Heavy Metals in Surface Soils in the Upper Reaches of the Heihe River, Northeastern Tibetan Plateau, China
Abstract
:1. Introduction
2. Materials and Methods
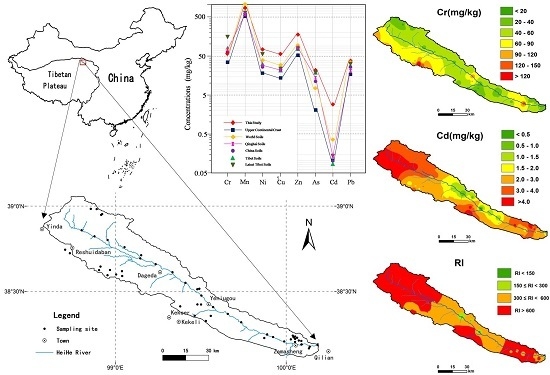
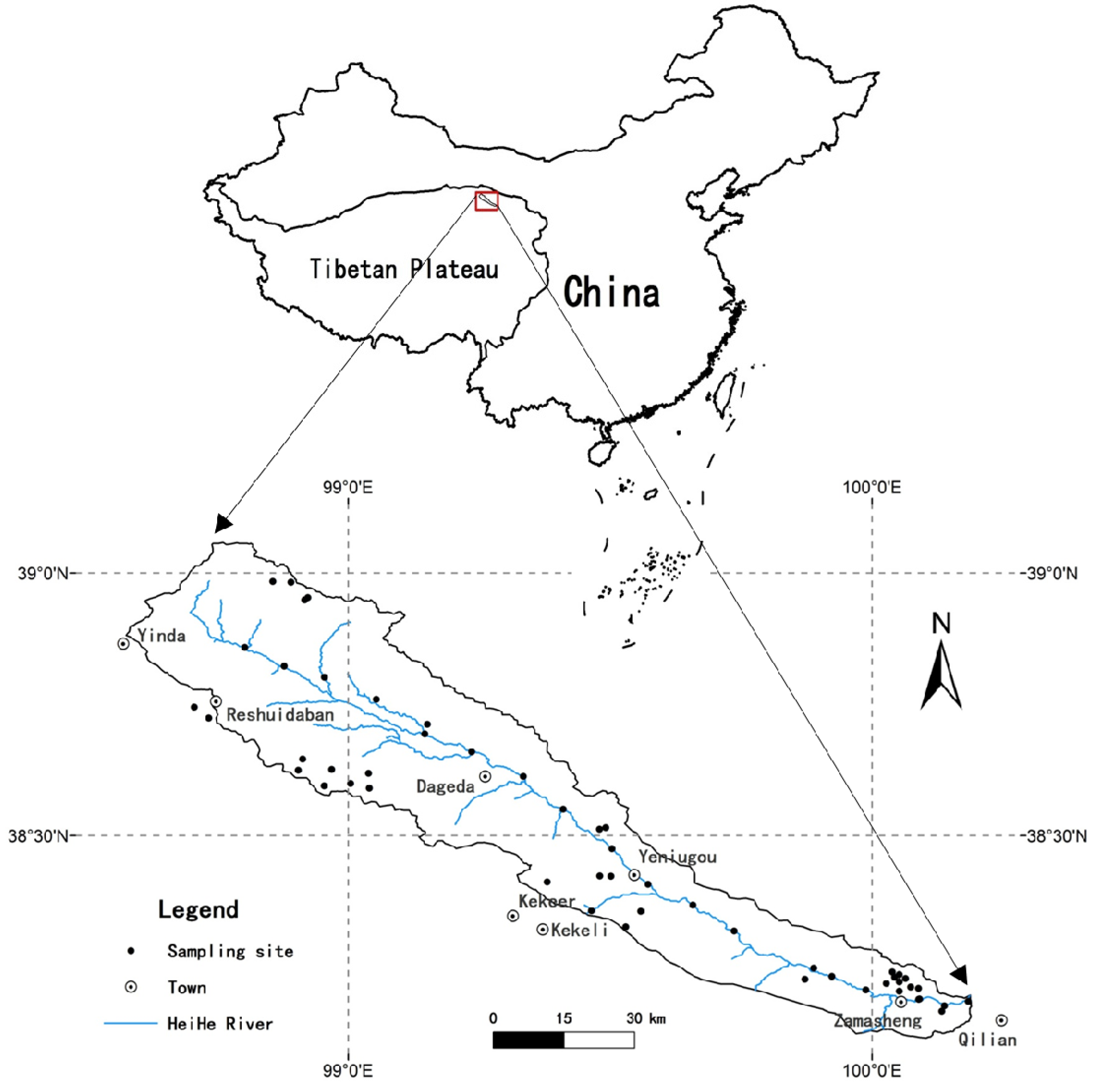
2.1. General Setting of the Study Area
2.2. Soil Sampling
2.3. Chemical Analyses
2.4. Statistics
2.5. Multivariate Analyses
2.6. Risk Assessment
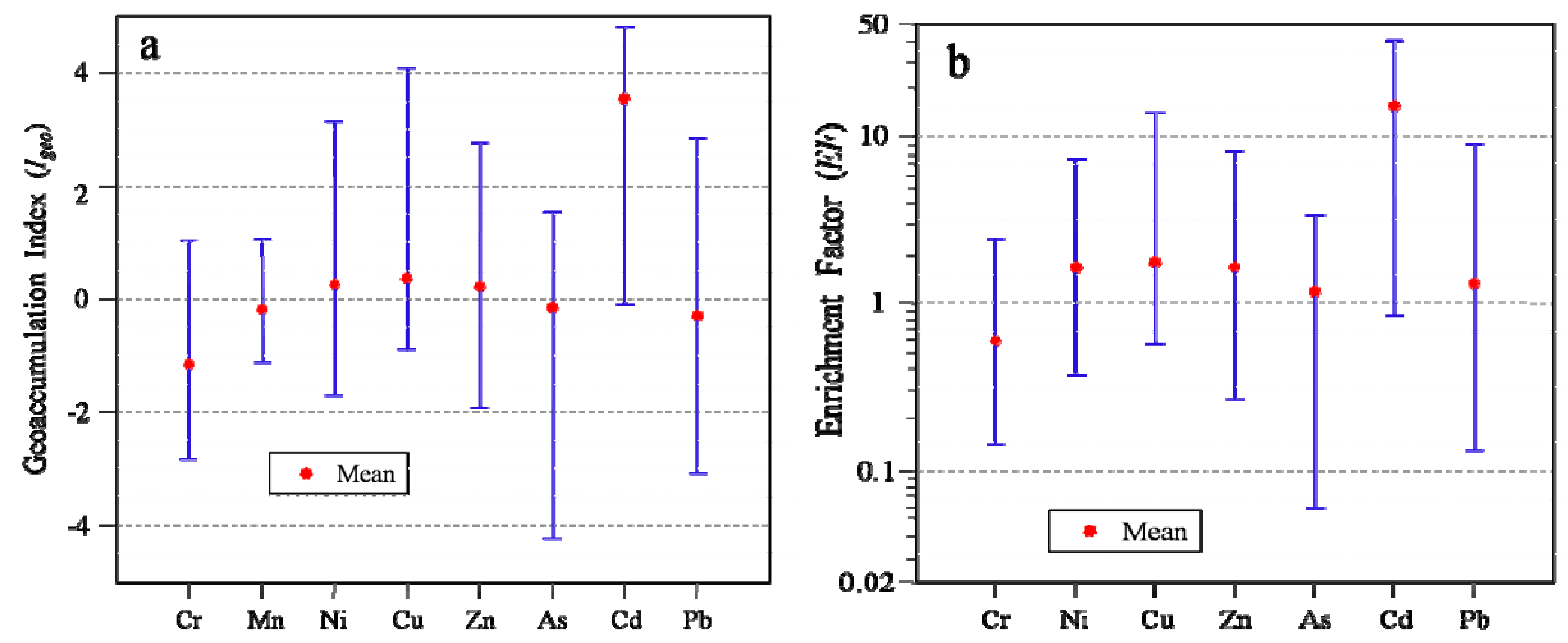
2.6.1. Geoaccumulation Index (Igeo)
2.6.2. Enrichment Factor (EF)
2.6.3. Potential Ecological Risk Index (PERI)
3. Results and Discussion
3.1. Basic Statistics and Concentration Comparisons
3.2. Probability Distribution
3.3. Correlation Coefficient Analysis (CCA)
3.4. Principal Component Analysis (PCA)
3.5. Cluster Analysis (CA)
3.6. Spatial Distributions
3.7. Environmental Risk Assessment
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, T.B.; Wong, J.W.C.; Zhou, H.Y.; Wong, M.H. Assessment of trace metal distribution and contamination in surface soils of hong kong. Environ. Pollut. 1997, 96, 61–68. [Google Scholar] [CrossRef]
- Yu, R.; Yuan, X.; Zhao, Y.H.; Hu, G.R.; Tu, X.L. Heavy metal pollution in intertidal sediments from quanzhou bay, china. J. Environ. Sci. 2008, 20, 664–669. [Google Scholar] [CrossRef]
- Chakraborty, P.; Babu, P.V.R.; Acharyya, T.; Bandyopadhyay, D. Stress and toxicity of biologically important transition metals (co, ni, cu and zn) on phytoplankton in a tropical freshwater system: An Investigation with Pigment Analysis by Hplc. Chemosphere 2010, 80, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Ahmed, M.K.; Habibullahalmamun, M.; Hoque, M.F. Preliminary assessment of heavy metal contamination in surface sediments from a river in bangladesh. Environ. Earth Sci. 2015, 73, 1837–1848. [Google Scholar] [CrossRef]
- Meza-Montenegro, M.M.; Gandolfi, A.J.; Klimecki, W.T.; Aguilar-Apodaca, M.G.; Río-Salas, R.D.; O-Villanueva, M.D.L.; Gómez-Alvarez, A.; Mendivil-Quijada, H.; Valencia, M. Metals in residential soils and cumulative risk assessment in yaqui and mayo agricultural valleys, northern mexico. Sci. Environ. 2012, 433, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Teng, Y.G.; Lu, S.J.; Wang, Y.Y.; Wang, J.S. Contamination features and health risk of soil heavy metals in china. Sci. Total Environ. 2015, 512–513, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.A.; Cheng, H.F. Application of stochastic models in identification and apportionment of heavy metal pollution sources in the surface soils of a large-scale region. Environ. Sci. Technol. 2013, 47, 3752–3760. [Google Scholar] [CrossRef] [PubMed]
- Alloway, B.J. Heavy metals in soils. Heavy Met. Soils 1995, 85, 1318–1324. [Google Scholar]
- Nriagu, J.O. A history of global metal pollution. Science 1996, 272, 223–224. [Google Scholar] [CrossRef]
- Ip, C.C.M.; Li, X.D.; Zhang, G.; Wai, O.W.H.; Li, Y.S. Trace metal distribution in sediments of the pearl river estuary and the surrounding coastal area, south china. Environ. Pollut. 2007, 147, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Chon, H.S.; Ohandja, D.G.; Voulvoulis, N. Implementation of E.U. Water framework directive: Source Assessment of Metallic Substances at Catchment Levels. J. Environ. Monit. 2010, 12, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Davutluoglu, O.I.; Seckin, G.; Ersu, C.B.; Yilmaz, T.; Sari, B. Heavy metal content and distribution in surface sediments of the seyhan river, turkey. J. Environ. Manag. 2011, 92, 2250–2259. [Google Scholar] [CrossRef] [PubMed]
- Giller, K.E.; Mcgrath, S.P. Pollution by toxic metals on agricultural soils. Nature 1988, 335, 676. [Google Scholar] [CrossRef]
- Abrahams, P.W. Soils: Their Implications to Human Health. Sci. Total Environ. 2002, 291, 1–32. [Google Scholar] [CrossRef]
- Zhang, L.P.; Ye, X.; Feng, H.; Jing, Y.H.; Yang, T.O.; Yu, X.T.; Liang, R.Y.; Gao, C.T.; Chen, W.Q. Heavy metal contamination in western xiamen bay sediments and its vicinity, china. Mar. Pollut. Bull. 2007, 54, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.M.; Cruz, N.; Coelho, C.; Henriques, B.; Carvalho, L.; Duarte, A.C.; Pereira, E.; Römkens, P.F.A.M. Risk assessment for cd, cu, pb and zn in urban soils: Chemical Availability as the Central Concept. Environ. Pollut. 2013, 183, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Culbard, E.B.; Thornton, I.; Watt, J.; Wheatley, M.; Moorcroft, S.; Thompson, M. Metal contamination in british urban dusts and soils. J. Environ. Qual. 1988, 17, 226–234. [Google Scholar] [CrossRef]
- Pouyat, R.V.; Nnell, M.J. Heavy metal accumulations in forest soils along an urban- rural gradient in southeastern new york, USA. Water Air Soil Pollut. 1991, 57–58, 797–807. [Google Scholar] [CrossRef]
- Steiger, B.V.; Webster, R.; Schulin, R.; Lehmann, R. Mapping heavy metals in polluted soil by disjunctive kriging. Environ. Pollut. 1996, 94, 205–215. [Google Scholar] [CrossRef]
- Raghunath, R.; Tripathi, R.M.; Kumar, A.V.; Sathe, A.P.; Khandekar, R.N.; Nambi, K.S. Assessment of pb, cd, cu, and zn exposures of 6- to 10-year-old children in mumbai. Environ. Res. 1999, 80, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Manta, D.S.; Angelone, M.; Bellanca, A.; Neri, R.; Sprovieri, M. Heavy metals in urban soils: A Case Study from the City of Palermo (Sicily), italy. Sci. Total Environ. 2003, 300, 229–243. [Google Scholar] [CrossRef]
- Micó, C.; Recatalá, L.; Peris, M.; Sánchez, J. Assessing heavy metal sources in agricultural soils of an european mediterranean area by multivariate analysis. Chemosphere 2006, 65, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.B.; Zhou, Q.X.; Xie, X.K.; Rui, L. Spatial, sources and risk assessment of heavy metal contamination of urban soils in typical regions of shenyang, china. J. Hazard. Mater. 2010, 174, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.Z.; Yang, R. Background concentrations of elements in surface soils and their changes as affected by agriculture use in the desert-oasis ecotone in the middle of heihe river basin, north-west china. J. Geochem. Explor. 2008, 98, 57–64. [Google Scholar] [CrossRef]
- Kang, S.C.; Zhang, Q.G. Scientific survey and monitoring of atmospheric pollution over the tibetan plateau. Chin. J. Nat. 2010, 32, 13–18. [Google Scholar]
- Yao, T.; Thompson, L.G.; Mosbrugger, V.; Zhang, F.; Ma, Y.; Luo, T.; Xu, B.; Yang, X.; Joswiak, D.R.; Wang, W. Third pole environment (TPE). Environ. Dev. 2012, 3, 52–64. [Google Scholar] [CrossRef]
- Cong, Z.Y.; Kang, S.C.; Zhang, Y.L.; Li, X.D. Atmospheric wet deposition of trace elements to central tibetan plateau. Appl. Geochem. 2010, 25, 1415–1421. [Google Scholar] [CrossRef]
- Zhang, J.L. Barriers to water markets in the heihe river basin in northwest china. Agric. Water Manag. 2007, 87, 32–40. [Google Scholar] [CrossRef]
- Qiu, J. China: The Third Pole. Nat. News 2008, 454, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.Y.; Long, X.; Ma, R. Water uptake by saltcedar (Tamarix ramosissima) in a desert riparian forest: Responses to Intra—Annual Water Table Fluctuation. Hydrol. Process. 2015. [Google Scholar] [CrossRef]
- Mason, B.J. Preparation of Soil Sampling Protocols: Sampling Techniques and Strategies; Environmental Protection Agency: Washington, DC, USA, 1992.
- Guo, M. Soil sampling and methods of analysis. J. Environ. Qual. 2009. [Google Scholar] [CrossRef]
- Zheng, W. The Technical Specification For Soil Environmental Monitoring (HJ/T 166–2004); Chinese Standard: Beijing, China, 2004; pp. 4–17. [Google Scholar]
- Akhter, M.S.; Madany, I.M. Heavy metals in street and house dust in bahrain. Water Air Soil Pollut. 1993, 66, 111–119. [Google Scholar] [CrossRef]
- Sheng, J.J.; Wang, X.P.; Gong, P.; Tian, L.D.; Yao, T.D. Heavy metals of the tibetan top soils. Environ. Sci. Pollut. Res. 2012, 19, 3362–3370. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.; Andrade, E.; López-Suárez, A.; Ledesma, R.; Cahill, T.A.; Wakabayashi, P.H. A receptor model for atmospheric aerosols from a southwestern site in mexico city. Atmos. Environ. 1996, 30, 3471–3479. [Google Scholar] [CrossRef]
- Dı́Az, R.V.; Aldape, F.; Flores, M.J. Identification of airborne particulate sources, of samples collected in ticomán, mexico, using pixe and multivariate analysis. Nucl. Instrum. Methods Phys. Res. 2002, 189, 249–253. [Google Scholar] [CrossRef]
- Loska, K.; Wiechuła, D. Application of principal component analysis for the estimation of source of heavy metal contamination in surface sediments from the rybnik reservoir. Chemosphere 2003, 51, 723–733. [Google Scholar] [CrossRef]
- Han, Y.M.; Du, P.X.; Cao, J.J.; Posmentier, E.S. Multivariate analysis of heavy metal contamination in urban dusts of xi'an, central china. Sci. Total Environ. 2006, 355, 176–186. [Google Scholar]
- Kaiser, H.F. The application of electronic computers to factor analysis. Educ. Psychol. Meas. 1960, 20, 141–151. [Google Scholar] [CrossRef]
- Bu, J.W.; Zhou, J.W.; Zhou, A.G.; Kong, F.L. The Comparison of Different Methods in Hydrochemical Classification Using Hierarchical Clustering Analysis. In 2011 International Conference on Remote Sensing, Environ. and Transportation Engineering (RSETE), Nanjing, China, 24–26 June 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 1783–1787. [Google Scholar]
- Wedepohl, K.H. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Bowen, H.J.M. Environmental Chemistry of the Elements; Academic Press: New York, NY, USA, 1979. [Google Scholar]
- Vinogradov, A.P. Geochemistry of Rare and Dispersed Chemical Elements in Soils; Consultants Bureau: New York, NY, USA, 1959. [Google Scholar]
- CNEMC. Background Values of Elements in Soils of China; China Environmental Science Press: Beijing, China, 1990. [Google Scholar]
- Cheng, Y.A.; Tian, J.L. Background Values of Elements in Tibetan Soil and Their Distribution; Science Press: Beijing, China, 1993. [Google Scholar]
- Udoguchi, A.; Gotoh, S. Fourth International KfK/TNO Conference on Contaminated Soil 3–7 May 1993, Berlin, Germany. In Environmental Quality Standard For Soils (GB 15618–1995); Springer: Berlin, Germany, 1993; pp. 149–153. [Google Scholar]
- Muller, G. Index of geoaccumulation in sediments of the rhine river. Geojournal 1969, 2, 108–118. [Google Scholar]
- Loska, K.; Wiechuła, D.; Barska, B.; Cebula, E.; Chojnecka, A. Assessment of arsenic enrichment of cultivated soils in southern poland. Polish J. Environ. Stud. 2003, 12, 187–192. [Google Scholar]
- Armid, A.; Shinjo, R.; Zaeni, A.; Sani, A.; Ruslan, R. The distribution of heavy metals including pb, cd and cr in kendari bay surficial sediments. Mar. Pollut. Bull. 2014, 84, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Loska, K.; Wiechuła, D.; Korus, I. Metal contamination of farming soils affected by industry. Environ. Int. 2004, 30, 159–165. [Google Scholar] [CrossRef]
- Ji, Y.Q.; Feng, Y.c.; Wu, J.H.; Zhu, T.; Bai, Z.P.; Duan, C.Q. Using geoaccumulation index to study source profiles of soil dust in china. J. Environ. Sci. 2008, 20, 571–578. [Google Scholar] [CrossRef]
- Quevauviller, P.; Lavigne, R.; Cortez, L. Impact of industrial and mine drainage wastes on the heavy metal distribution in the drainage basin and estuary of the sado river (Portugal). Environ. Pollut. 1989, 59, 267–286. [Google Scholar] [CrossRef]
- Lee, C.L.; Fang, M.D.; Hsieh, M.T. Characterization and distribution of metals in surficial sediments in southwestern taiwan. Mar. Pollut. Bull. 1998, 36, 464–471. [Google Scholar] [CrossRef]
- Reimann, C.; Caritat, P. Intrinsic flaws of element enrichment factors (EFS) in environmental geochemistry. Environ. Sci. Technol. 2000, 34, 5084–5091. [Google Scholar] [CrossRef]
- Bergamaschi, L.; Rizzio, E.; Valcuvia, M.; Verza, G.; Profumo, A.; Gallorini, M. Determination of trace elements and evaluation of their enrichment factors in himalayan lichens. Environ. Pollut. 2002, 120, 137–144. [Google Scholar] [CrossRef]
- Aloupi, M.; Angelidis, M. Normalization to lithium for the assessment of metal contamination in coastal sediment cores from the aegean sea, greece. Mar. Environ. Res. 2001, 52, 1–12. [Google Scholar] [CrossRef]
- Schiff, K.; Weisberg, S.B. Iron as a reference element for determining trace metal enrichment in southern california coastal shelf sediments. Mar. Environ. Res. 1999, 48, 161–176. [Google Scholar] [CrossRef]
- Chabukdhara, M.; Nema, A.K. Assessment of heavy metal contamination in hindon river sediments: A Chemometric and Geochemical Approach. Chemosphere 2012, 87, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Gowd, S.S.; Reddy, M.R.; Govil, P. Assessment of heavy metal contamination in soils at Jajmau (Kanpur) and unnao industrial areas of the ganga plain, uttar pradesh, India. J. Hazard. Mater. 2010, 174, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Hakanson, L. Ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Hakanson, L. Aquatic contamination and ecological risk. An attempt to a conceptual framework. Water Res. 1984, 18, 1107–1118. [Google Scholar] [CrossRef]
- Suresh, G.; Sutharsan, P.; Ramasamy, V.; Venkatachalapathy, R. Assessment of spatial distribution and potential ecological risk of the heavy metals in relation to granulometric contents of veeranam lake sediments, india. Ecotoxicol. Environ. Saf. 2012, 84, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.H.; Liu, X.B.; Liu, Z.G.; Li, G.F. Pollution and potential ecological risk evaluation of heavy metals in the sediments around dongjiang harbor, tianjin. Proced. Environ. Sci. 2010, 2, 729–736. [Google Scholar] [CrossRef]
- Joaquín, D.; Cinta, B.B.; José Miguel, N.; Tomasz, B. Speciation and ecological risk of toxic elements in estuarine sediments affected by multiple anthropogenic contributions (guadiana saltmarshes, sw iberian peninsula): I. Surficial Sediments. Sci. Total Environ. 2011, 409, 3666–3679. [Google Scholar]
- Deng, H.G.; Zhang, J.; Wang, D.Q.; Chen, Z.L.; Xu, S.Y. Heavy metal pollution and assessment of the tidal flat sediments near the coastal sewage outfalls of shanghai, china. Environ. Earth Sci. 2010, 60, 57–63. [Google Scholar] [CrossRef]
- Wu, Y.G.; Xu, Y.N.; Zhang, J.H.; Hu, S.H. Evaluation of ecological risk and primary empirical research on heavy metals in polluted soil over xiaoqinling gold mining region, shaanxi, china. Trans. Nonferr. Met. Soc. China 2010, 20, 688–694. [Google Scholar] [CrossRef]
- Xu, Z.Q.; Ni, S.J.; Tuo, X.G.; Zhang, C.j. Calculation of heavy metals' toxicity coefficient in the evaluation of potential ecological risk index. Environ. Sci. Technol. 2008, 2, 029. [Google Scholar]
- Jenkins, D.A.; Jones, R.G.W. Trace Elements in Rocks, Soils, Plants, and Animals: Introduction; Wiley: New York, NY, USA, 1979. [Google Scholar]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell: Oxford, UK, 1985. [Google Scholar]
- Tiller, K.G. Heavy Metals in Soils and Their Environmental Significance; Springer: New York, NY, USA, 1989. [Google Scholar]
- Yin, A.; Harrison, T.M. Geologic evolution of the himalayan-tibetan orogen. Ann. Rev. Earth Planet. Sci. 2000, 28, 211–280. [Google Scholar] [CrossRef]
- Li, C.L.; Kang, S.C.; Wang, X.P.; Ajmone-Marsan, F.; Zhang, Q.G. Heavy metals and rare earth elements (rees) in soil from the nam co basin, tibetan plateau. Environ. Geol. 2008, 53, 1433–1440. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, J.X.; Tu, S.D.; Wang, Y.G.; Qian, Q.F.; Chen, B.R. Content and distribution of ree in the soils from mt. Qomolangma region. Geogr. Res. 1990, 9, 58–66. [Google Scholar]
- Zhang, X.P.; Deng, W.; Yang, X.M. The background concentrations of 13 soil trace elements and their relationships to parent materials and vegetation in xizang (tibet), china. J. Asian Earth Sci. 2002, 21, 167–174. [Google Scholar] [CrossRef]
- Chen, Y.L. Geochemistry of Granitoids from the Eastern Tianshan Mountains and Northern Qinling Belt; Geological Publishing House: Beijing, China, 1999. [Google Scholar]
- Luo, J.; Niu, F.J.; Lin, Z.J.; Liu, M.H.; Yin, G.A. Thermokarst lake changes between 1969 and 2010 in the beilu river basin, qinghai–tibet plateau, China. Sci. Bull. 2015, 60, 556–564. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Hinzman, L.D. Shrinking thermokarst ponds and groundwater dynamics in discontinuous permafrost near council, alaska. Permafr. Periglac. Process. 2003, 14, 151–160. [Google Scholar] [CrossRef]
- Marsh, P.; Russell, M.; Pohl, S.; Haywood, H.; Onclin, C. Changes in thaw lake drainage in the western canadian arctic from 1950 to 2000. Hydrol. Process. 2009, 23, 145–158. [Google Scholar] [CrossRef]
- Arp, C.D.; Jones, B.M.; Urban, F.E.; Grosse, G. Hydrogeomorphic processes of thermokarst lakes with grounded—Ice and floating—Ice regimes on the arctic coastal plain, alaska. Hydrol. Process. 2011, 25, 2422–2438. [Google Scholar] [CrossRef]
- Sun, Z.Y.; Ma, R.; Wang, Y.X.; Hu, Y.L.; Sun, L.J. Hydrogeological and hydrogeochemical control of groundwater salinity in an arid inland basin: Dunhuang Basin, Northwestern China. Hydrol. Process. 2015. [Google Scholar] [CrossRef]
- Vine, J.D.; Tourtelot, E.B. Geochemistry of black shale deposits; a summary report. Econ. Geol. 1970, 65, 253–272. [Google Scholar] [CrossRef]
- Zhang, B.R.; Fu, J.M. Advances in Geochemistry; Chemical Industry Press: Beijing, China, 2005. [Google Scholar]
- Obrist, D.; Johnson, D.; Lindberg, S.; Luo, Y.; Hararuk, O.; Bracho, R.; Battles, J.; Dail, D.; Edmonds, R.; Monson, R. Mercury distribution across 14 us forests. Part I: Spatial Patterns of Concentrations in Biomass, Litter, and Soils. Environ. Sci. Technol. 2011, 45, 3974–3981. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Cao, L.M.; Li, Z.L.; Wang, H.N.; Chu, T.Q.; Zhang, J.R. Geochemistry of Element; Science Press: Beijing, China, 1984. [Google Scholar]





| Element | Raw Data | Log-Transformed Data | UCC a | World Soils | China Soils d | Tibet Soils e | Latest Tibet Soils f | Qinghai Soils g | China Soil Quality Standard h | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | Median | S.D. | V.C. | Skewness | Kurtosis | K-S p | Skewness | Kurtosis | K-S p | Mean | Mean | Mean | Mean | Median | Min | Max | Mean | Median | Mean | Median | Class 1 | Class 2 | Class 3 | |
| Cr | 14.91 | 214.25 | 57.29 | 44.30 | 38.91 | 0.68 | 1.881 | 4.496 | 0.082 | 0.281 | −0.223 | 0.949 | 35 | 70 b | 61.0 | 77.4 | 69.6 | 17.77 | 3429 | 155.54 | 60.06 | 70.1 | 63.3 | 90 | 350 | 400 |
| Mn | 413.19 | 1807.32 | 818.84 | 794.29 | 257.96 | 0.32 | 1.978 | 6.168 | 0.349 | 0.573 | 1.579 | 0.835 | 527 | 1000 b | 583 | 626 | 586 | 224.6 | 1776 | 617.36 | 591.45 | 580 | 570 | |||
| Ni | 13.97 | 395.73 | 70.22 | 40.78 | 71.04 | 1.01 | 2.917 | 9.691 | 0.004 | 1.023 | 0.807 | 0.092 | 18.6 | 40 c | 26.9 | 32.1 | 29.8 | 6.108 | 2045 | 55.86 | 26.55 | 29.6 | 29.1 | 40 | 60 | 200 |
| Cu | 18.04 | 551.01 | 56.38 | 37.49 | 75.16 | 1.33 | 5.863 | 38.303 | 0.000 | 1.728 | 5.231 | 0.417 | 14.3 | 30 b | 22.6 | 21.9 | 19.7 | 6.14 | 107.9 | 24.27 | 22.30 | 22.2 | 22.3 | 35 | 100 | 400 |
| Zn | 31.87 | 830.06 | 178.68 | 135.81 | 151.31 | 0.85 | 2.726 | 8.493 | 0.002 | 0.494 | 0.925 | 0.481 | 52 | 90 b | 74.2 | 73.7 | 71.9 | 31.39 | 315 | 75.59 | 64.45 | 80.3 | 75.7 | 100 | 300 | 500 |
| As | 1.12 | 60.80 | 21.60 | 19.73 | 10.40 | 0.48 | 1.774 | 4.517 | 0.054 | −2.369 | 13.053 | 0.156 | 2 | 7.2 b | 11.2 | 18.7 | 17.2 | 1.834 | 154.5 | 19.27 | 15.32 | 14.0 | 13.0 | 15 | 25 | 40 |
| Cd | 0.20 | 5.90 | 2.93 | 2.92 | 1.47 | 0.50 | 0.180 | −0.664 | 0.809 | −1.687 | 3.621 | 0.181 | 0.102 | 0.35 b | 0.097 | 0.08 | 0.074 | 0.028 | 0.849 | 0.141 | 0.108 | 0.137 | 0.132 | 0.2 | 0.6 | 1 |
| Pb | 3.78 | 225.52 | 37.35 | 22.72 | 41.32 | 1.11 | 2.753 | 8.687 | 0.001 | 0.419 | 0.419 | 0.518 | 17 | 35 b | 26.0 | 28.9 | 27.7 | 9.787 | 153.9 | 32.15 | 26.08 | 20.9 | 20.4 | 35 | 350 | 500 |
| TOC | 0.44 | 12.33 | 3.41 | 2.37 | 2.65 | 0.78 | 1.354 | 1.677 | 0.108 | −0.126 | −0.631 | 0.969 | ||||||||||||||
| Value | Soil Quality | Value | Enrichment Level | Value | Ecological Risk |
|---|---|---|---|---|---|
| Igeo < 0 | Practically uncontaminated | EF < 2 | Deficiency to minimal enrichment | RI < 150 | Low risk |
| 0 < Igeo < 1 | Uncontaminated to moderately contaminated | 2 < EF < 5 | Moderate enrichment | 150 ≤ RI < 300 | Moderate risk |
| 1 < Igeo < 2 | Moderately contaminated | 5 < EF < 20 | Significant enrichment | 300 ≤ RI < 600 | Considerable risk |
| 2 < Igeo < 3 | Moderately to heavily contaminated | 20 < EF < 40 | Very high enrichment | 600 ≤ RI | High risk |
| 3 < Igeo < 4 | Heavily contaminated | 40 < EF | Extremely high enrichment | ||
| 4 < Igeo < 5 | Heavily to extremely contaminated | ||||
| 5 < Igeo | Extremely contaminated |
| Cr | Mn | Ni | Cu | Zn | As | Cd | Pb | TOC | |
|---|---|---|---|---|---|---|---|---|---|
| Cr | 0.412 | 0.000 | 0.173 | 0.124 | 0.001 | 0.302 | 0.013 | 0.063 | |
| Mn | 0.031 | 0.105 | 0.021 | 0.227 | 0.075 | 0.105 | 0.245 | 0.450 | |
| Ni | 0.671 | 0.177 | 0.208 | 0.065 | 0.003 | 0.335 | 0.045 | 0.001 | |
| Cu | 0.134 | 0.283 | 0.115 | 0.286 | 0.046 | 0.007 | 0.274 | 0.296 | |
| Zn | −0.163 | −0.106 | −0.213 | 0.080 | 0.295 | 0.000 | 0.001 | 0.311 | |
| As | 0.441 | 0.203 | 0.383 | 0.236 | −0.076 | 0.083 | 0.365 | 0.142 | |
| Cd | −0.074 | 0.177 | 0.061 | 0.337 | 0.555 | 0.195 | 0.002 | 0.260 | |
| Pb | −0.309 | −0.098 | −0.237 | 0.085 | 0.416 | −0.049 | 0.400 | 0.242 | |
| TOC | 0.215 | −0.018 | 0.427 | -0.076 | −0.070 | 0.151 | 0.091 | 0.099 |
| Element | Rotated Component Matrix | Communities | |||
|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | ||
| Cr | 0.885 | −0.179 | −0.094 | 0.061 | 0.827 |
| Mn | 0.015 | −0.099 | 0.908 | 0.081 | 0.842 |
| Ni | 0.755 | −0.145 | 0.114 | 0.419 | 0.780 |
| Cu | 0.311 | 0.298 | 0.574 | −0.307 | 0.609 |
| Zn | −0.058 | 0.822 | −0.172 | −0.165 | 0.736 |
| As | 0.695 | 0.107 | 0.239 | 0.010 | 0.552 |
| Cd | 0.136 | 0.816 | 0.292 | 0.040 | 0.771 |
| Pb | −0.299 | 0.719 | −0.021 | 0.223 | 0.656 |
| TOC | 0.210 | 0.077 | -0.032 | 0.907 | 0.874 |
| Initial Eigenvalue | 2.455 | 2.033 | 1.282 | 0.878 | |
| Percent of variance | 27.277 | 22.593 | 14.239 | 9.754 | |
| Cumulative percent | 27.277 | 49.869 | 64.109 | 73.862 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bu, J.; Sun, Z.; Zhou, A.; Xu, Y.; Ma, R.; Wei, W.; Liu, M. Heavy Metals in Surface Soils in the Upper Reaches of the Heihe River, Northeastern Tibetan Plateau, China. Int. J. Environ. Res. Public Health 2016, 13, 247. https://doi.org/10.3390/ijerph13030247
Bu J, Sun Z, Zhou A, Xu Y, Ma R, Wei W, Liu M. Heavy Metals in Surface Soils in the Upper Reaches of the Heihe River, Northeastern Tibetan Plateau, China. International Journal of Environmental Research and Public Health. 2016; 13(3):247. https://doi.org/10.3390/ijerph13030247
Chicago/Turabian StyleBu, Jianwei, Ziyong Sun, Aiguo Zhou, Youning Xu, Rui Ma, Wenhao Wei, and Meng Liu. 2016. "Heavy Metals in Surface Soils in the Upper Reaches of the Heihe River, Northeastern Tibetan Plateau, China" International Journal of Environmental Research and Public Health 13, no. 3: 247. https://doi.org/10.3390/ijerph13030247