The Detoxification and Degradation of Benzothiazole from the Wastewater in Microbial Electrolysis Cells
Abstract
:1. Introduction
2. Materials and Methods
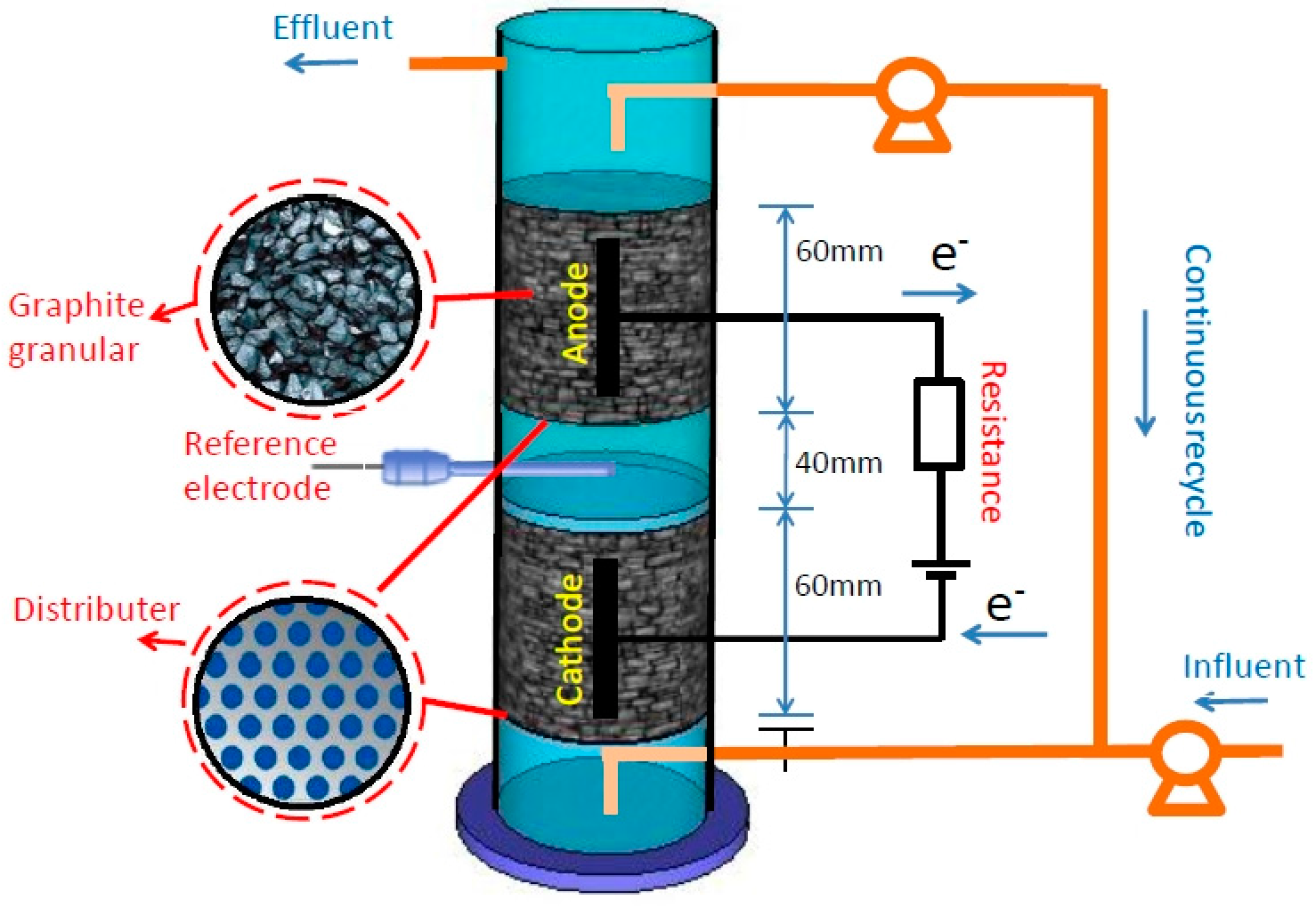
2.1. Experimental Setup
2.2. Microbial Inoculum and Wastewater
2.3. Operation
2.4. Analysis
2.5. Antibacterial Activity Measurement
3. Results and Discussion
3.1. COD Removal and BTH Degradation at Different BTH Concentrations
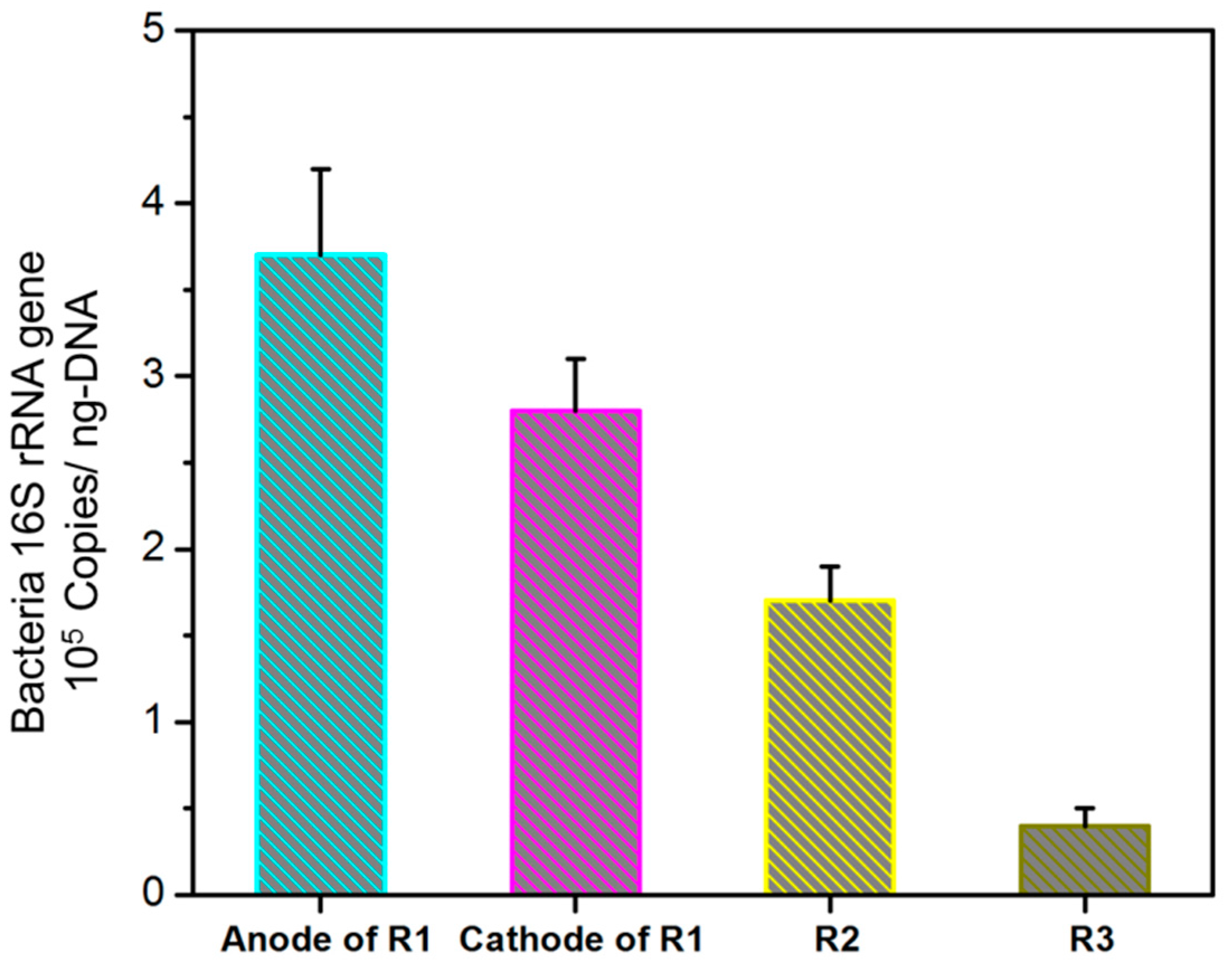
3.2. Effects of Supplied Voltages on the Enrichment of Bacteria and Sludge Characteristics
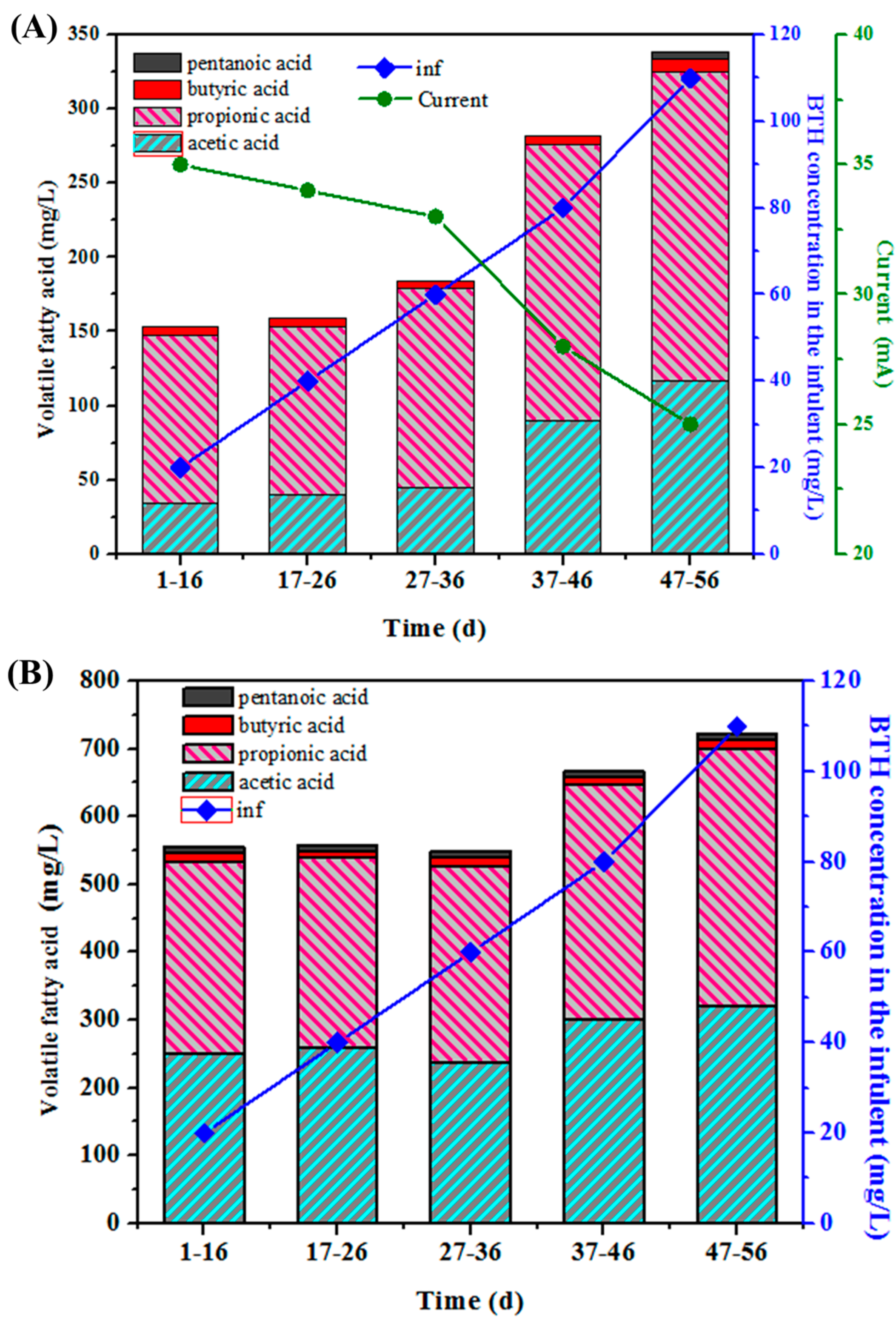
3.3. Effects of BTH Concentration on Current Value and VFA Production
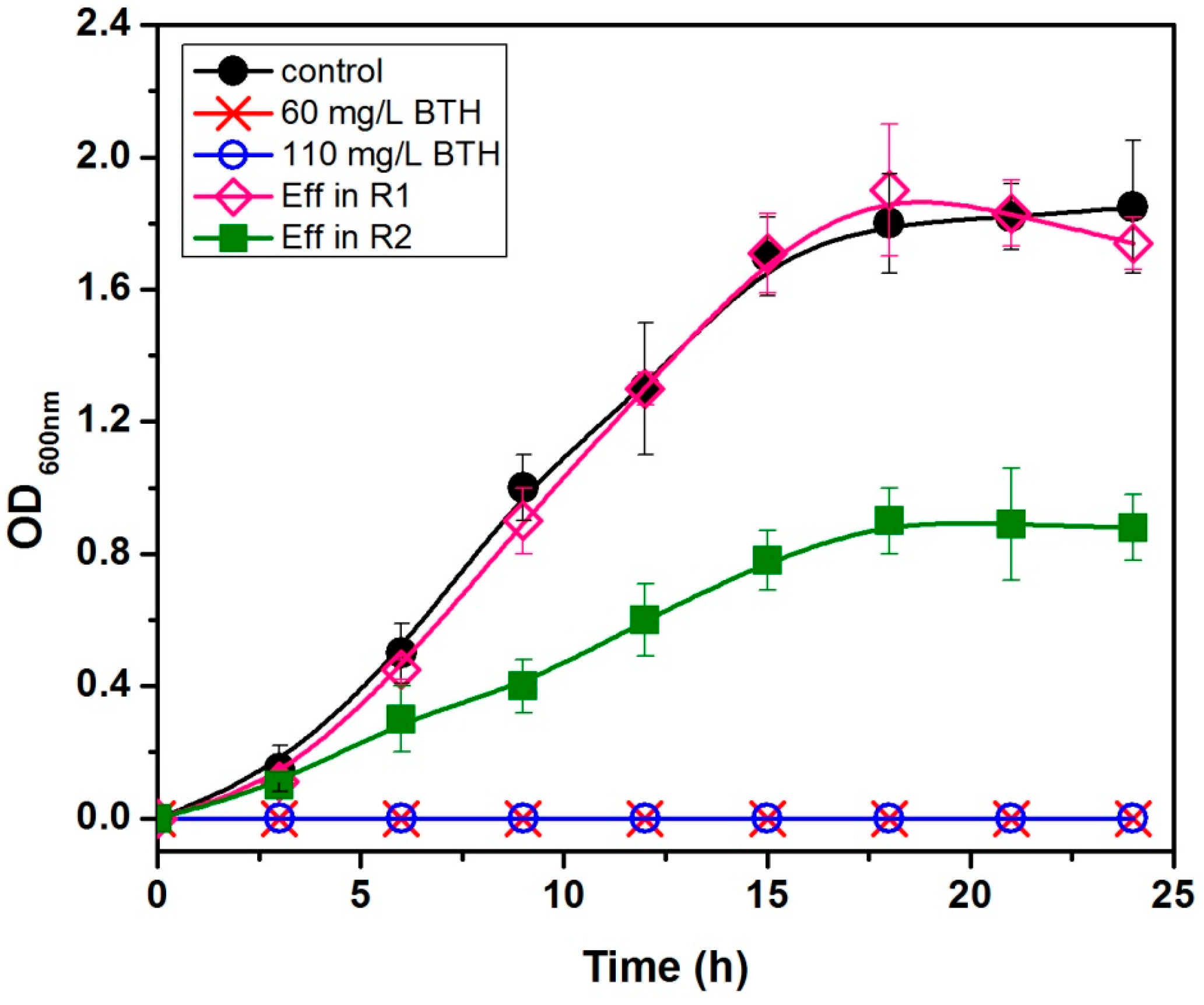
3.4. Antibacterial Activity Elimination Due to BTH Concentration
4. Conclusions
- (1)
- An up-flow internal circulation microbial electrolysis reactor (UICMER) was shown to be an effective method for BTH degradation. Benzothiazole and its intermediates can be degraded completely to simple organic acids by MEC treatment.
- (2)
- In UICMER, the electric field significantly improved the treatment performance for high concentration BTH wastewater in both COD removal and BTH degradation with intermediate biodegradability, and led to more EPS production, which was favorable for the enrichment of bacteria in the anode and cathode biofilm.
- (3)
- The right amount of VFA production could provide more substance for the growth of exoelectrogens; the presence of an electric field improves the degradation of BTH in UICMER.
- (4)
- The reduction of BTH using a microbial electrochemical method would have ecological significance in the elimination of the selection pressure of antibiotic for the generation of antibiotic resistant bacteria/genes in the environments.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De Wever, H.; Verachtert, H. Biodegradation and toxicity of benzothiazoles. Water Res. 1997, 31, 2673–2684. [Google Scholar] [CrossRef]
- Richardson, S.D. Environmental Mass Spectrometry: Emerging Contaminants and Current Issues. Anal. Chem. 2011, 84, 747–778. [Google Scholar] [CrossRef] [PubMed]
- Thomaidis, N.S.; Asimakopoulos, A.G.; Bletsou, A.A. Emerging contaminants: A tutorial mini-review. Glob. Nest J. 2012, 14, 72–79. [Google Scholar]
- Asimakopoulos, A.G.; Wang, L.; Thomaidis, N.S.; Kannan, K. Benzotriazoles and benzothiazoles in human urine from several countries: A perspective on occurrence, biotransformation, and human exposure. Environ. Int. 2013, 59, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Kloepfer, A.; Jekel, M.; Reemtsma, T. Occurrence, sources, and fate of benzothiazoles in municipal wastewater treatment plants. Environ. Sci. Technol. 2005, 39, 3792–3798. [Google Scholar] [CrossRef] [PubMed]
- Catallo, W.J.; Junk, T. Transformation of benzothiazole in estuarine sediments. J. Environ. Qual. 2005, 34, 1746–1754. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Weiss, S.C.; Beißwenger, J.; Leukhardt, H.G.; Schulz, W.; Seitz, W.; Ruck, W.K.L.; Weber, W.H. Identification of ozonation by-products of 4- and 5-methyl-1H-benzotriazole during the treatment of surface water to drinking water. Water Res. 2012, 46, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Dewever, H.; Demoor, K.; Verachtert, H. Toxicity of 2-Mercaptobenzothiazole towards Bacterial-Growth and Respiration. Appl. Microbiol. Biotechnol. 1994, 42, 631–635. [Google Scholar] [CrossRef]
- Haroune, N.; Combourieu, B.; Besse, P.; Sancelme, M.; Reemtsma, T.; Kloepfer, A.; Diab, A.; Knapp, J.S.; Baumberg, S.; Delort, A.M. Benzothiazole degradation by Rhodococcus pyridinovorans strain PA: Evidence of a catechol 1,2-dioxygenase activity. Appl. Environ. Microbiol. 2002, 68, 6114–6120. [Google Scholar] [CrossRef] [PubMed]
- Chipinda, I.; Hettick, J.M.; Simoyi, R.H.; Siegel, P.D. Oxidation of 2-mercaptobenzothiazole in latex gloves and its possible haptenation pathway. Chem. Res. Toxicol. 2007, 20, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Gold, L.S.; Slone, T.H.; Stern, B.R.; Bernstein, L. Comparison of Target Organs of Carcinogenicity for Mutagenic and Nonmutagenic Chemicals. Mutat. Res. 1993, 286, 75–100. [Google Scholar] [CrossRef]
- Janna, H.; Scrimshaw, M.D.; Williams, R.J.; Sumpter, J.P. From Dishwasher to Tap? Xenobiotic Substances Benzotriazole and Tolyltriazole in the Environment. Environ. Sci. Technol. 2011, 45, 3858–3864. [Google Scholar] [CrossRef] [PubMed]
- Richter, D.; Massmann, G.; Dunnbier, U. Identification and significance of sulphonamides (p-TSA, o-TSA, BSA) in an urban water cycle (Berlin, Germany). Water Res. 2008, 42, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- De Wever, H.; Vereecken, K.; Stolz, A.; Verachtert, H. Initial transformations in the biodegradation of benzothiazoles by Rhodococcus isolates. Appl. Environ. Microbiol. 1998, 64, 3270–3274. [Google Scholar] [PubMed]
- Hassan, N.; Verdes, P.V.; Ruso, J.M. Assessment of interactions between four proteins and benzothiazole derivatives by DSC and CD. J. Chem. Thermodyn. 2011, 43, 399–404. [Google Scholar] [CrossRef]
- Kannan, K.; Tao, L.; Sinclair, E.; Pastva, S.; Jude, D.J.; Giesy, J.P. Perfluorinated Compounds in Aquatic Organisms at Various Trophic Levels in a Great Lakes Food Chain. Arch. Environ. Contam. Toxicol. 2005, 48, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Haroune, N.; Combourieu, B. Liquid and solid state NMR study of benzothiazole degradation by Rhodococcus isolates. Eur. Symp. Environ. Biotechnol. 2004, 2004, 705–708. [Google Scholar]
- Bunescu, A.; Besse-Hoggan, P.; Sancelme, M.; Mailhot, G.; Delort, A.M. Comparison of microbial and photochemical processes and their combination for degradation of 2-aminobenzothiazole. Appl. Environ. Microbiol. 2008, 74, 2976–2984. [Google Scholar] [CrossRef] [PubMed]
- Chorao, C.; Charmantray, F.; Besse-Hoggan, P.; Sancelme, M.; Cincilei, A.; Traikia, M.; Mailhot, G.; Delort, A.M. 2-Aminobenzothiazole degradation by free and Ca-alginate immobilized cells of Rhodococcus rhodochrous. Chemosphere 2009, 75, 121–128. [Google Scholar] [CrossRef] [PubMed]
- El-Bassi, L.; Iwasaki, H.; Oku, H.; Shinzato, N.; Matsui, T. Biotransformation of benzothiazole derivatives by the Pseudomonas putida strain HKT554. Chemosphere 2010, 81, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Gaja, M.A.; Knapp, J.S. Removal of 2-mercaptobenzothiazole by activated sludge: A cautionary note. Water Res. 1998, 32, 3786–3789. [Google Scholar] [CrossRef]
- Mu, Y.; Rozendal, R.A.; Rabaey, K.; Keller, J. Nitrobenzene Removal in Bioelectrochemical Systems. Environ. Sci. Technol. 2009, 43, 8690–8695. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.A.; Chigome, S.; Hägerhäll, C.; Torto, N.; Gorton, L. Electrospun carbon nanofibers from polyacrylonitrile blended with activated or graphitized carbonaceous materials for improving anodic bioelectrocatalysis. Bioresour. Technol. 2013, 132, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Angelidaki, I. Microbial electrolysis cells turning to be versatile technology: Recent advances and future challenges. Water Res. 2014, 56, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Angelidaki, I. Bioelectrochemical recovery of waste-derived volatile fatty acids and production of hydrogen and alkali. Water Res. 2015, 81, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-W.; Choi, S.-J.; Lee, T.-H.; Lee, G.-Y.; Cha, J.-H.; Kim, C.-W. Application of biocathode in microbial fuel cells: Cell performance and microbial community. Appl. Microbiol. Biotechnol. 2008, 79, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Call, D.; Cheng, S.; Hamelers, H.V.M.; Sleutels, T.H.J.A.; Jeremiasse, A.W.; Rozendal, R.A. Microbial Electrolysis Cells for High Yield Hydrogen Gas Production from Organic Matter. Environ. Sci. Technol. 2008, 42, 8630–8640. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.S.; Clauwaert, P.; Green, S.J.; Verstraete, W.; Nerenberg, R. Bioelectrochemical Perchlorate Reduction in a Microbial Fuel Cell. Environ. Sci. Technol. 2010, 44, 4685–4691. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Kong, F.Y.; Zheng, H.T.; Yin, J.L. Electricity generation from synthetic penicillin wastewater in an air-cathode single chamber microbial fuel cell. Chem. Eng. J. 2011, 168, 572–576. [Google Scholar] [CrossRef]
- Wen, Q.; Kong, F.Y.; Zheng, H.T.; Yin, J.L.; Cao, D.X.; Ren, Y.M.; Wang, G.L. Simultaneous processes of electricity generation and ceftriaxone sodium degradation in an air-cathode single chamber microbial fuel cell. J. Power Sources 2011, 196, 2567–2572. [Google Scholar] [CrossRef]
- Harnisch, F.; Gimkiewicz, C.; Bogunovic, B.; Kreuzig, R.; Schroder, U. On the removal of sulfonamides using microbial bioelectrochemical systems. Electrochem. Commun. 2013, 26, 77–80. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Quan, X.; Chen, S. Effects of ferric iron on the anaerobic treatment and microbial biodiversity in a coupled microbial electrolysis cell (MEC)—Anaerobic reactor. Water Res. 2013, 47, 5719–5728. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Xing, D.; Liu, B.; Ren, N. Enhanced hydrogen production from waste activated sludge by cascade utilization of organic matter in microbial electrolysis cells. Water Res. 2012, 46, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Miron, Y.; Zeeman, G.; van Lier, J.B.; Lettinga, G. The role of sludge retention time in the hydrolysis and acidification of lipids, carbohydrates and proteins during digestion of primary sludge in CSTR systems. Water Res. 2000, 34, 1705–1713. [Google Scholar] [CrossRef]
- Reemtsma, T.; Fiehn, O.; Kalnowski, G.; Jekel, M. Microbial Transformations and Biological Effects of Fungicide-Derived Benzothiazoles Determined in Industrial Waste-Water. Environ. Sci. Technol. 1995, 29, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Thrash, J.C.; Coates, J.D. Review: Direct and indirect electrical stimulation of microbial metabolism. Environ. Sci. Technol. 2008, 42, 3921–3931. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Quan, X.; Li, Y.; Chen, S.; Zhao, H.; Wang, D. An anaerobic reactor packed with a pair of Fe-graphite plate electrodes for bioaugmentation of azo dye wastewater treatment. Biochem. Eng. J. 2012, 63, 31–37. [Google Scholar] [CrossRef]
- Reguera, G.; McCarthy, K.D.; Mehta, T.; Nicoll, J.S.; Tuominen, M.T.; Lovley, D.R. Extracellular electron transfer via microbial nanowires. Nature 2005, 435, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Hamelers, B.; Rozendal, R.A.; Schrorder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, H.L.; Yang, S.F.; Tay, J.H. Mechanisms and models for anaerobic granulation in upflow anaerobic sludge blanket reactor. Water Res. 2003, 37, 661–673. [Google Scholar] [CrossRef]
- Wrana, N.; Sparling, R.; Cicek, N.; Levin, D.B. Hydrogen gas production in a microbial electrolysis cell by electrohydrogenesis. J. Clean. Prod. 2010, 18, S105–S111. [Google Scholar] [CrossRef]
- Lalaurette, E.; Thammannagowda, S.; Mohagheghi, A.; Maness, P.C.; Logan, B.E. Hydrogen production from cellulose in a two-stage process combining fermentation and electrohydrogenesis. Int. J. Hydrogen Energy 2009, 34, 6201–6210. [Google Scholar] [CrossRef]
- Zhang, X.X.; Zhang, T.; Fang, H. Antibiotic resistance genes in water environment. Appl. Microbiol. Biotechnol. 2009, 82, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Cheng, H.-Y.; Kong, D.-Y.; Gao, S.-H.; Sun, F.; Cui, D.; Kong, F.-Y.; Zhou, A.-J.; Liu, W.-Z.; Ren, N.-Q.; et al. Accelerated Reduction of Chlorinated Nitroaromatic Antibiotic Chloramphenicol by Biocathode. Environ. Sci. Technol. 2013, 47, 5353–5361. [Google Scholar] [CrossRef] [PubMed]


| Sample | EPS | ||
|---|---|---|---|
| Polypeptides | Polysaccharides | Sum | |
| (mg Protein/g VSS) | (mg Glucose/g VSS) | (mg EPS/g VSS) | |
| Seed sludge | 11.23 ± 0.69 | 7.8 ± 0.41 | 19.03 ± 1.1 |
| Sludge of R1 | 23.43 ± 0.87 | 15.58 ± 0.57 | 39.01 ± 1.44 |
| Sludge of R2 | 13.34 ± 0.78 | 8.89 ± 0.37 | 22.23 ± 1.15 |
| Anode surface | 29.9 ± 1.02 | 19.25 ± 0.67 | 49.15 ± 1.69 |
| Cathode surface | 27.3 ± 0.89 | 17.51 ± 0.48 | 44.81 ± 1.37 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Ding, J.; Ren, N.; Tong, Q.; Zhang, L. The Detoxification and Degradation of Benzothiazole from the Wastewater in Microbial Electrolysis Cells. Int. J. Environ. Res. Public Health 2016, 13, 1259. https://doi.org/10.3390/ijerph13121259
Liu X, Ding J, Ren N, Tong Q, Zhang L. The Detoxification and Degradation of Benzothiazole from the Wastewater in Microbial Electrolysis Cells. International Journal of Environmental Research and Public Health. 2016; 13(12):1259. https://doi.org/10.3390/ijerph13121259
Chicago/Turabian StyleLiu, Xianshu, Jie Ding, Nanqi Ren, Qingyue Tong, and Luyan Zhang. 2016. "The Detoxification and Degradation of Benzothiazole from the Wastewater in Microbial Electrolysis Cells" International Journal of Environmental Research and Public Health 13, no. 12: 1259. https://doi.org/10.3390/ijerph13121259





