potency of Cigarette Smoke by Assessing the Thioredoxin Reductase Inhibitory Capacity of Cigarette Smoke Extract" International Journal of Environmental Research and Public Health 13, no. 3: 348. https://doi.org/10.3390/ijerph13030348




