Heavy Metal Accumulation is Associated with Molecular and Pathological Perturbations in Liver of Variola louti from the Jeddah Coast of Red Sea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Handling
2.2. Preparation of Tissue Homogenates
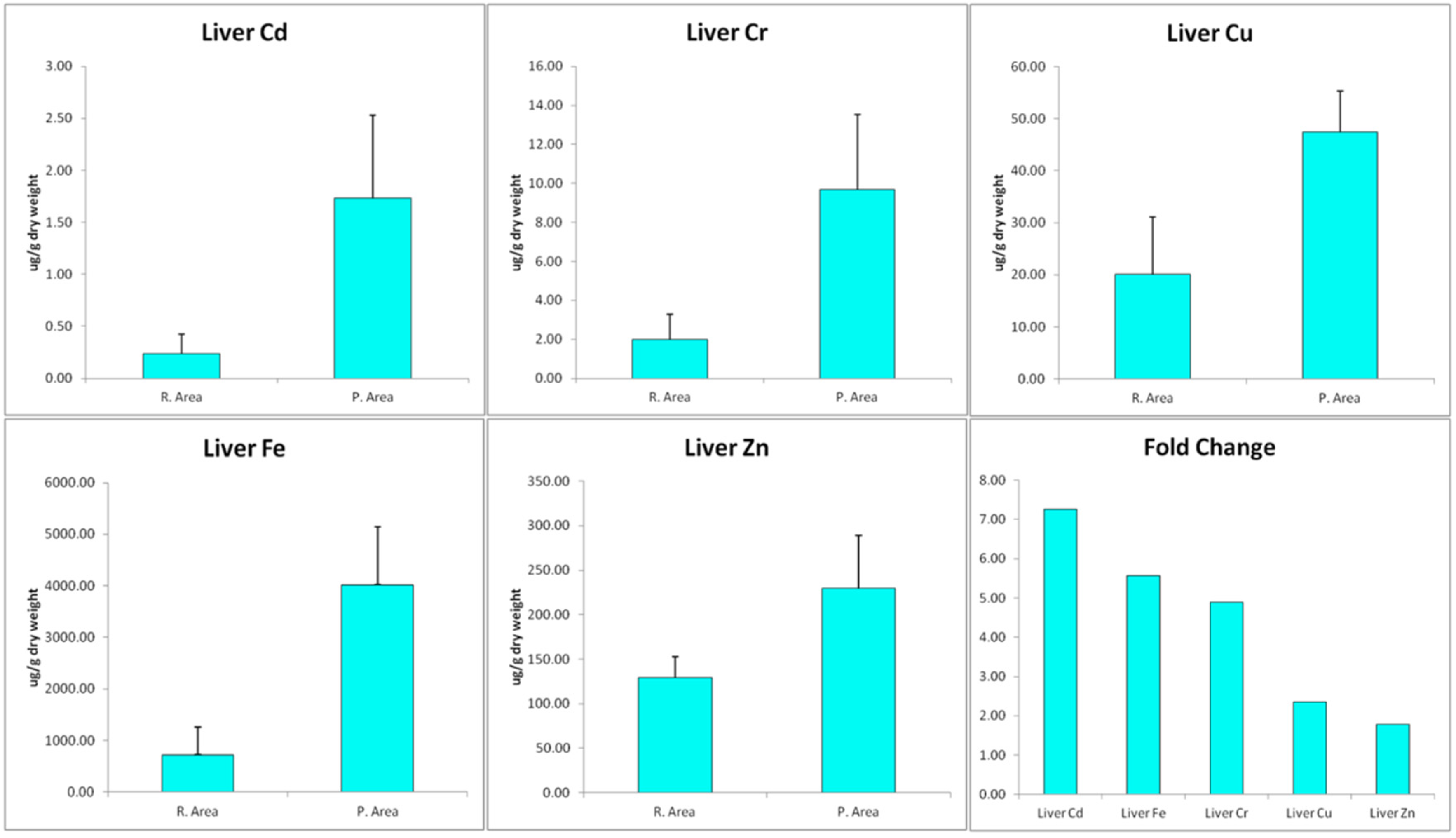
2.3. Trace Metals Determination
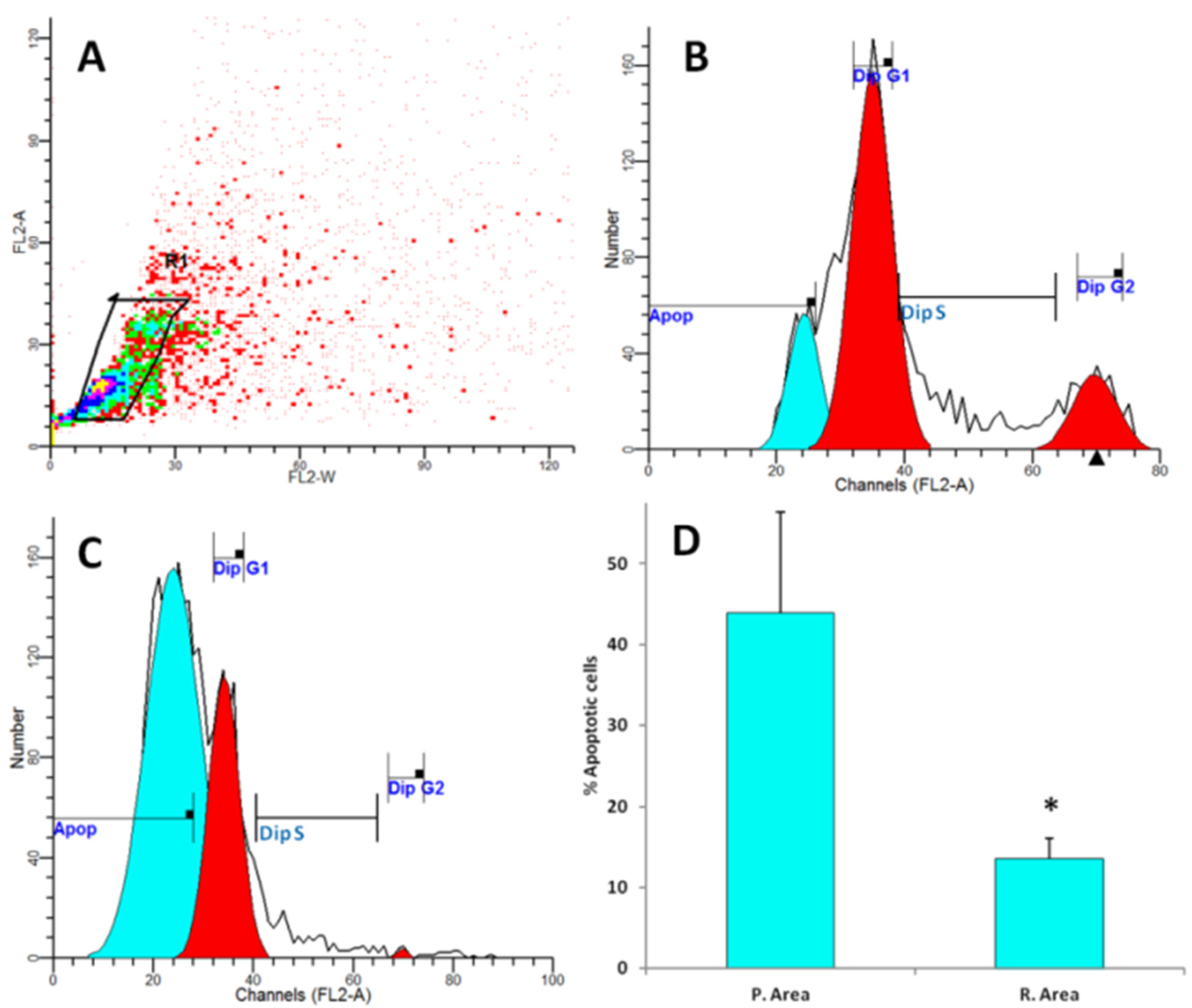
2.4. DNA Flow Cytometry Analysis
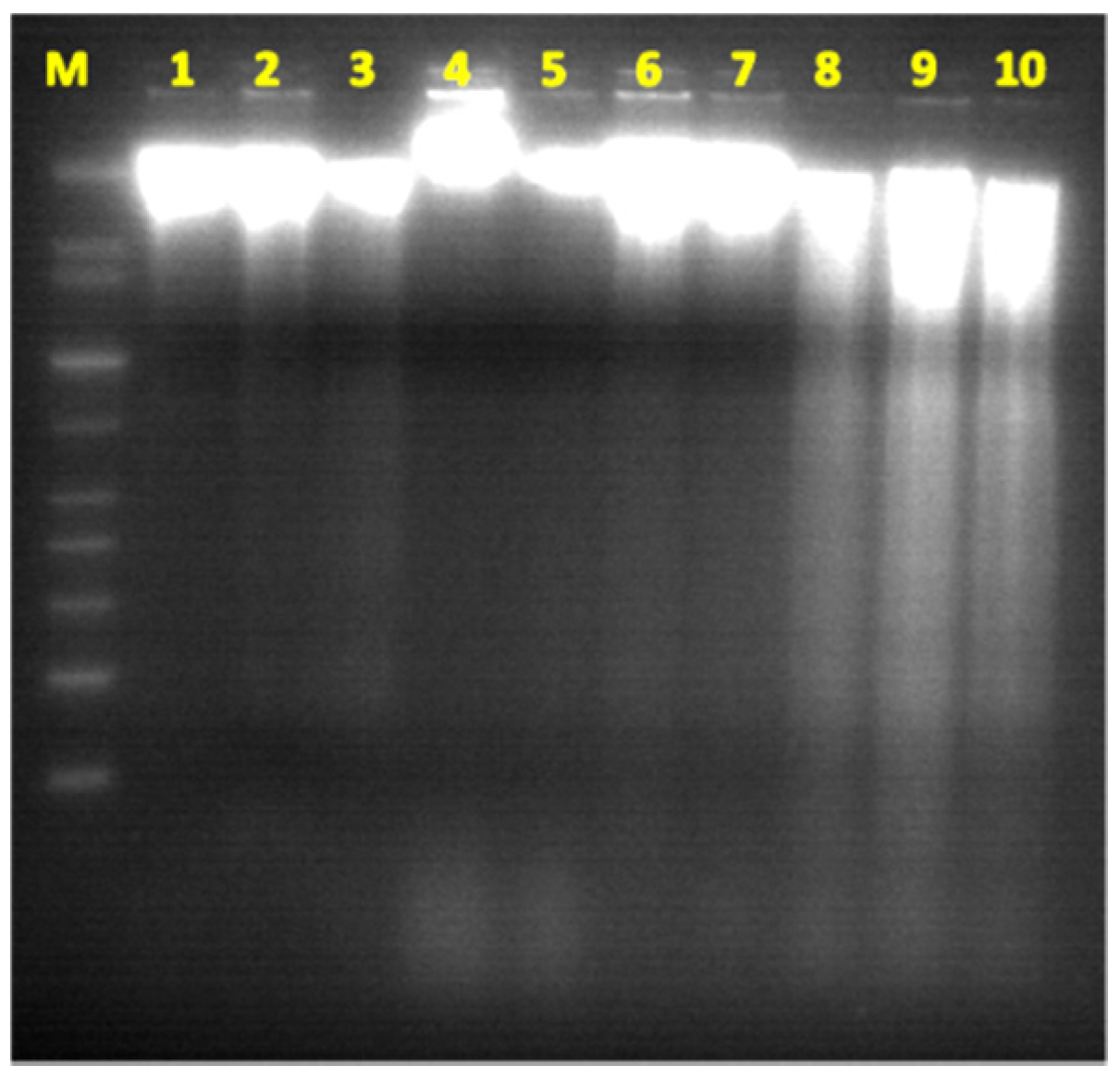
2.5. DNA-Ladder Fragmentation Assay
2.6. SDS-Polyacrylamide Gel Electrophoresis
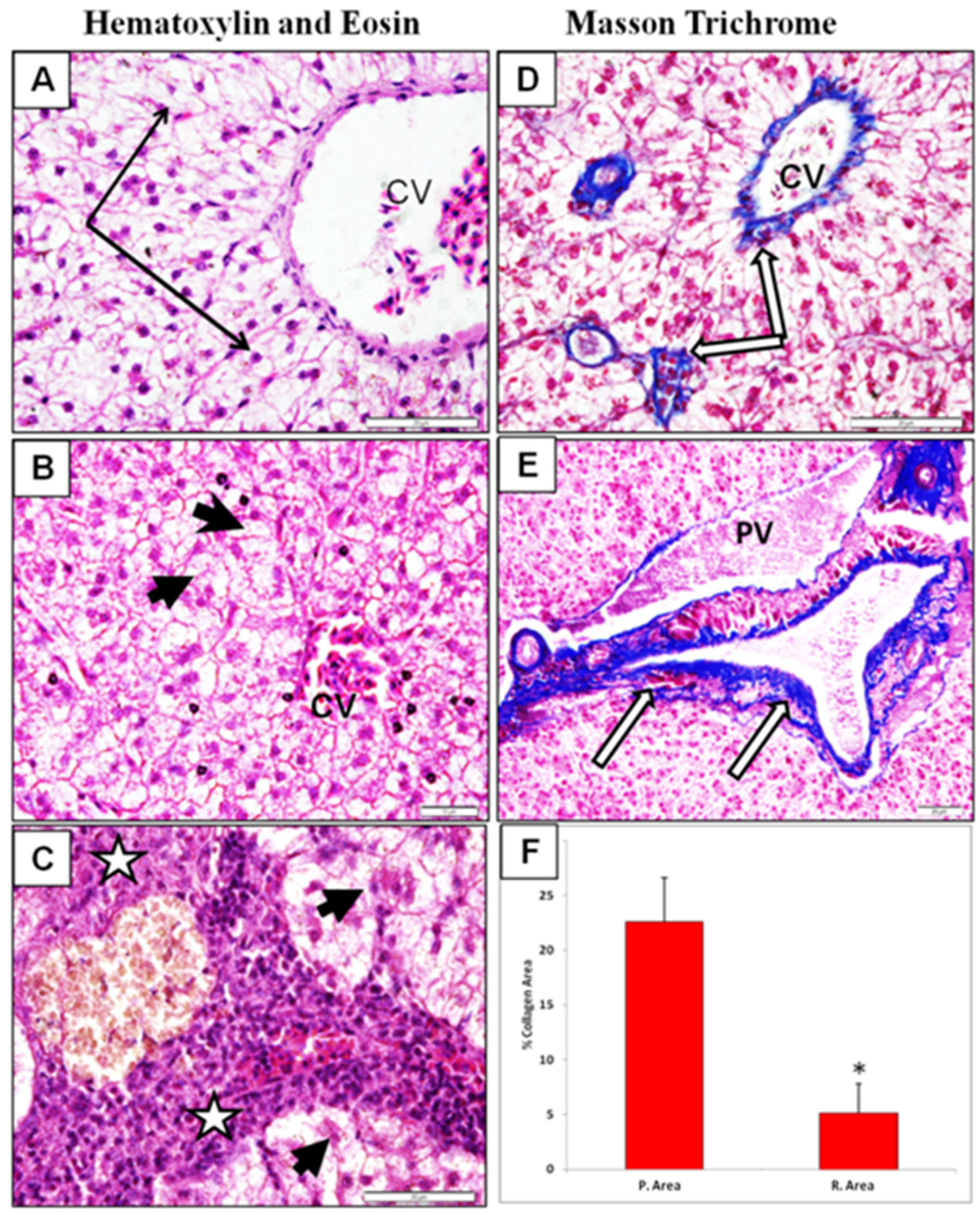
2.7. Light Microscopy
2.8. Statistical Analysis
3. Results Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| R. Area | Reference area |
| P. Area | Polluted area |
| CV | central vein |
| OD | optic density |
| FL-A | peaks area |
| FL-W | peaks width |
| FCM | flow cytometry |
References
- Pena-Garcia, D.; Ladwig, N.; Turki, A.J.; Mudarris, M.S. Input and dispersion of nutrients from the Jeddah Metropolitan area, Red Sea. Mar. Pollut. Bull. 2014, 80, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Doust, J.L.; Schmidt, M.; Doust, L.L. Biological assessment of aquatic pollution: A review, with emphasis on plants as biomonitors. Biol. Rev. Camb. Philos. Soc. 1994, 69, 147–186. [Google Scholar] [CrossRef] [PubMed]
- Araujo, R.M.; Arribas, R.M.; Pares, R. Distribution of aeromonas species in waters with different levels of pollution. J. Appl. Bacteriol. 1991, 71, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Aslam, Z.; Song, G.C.; Kim, S.W.; Jeon, C.O.; Chon, T.S.; Chung, Y.R. Flavobacterium sasangense sp. Nov., isolated from a wastewater stream polluted with heavy metals. Int. J. Syst. Evolut. Microbiol. 2009, 59, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Panero, C.; Grasso, A.; La Cauza, C.; Ragazzini, F.; Bosco, G. Drinking water pollution and Pseudomonas aeruginosa infections in a department for premature infants. G Batteriol. Virol. Immunol. 1970, 63, 517–527. [Google Scholar] [PubMed]
- Santos, I.; Diniz, M.S.; Carvalho, M.L.; Santos, J.P. Assessment of essential elements and heavy metals content on Mytilus galloprovincialis from river Tagus estuary. Biol. Trace Element Res. 2014, 159, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Saeedi Saravi, S.S.; Shokrzadeh, M. Heavy metals contamination in water and three species of most consumed fish sampled from Caspian sea, 2011. Environ. Monit. Assess. 2013, 185, 10333–10337. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, Y.; Cao, Y.; Chen, A.; Ren, M.; Ge, Y.; Yu, Z.; Wan, S.; Hu, A.; Bo, Q.; et al. Potential health risks of heavy metals in cultivated topsoil and grain, including correlations with human primary liver, lung and gastric cancer, in Anhui province, Eastern China. Sci. Total Environ. 2014, 470–471, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Chesman, B.S.; O’Hara, S.; Burt, G.R.; Langston, W.J. Hepatic metallothionein and total oxyradical scavenging capacity in atlantic cod Gadus morhua caged in open sea contamination gradients. Aquat. Toxicol. 2007, 84, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Canesi, L.; Ciacci, C.; Piccoli, G.; Stocchi, V.; Viarengo, A.; Gallo, G. In vitro and in vivo effects of heavy metals on mussel digestive gland hexokinase activity: The role of glutathione. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1998, 120, 261–268. [Google Scholar] [CrossRef]
- Wang, S.; Shi, X. Molecular mechanisms of metal toxicity and carcinogenesis. Mol. Cell. Biochem. 2001, 222, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Frieden, E. The evolution of metals as essential elements (with special reference to iron and copper). Adv. Exp. Med. Biol. 1974, 48, 1–29. [Google Scholar] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology; Springer: Berlin, Germany, 2012; pp. 133–164. [Google Scholar]
- Badisa, V.L.; Latinwo, L.M.; Odewumi, C.O.; Ikediobi, C.O.; Badisa, R.B.; Brooks-Walter, A.; Lambert, A.T.; Nwoga, J. Cytotoxicity and stress gene microarray analysis in cadmium-exposed crl-1439 normal rat liver cells. Int. J. Mol. Med. 2008, 22, 213–219. [Google Scholar] [PubMed]
- Flores, A.; Perez, J.M. Cytotoxicity, apoptosis, and in vitro DNA damage induced by potassium chromate. Toxicol. Appl. Pharmacol. 1999, 161, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Gabbianelli, R.; Lupidi, G.; Villarini, M.; Falcioni, G. DNA damage induced by copper on erythrocytes of gilthead sea bream sparus aurata and mollusk scapharca inaequivalvis. Arch. Environ. Contam. Toxicol. 2003, 45, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Glei, M.; Knobel, Y.; Pool-Zobel, B.L. Blood mononucleocytes are sensitive to the DNA damaging effects of iron overload—in vitro and ex vivo results with human and rat cells. Mutat. Res. 2007, 619, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Roney, N.; Osier, M.; Paikoff, S.J.; Smith, C.V.; Williams, M.; De Rosa, C.T. ATSDR evaluation of the health effects of zinc and relevance to public health. Toxicol. Ind. Health 2006, 22, 423–493. [Google Scholar] [CrossRef] [PubMed]
- Baatrup, E. Structural and functional effects of heavy metals on the nervous system, including sense organs of fish. Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 1991, 100, 253–257. [Google Scholar] [CrossRef]
- Khan, M.A.; Castro-Guerrero, N.; Mendoza-Cozatl, D.G. Moving toward a precise nutrition: Preferential loading of seeds with essential nutrients over non-essential toxic elements. Front. Plant Sci. 2014, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Mance, G. Pollution Threat of Heavy Metals in Aquatic Environments; Springer: Berlin, Germany, 1987. [Google Scholar]
- Qian, L.; Murakami, T.; Kimura, Y.; Takahashi, M.; Okita, K. Saikosaponin a-induced cell death of a human hepatoma cell line (Huh-7): The significance of the “sub-g1 peak” in a DNA histogram. Pathol. Int. 1995, 45, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Hirt, B. Selective extraction of polyoma DNA from infected mouse cell cultures. J. Mol. Biol. 1967, 26, 365–369. [Google Scholar] [CrossRef]
- Boesenberg-Smith, K.A.; Pessarakli, M.M.; Wolk, D.M. Assessment of DNA yield and purity: An overlooked detail of PCR troubleshooting. Clin. Microbiol. Newslett. 2012, 34, 1–6. [Google Scholar] [CrossRef]
- Laemmli, U. Most commonly used discontinuous buffer system for SDS electrophoresis. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Delafield, F. Haematoxylin and eosin for general staining. In Staining of the Animal Tissues Practical and Theoretical; Oxford University Press: London, UK, 1984. [Google Scholar]
- Yokoo, T.; Wolfson, T.; Iwaisako, K.; Peterson, M.R.; Mani, H.; Goodman, Z.; Changchien, C.; Middleton, M.S.; Gamst, A.C.; Mazhar, S.M.; et al. Evaluation of liver fibrosis using texture analysis on combined-contrast-enhanced magnetic resonance images at 3.0T. BioMed Res. Int. 2015, 2015, 387653. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, P.; Kloner, R.A.; Boughner, D.R.; Pickering, J.G. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res. Cardiol. 1994, 89, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, P.; Andreji, J.; Dvorakova-Liskova, Z.; Vejsada, P. Assessment of selected heavy metals pollution in water, sediments and fish in the basin Dyje, Czech republic. Neuro Endocrinol. Lett. 2014, 35, 26–34. [Google Scholar] [PubMed]
- Adeniyi, A.A.; Yusuf, K.A.; Okedeyi, O.O. Assessment of the exposure of two fish species to metals pollution in the Ogun river catchments, Ketu, Lagos, Nigeria. Environ. Monit. Assess. 2008, 137, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Bosnir, J.; Puntaric, D.; Skes, I.; Klaric, M.; Simic, S.; Zoric, I.; Galic, R. Toxic metals in freshwater fish from the Zagreb area as indicators of environmental pollution. Coll. Antropol. 2003, 27, 31–39. [Google Scholar] [PubMed]
- Song, J.K.; Luo, H.; Yin, X.H.; Huang, G.L.; Luo, S.Y.; Lin du, R.; Yuan, D.B.; Zhang, W.; Zhu, J.G. Association between cadmium exposure and renal cancer risk: A meta-analysis of observational studies. Sci. Rep. 2015, 5, 17976. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Huang, Y.; Zhang, J.; Peng, Y.; Lin, X.; Wu, K.; Huo, X. Cadmium exposure and the risk of breast cancer in Chaoshan population of southeast China. Environ. Sci. Pollut. Res. Int. 2015, 22, 19870–19878. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xun, P.; Nishijo, M.; Sekikawa, A.; He, K. Cadmium exposure and risk of pancreatic cancer: A meta-analysis of prospective cohort studies and case-control studies among individuals without occupational exposure history. Environ. Sci. Pollut. Res. Int. 2015, 22, 17465–17474. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.V.; Quraishi, S.M.; Shafer, M.M.; Passarelli, M.N.; Freney, E.P.; Chlebowski, R.T.; Luo, J.; Meliker, J.R.; Mu, L.; Neuhouser, M.L.; et al. Dietary cadmium exposure and risk of breast, endometrial, and ovarian cancer in the women’s health initiative. Environ. Health Perspect. 2014, 122, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, K.T.; Halkjaer, J.; Sorensen, M.; Meliker, J.R.; McElroy, J.A.; Tjonneland, A.; Raaschou-Nielsen, O. Dietary cadmium intake and risk of breast, endometrial and ovarian cancer in danish postmenopausal women: A prospective cohort study. PLoS ONE 2014, 9, e100815. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Caffrey, J.L.; Lin, J.W.; Bayliss, D.; Faramawi, M.F.; Bateson, T.F.; Sonawane, B. Increased risk of cancer mortality associated with cadmium exposures in older Americans with low zinc intake. J. Toxicol. Environ. Health Part A 2013, 76, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hengstler, J.G.; Bolm-Audorff, U.; Faldum, A.; Janssen, K.; Reifenrath, M.; Gotte, W.; Jung, D.; Mayer-Popken, O.; Fuchs, J.; Gebhard, S.; et al. Occupational exposure to heavy metals: DNA damage induction and DNA repair inhibition prove co-exposures to cadmium, cobalt and lead as more dangerous than hitherto expected. Carcinogenesis 2003, 24, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Bertin, G.; Averbeck, D. Cadmium: Cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (A review). Biochimie 2006, 88, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239. [Google Scholar] [CrossRef] [PubMed]
- Arends, M.; Morris, R.; Wyllie, A. Apoptosis. The role of the endonuclease. Am. J. Pathol. 1990, 136, 593. [Google Scholar] [PubMed]
- Lohmann, R.D.; Beyersmann, D. Cadmium and zinc mediated changes of the Ca(2+)-dependent endonuclease in apoptosis. Biochem. Biophys. Res. Commun. 1993, 190, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- FAO. Compilation of legal limits for hazardous substances in fish and fishery products. FAO Fish. Circ. 1983, 464, 5–100. [Google Scholar]
- Lurie, P.; Wolfe, S.M. Continuing exposure to hexavalent chromium, a known lung carcinogen: An analysis of Osha compliance inspections, 1990–2000. Am. J. Ind. Med. 2002, 42, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, T.F. Chromium as an industrial carcinogen: Part I. Am. J. Ind. Med. 1997, 31, 129–139. [Google Scholar] [CrossRef]
- Prasad, R.; Kaur, G.; Nath, R.; Walia, B.N. Molecular basis of pathophysiology of Indian childhood cirrhosis: Role of nuclear copper accumulation in liver. Mol. Cell. Biochem. 1996, 156, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Ishida, M.; Nakagawara, G.; Imamura, Y.; Fukuda, M. Iron and copper deposition in chronic active hepatitis and liver cirrhosis: Pathogenetic role in progressive liver cell damage. Eur. J. Histochem. 1995, 39, 221–236. [Google Scholar] [PubMed]
- Papanikolaou, G.; Pantopoulos, K. Iron metabolism and toxicity. Toxicol. Appl. Pharmacol. 2005, 202, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhang, J.; Li, W.; Xu, M.; Liu, S. Disruption of iron homeostasis and resultant health effects upon exposure to various environmental pollutants: A critical review. J. Environ. Sci. 2015, 34, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Okada, S.; Yu, Y.; Zheng, P.; Yamaguchi, R.; Kasai, H. Vitamin e inhibits apoptosis, DNA modification, and cancer incidence induced by iron-mediated peroxidation in wistar rat kidney. Cancer Res. 1997, 57, 2410–2414. [Google Scholar] [PubMed]
- Ramm, G.A.; Ruddell, R.G. Hepatotoxicity of iron overload: Mechanisms of iron-induced hepatic fibrogenesis. Semin. Liver Dis. 2005, 25, 433–449. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, K.; Saile, B.; Ramadori, G. Liver fibrosis and altered matrix synthesis. Can. J. Gastroenterol. 2001, 15, 187–193. [Google Scholar] [PubMed]
- Biagini, G.; Ballardini, G. Liver fibrosis and extracellular matrix. J. Hepatol. 1989, 8, 115–124. [Google Scholar] [CrossRef]
- Attallah, A.M.; Mosa, T.E.; Omran, M.M.; Abo-Zeid, M.M.; El-Dosoky, I.; Shaker, Y.M. Immunodetection of collagen types I, II, III, and IV for differentiation of liver fibrosis stages in patients with chronic HCV. J. Immunoass. Immunochem. 2007, 28, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, F.A. Bioaccumulation of selected metals and histopathological alterations in tissues of Oreochromis niloticus and lates niloticus from lake Nasser, Egypt. Glob. Vet. 2008, 2, 205–218. [Google Scholar]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, S.A.; Elshal, M.F.; Kumosani, T.A.; Mal, A.O.; Ahmed, Y.M.; Almulaiky, Y.Q.; Asseri, A.H.; Zamzami, M.A. Heavy Metal Accumulation is Associated with Molecular and Pathological Perturbations in Liver of Variola louti from the Jeddah Coast of Red Sea. Int. J. Environ. Res. Public Health 2016, 13, 342. https://doi.org/10.3390/ijerph13030342
Mohamed SA, Elshal MF, Kumosani TA, Mal AO, Ahmed YM, Almulaiky YQ, Asseri AH, Zamzami MA. Heavy Metal Accumulation is Associated with Molecular and Pathological Perturbations in Liver of Variola louti from the Jeddah Coast of Red Sea. International Journal of Environmental Research and Public Health. 2016; 13(3):342. https://doi.org/10.3390/ijerph13030342
Chicago/Turabian StyleMohamed, Saleh A., Mohamed F. Elshal, Taha A. Kumosani, Ahmad O. Mal, Youssri M. Ahmed, Yaaser Q. Almulaiky, Amer H. Asseri, and Mazin A. Zamzami. 2016. "Heavy Metal Accumulation is Associated with Molecular and Pathological Perturbations in Liver of Variola louti from the Jeddah Coast of Red Sea" International Journal of Environmental Research and Public Health 13, no. 3: 342. https://doi.org/10.3390/ijerph13030342




