An Evaluation of the Efficacy of Selective Alpha-Blockers in the Treatment of Children with Neurogenic Bladder Dysfunction—Preliminary Findings
Abstract
:1. Introduction
Aim of the Study
2. Materials and Methods
- patients with body weight of up to 20 kg doxazosin 0.5 mg to 1 mg,
- patients with body weight between 20 kg and 40 kg: doxazosin 1 mg to 2 mg, and
- patients with body weight above 40 kg: 2 mg doxazosin in a single dose in the evening.
Ethical Statement
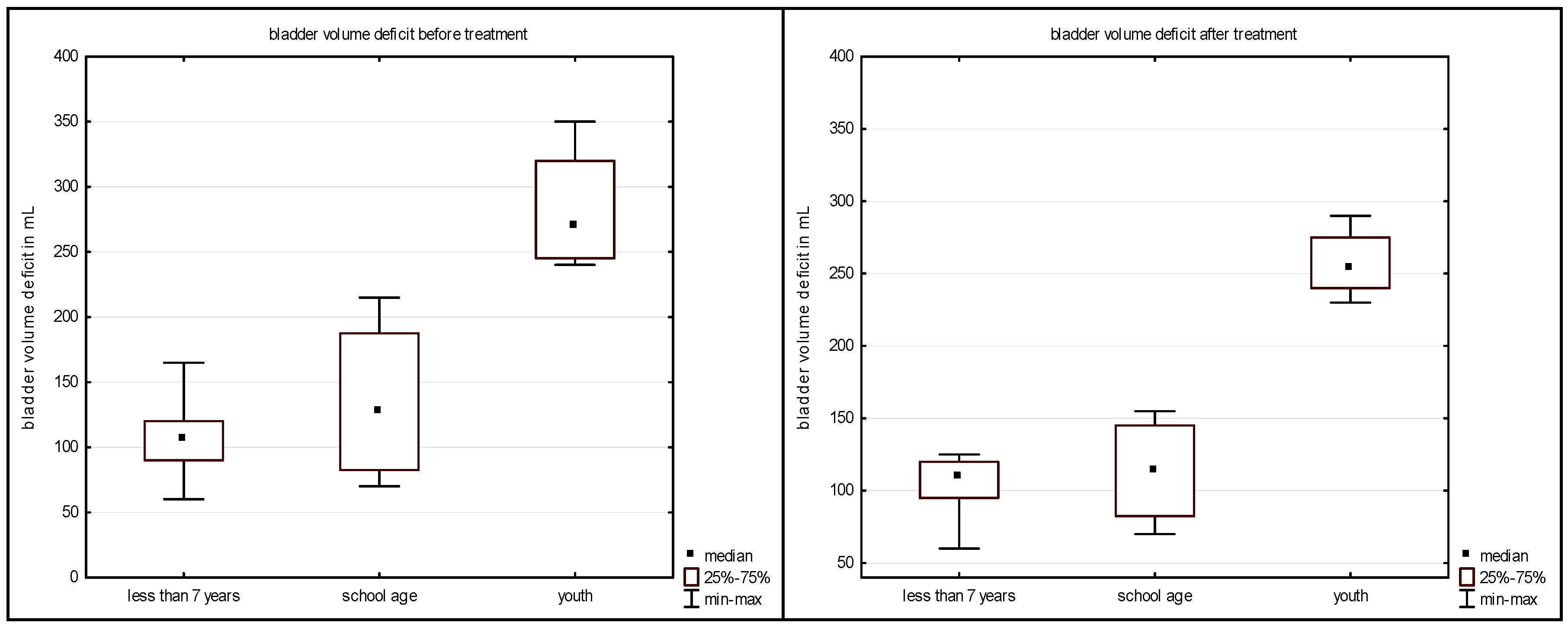
3. Results
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Verpoorten, C.; Buyse, G.M. The neurogenic bladder: Medical treatment. Pediatr. Nephrol. 2008, 23, 717–725. [Google Scholar] [CrossRef] [PubMed]
- McGuire, E.J.; Woodside, J.R.; Bordin, T.A.; Weiss, R.M. Prognostic value of urodynamic testing in myelodysplastic patients. J. Urol. 1981, 136, 205–209. [Google Scholar] [CrossRef]
- Austin, P.F.; Bauer, S.B.; Bower, W.; Chase, J.; Franco, I.; Hoebeke, P.; Rittig, S.; Vande Walle, J.; Von Gontard, A.; Wright, A.; et al. The standardization of terminology of lower urinary tract function in children and adolescents: Update report from the Standardization Committee of the International Children’s Continence Society. J. Urol. 2014, 191, 1863–1865. [Google Scholar] [CrossRef] [PubMed]
- Hendren, W.H. Some alternatives to urinary diversion in children. J. Urol. 1978, 119, 652–660. [Google Scholar] [PubMed]
- Morrisroe, S.N.; O’Connor, R.C.; Nanigian, D.K.; Kurzrock, E.A.; Stone, A.R. Vesicostomy revisited: The best treatment for the hostile bladder in myelodysplastic children? BJU 2005, 97, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Abrams, P.; Andersson, K.E. Muscarinic receptor antagonists for overactive bladder. BJU Int. 2007, 100, 987–1006. [Google Scholar] [CrossRef] [PubMed]
- Hegde, S.S.; Mammen, M.; Jasper, J.R. Antimuscarinics for the treatment of overactive bladder: Current opinions and emerging therapies. Curr. Opin. Investig. Drugs 2004, 5, 40–49. [Google Scholar] [PubMed]
- Hansen, B.J.; Nordling, J.; Mensink, H.J.; Walter, S.; Meyhoff, H.H. Alfuzosin in the treatment of benign prostatic hyperplasia: Effects on symptom scores, urinary flow rates and residual volume. A multicentre, double-blind, placebo-controlled trial. ALFECH Study Group. Scand. J. Urol. Nephrol. Suppl. 1994, 157, 169–176. [Google Scholar] [PubMed]
- Shim, S.R.; Kim, J.H.; Choi, H.; Lee, W.J.; Kim, H.J.; Bae, M.Y.; Hwang, S.D.; Kim, K.H.; Bae, J.H.; Yoon, S.J. General effect of low-dose tamsulosin (0.2 mg) as a first-line treatment for lower urinary tract symptoms associated with benign prostatic hyperplasia: A systematic review and meta-analysis. Curr. Med. Res. Opin. 2015, 31, 353–265. [Google Scholar] [CrossRef] [PubMed]
- Mokhless, I.; Zahran, A.-R.; Youssif, M.; Fahmy, A. Tamsulosin for the management of distal ureteral stones in children: A prospective randomized study. J. Pediatr. Urol. 2012, 8, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Erturhan, S.; Bayrak, O.; Sarica, K.; Seckiner, I.; Baturu, M.; Sen, H. Efficacy of medical expulsive treatment with doxazosin in pediatric patients. Urology 2013, 81, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Abrams, P.; Amarenco, G.; Bakke, A.; Buczyński, A.; Castro-Diaz, D.; Harrison, S.; Kramer, G.; Marsik, R.; Prajsner, A.; Stöhrer, M.; et al. Tamsulosin: Efficacy and safety in patients with neurogenic lower urinary tract dysfunction due to suprasacral spinal cord injury. J. Urol. 2003, 170, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- El-Hefnawy, A.S.; Helmy, T.; El-Assmy, M.M.; Sarhan, O.; Hafez, A.T.; Dawaba, M. Doxazosin versus tizanidine for treatment of dysfunctional voiding in children: A prospective randomized open-labeled trial. Urology 2012, 79, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.F.; Hossy, Y.L.; Masel, J.L.; Cain, M.P.; Casale, A.J.; Rink, R.C. Alpha-adrenergic blockade in children with neuropathic and nonneuropathic voiding dysfunction. J. Urol. 1999, 162, 1064–1067. [Google Scholar] [CrossRef]
- Bogaert, G.; Beckers, G.; Lombaerts, R. The use and rationale of selective alpha blockade in children with non-neurogenic neurogenic bladder dysfunction. Int. Braz. J. Urol. 2004, 30, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Cain, M.P.; Wu, S.D.; Austin, P.F.; Herndon, C.D.; Rink, R.C. Alpha blocker therapy for children with dysfunctional voiding and urinary retention. J. Urol. 2003, 170, 1514–1515. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, J.M.; Combs, A.J.; Glassberg, K.I. Primary bladder neck dysfunction in children and adolescents II: Results of treatment with alpha-adrenergic antagonists. J. Urol. 2005, 73, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, Y.; Tatami, S.; Yamamura, N.; Tadayasu, Y.; Sarashina, A.; Liesenfeld, K.H.; Staab, A.; Schäfer, H.G.; Ieiri, I.; Higuchi, S. Population pharmacokinetics of tamsulosin hydrochloride in paediatric patients with neuropathic and non-neuropathic bladder. Br. J. Clin. Pharmacol. 2010, 70, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Homsy, Y.; Arnold, P.; Zhang, W. Phase IIb/III dose ranging study of tamsulosin as treatment for children with neuropathic bladder. J. Urol. 2011, 186, 2033–2039. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Baukloh, H.; Michael, T.; Miller, K.; Knispel, H.H. Alfuzosin in the treatment of high leak-point pressure in children with neurogenic bladder. BJU Int. 2002, 90, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Abud, E.M.; Ichiyama, R.M.; Havton, L.A.; Chang, H.H. Spinal stimulation of the upper lumbar spinal cord modulates urethral sphincter activity in rats after spinal cord injury. Am. J. Physiol. Renal. Physiol. 2015, 308, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Thor, K.B. Serotonin and norepinephrine involvement in efferent pathways to the urethral rhabdosphincter: Implications for treating stress urinary incontinence. Urology 2003, 62, 3–9. [Google Scholar] [CrossRef]
- Yoshiyama, M.; Nezu, F.M.; Yokoyama, O.; Chancellor, M.B.; De Groat, W.C. Influence of glutamate receptor antagonists on micturition in rats with spinal cord injury. Exp. Neurol. 1999, 159, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Herman, R.M.; Wainberg, M.C. Clonidine inhibits vesico-sphincter reflexes in patients with chronic spinal lesions. Arch. Phys. Med. Rehabil. 1991, 72, 539–545. [Google Scholar] [PubMed]
- Marte, A.; Vessella, A.; Cautiero, P.; Romano, M.; Borrelli, M.; Noviello, C.; Del Gado, R.; Parmeggiani, P. Efficacy of botulinum toxin-A for treating intractable bladder hyperactiviy in children affected by neuropathic bladder secondary to myelomeningocele: An alternative to enterocystoplasty. Minerva Pediatr. 2005, 57, 35–40. [Google Scholar] [PubMed]
- Popat, R.; Apostolidis, A.; Kalsi, V.; Gonzales, G.; Fowler, C.J.; Dasgupta, P. A comparison between the response of patients with idiopathic detrusor overactivity and neurogenic detrusor overactivity to the first intradetrusor injection of botulinum-A toxin. J. Urol. 2005, 174, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Altaweel, W.; Jednack, R.; Bilodeau, C.; Corcos, J. Repeated intradetrusor botulinum toxin type A in children with neurogenic bladder due to myelomeningocele. J. Urol. 2006, 175, 1102–1105. [Google Scholar] [CrossRef]
- Schurch, B.; Holder, J.; Rodia, B. Botulinum A toxin as a treatment of detrusor-sphincter dyssynergia in patients with spinal cord injury: MRI controlled transperineal injections. J. Neurol. Neurosurg. Psychiat. 1997, 63, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.P.; Nishiguchi, J.; O’Leary, M.; Yoshimura, N.; Chancellor, M.B. Single-institution experience in 110 patients with botulinum toxin A injection into bladder or urethra. Urology 2005, 65, 37–41. [Google Scholar] [CrossRef] [PubMed]
| Patient | Age (Years) | LPP1 | LPV1 | LPP2 | LPV2 | HDN1 | HDN2 |
|---|---|---|---|---|---|---|---|
| 1 | 2 | 90 | 30 | 45 | 30 | ||
| 2 | 7 | 135 | 145 | 70 | 145 | 20 | 20 |
| 3 | 9 | 100 | 230 | 50 | 230 | 30 | 10 |
| 4 | 4 | 70 | 60 | 30 | 55 | ||
| 5 | 11 | 60 | 145 | 60 | 225 | ||
| 6 | 5 | 90 | 70 | 90 | 60 | ||
| 7 | 14 | 100 | 130 | 95 | 230 | 10 | 10 |
| 8 | 5 | 55 | 75 | 55 | 75 | ||
| 9 | 16 | 190 | 290 | 180 | 280 | 30 | 20 |
| 10 | 6 | 110 | 45 | 120 | 85 | 20 | 20 |
| 11 | 16 | 210 | 250 | 200 | 250 | 20 | 20 |
| 12 | 9 | 90 | 140 | 90 | 145 | 20 | 10 |
| 13 | 5 | 95 | 60 | 100 | 65 | ||
| 14 | 15 | 170 | 270 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kroll, P.; Gajewska, E.; Zachwieja, J.; Sobieska, M.; Mańkowski, P. An Evaluation of the Efficacy of Selective Alpha-Blockers in the Treatment of Children with Neurogenic Bladder Dysfunction—Preliminary Findings. Int. J. Environ. Res. Public Health 2016, 13, 321. https://doi.org/10.3390/ijerph13030321
Kroll P, Gajewska E, Zachwieja J, Sobieska M, Mańkowski P. An Evaluation of the Efficacy of Selective Alpha-Blockers in the Treatment of Children with Neurogenic Bladder Dysfunction—Preliminary Findings. International Journal of Environmental Research and Public Health. 2016; 13(3):321. https://doi.org/10.3390/ijerph13030321
Chicago/Turabian StyleKroll, Paweł, Ewa Gajewska, Jacek Zachwieja, Magdalena Sobieska, and Przemysław Mańkowski. 2016. "An Evaluation of the Efficacy of Selective Alpha-Blockers in the Treatment of Children with Neurogenic Bladder Dysfunction—Preliminary Findings" International Journal of Environmental Research and Public Health 13, no. 3: 321. https://doi.org/10.3390/ijerph13030321





