On-Line Monitoring the Growth of E. coli or HeLa Cells Using an Annular Microelectrode Piezoelectric Biosensor
Abstract
:1. Introduction
2 Materials and Methods
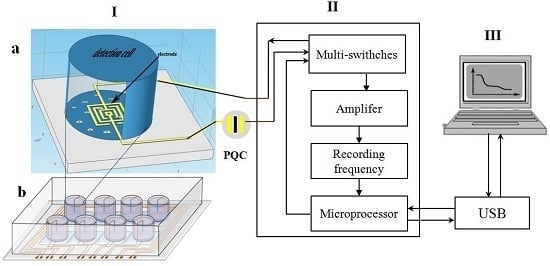
2.1. Apparatus
2.2. Materials and Reagents
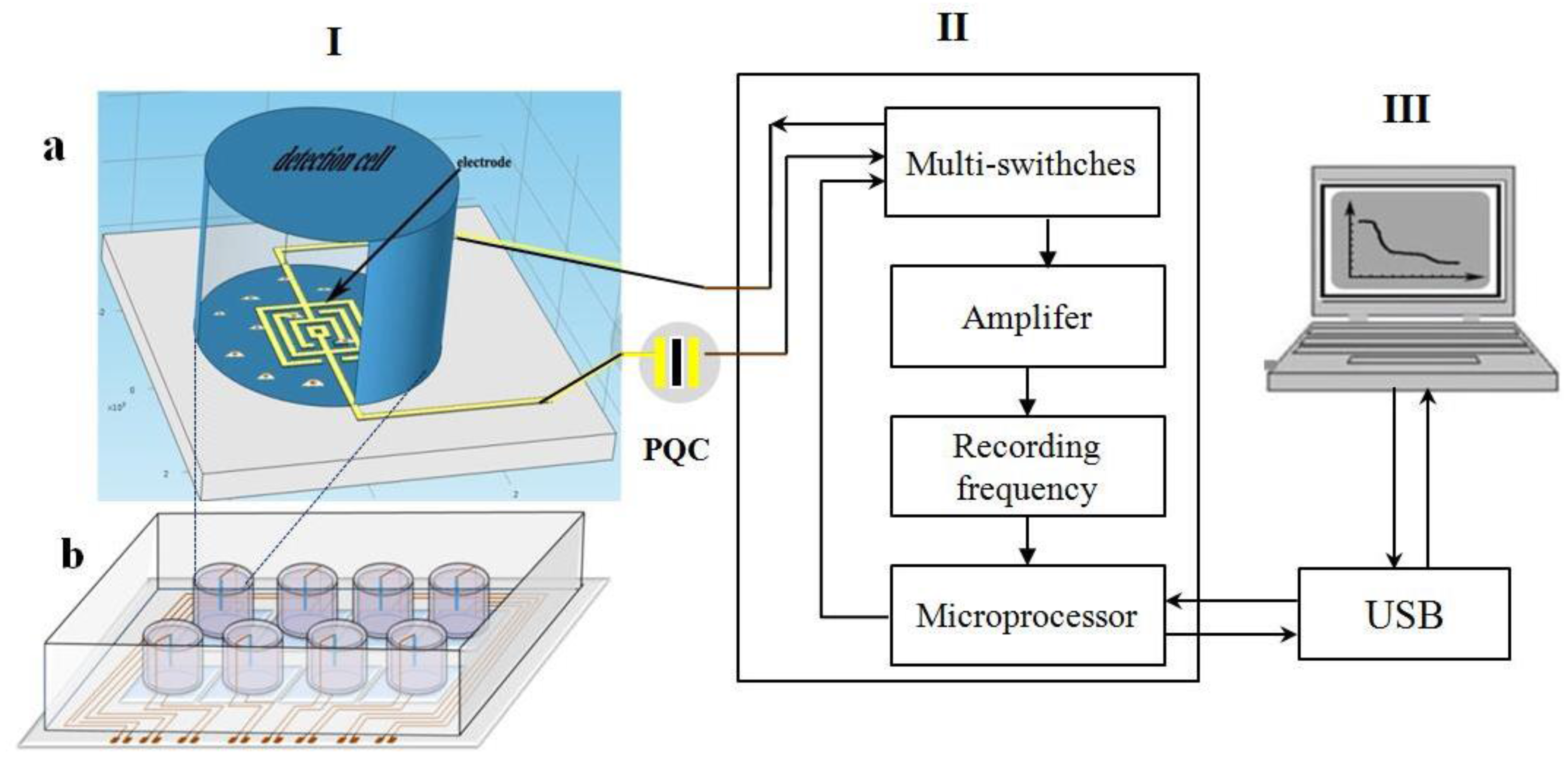
2.3. Design and Manufacture of the Annular Microelectrode
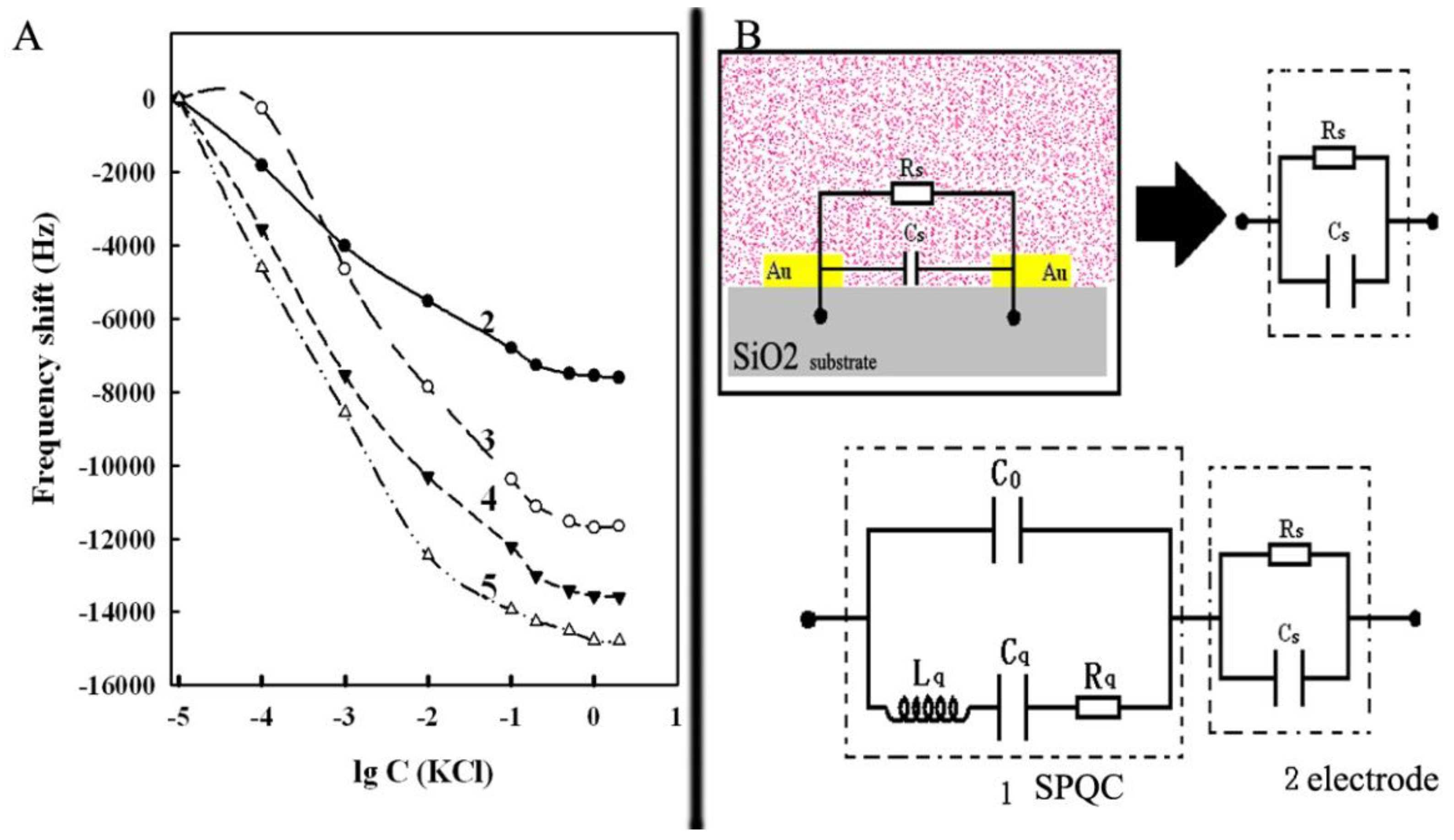
2.4. Response Characteristics of the Piezoelectric Sensor with Microelectrode
2.5. Detection of E. coli or HeLa Cells Using the Annular Microelectrode Series Piezoelectric Biosensor
3. Result and Discussion
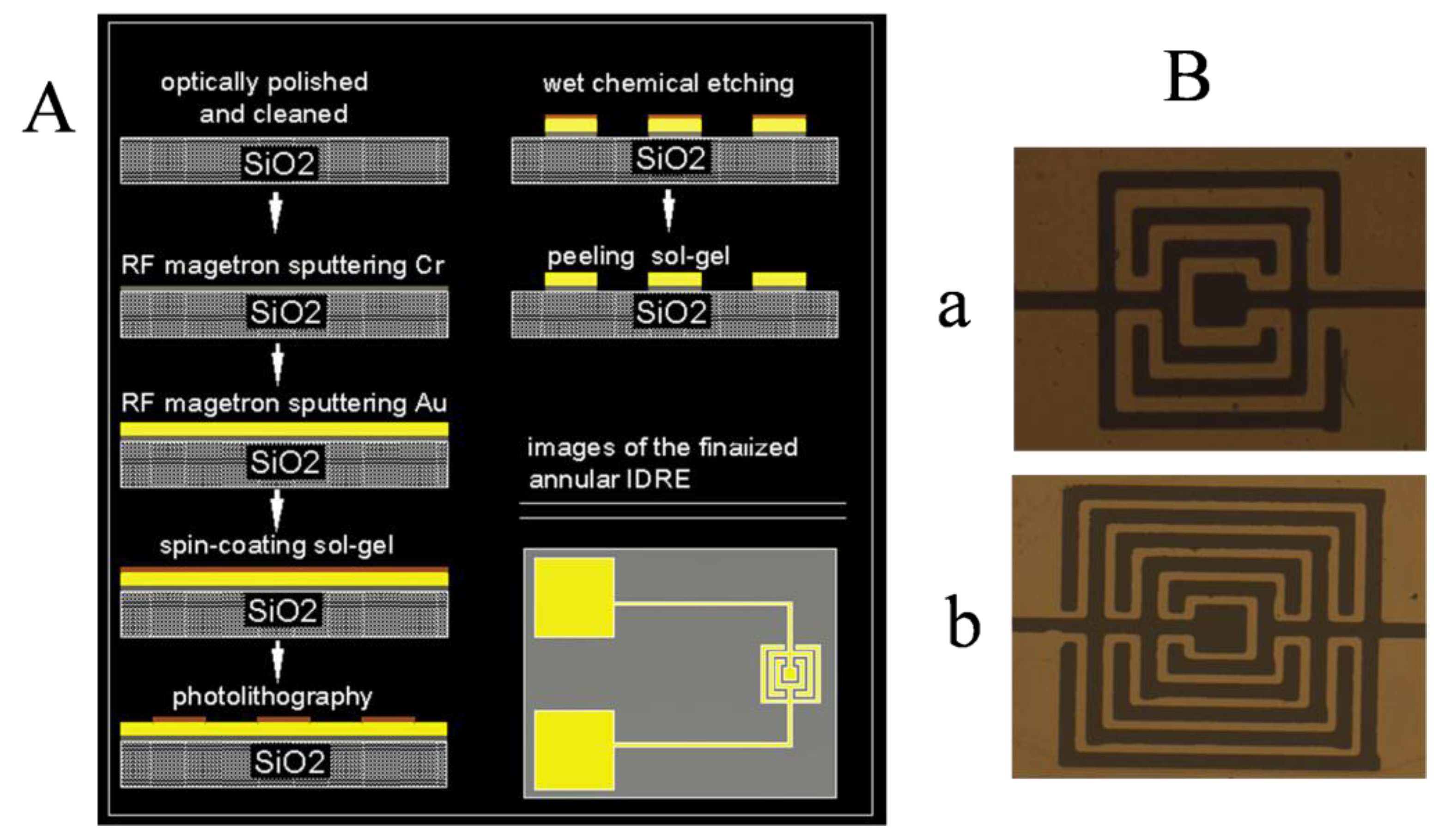
3.1. Multiphysics Simulation of the Microelectrode
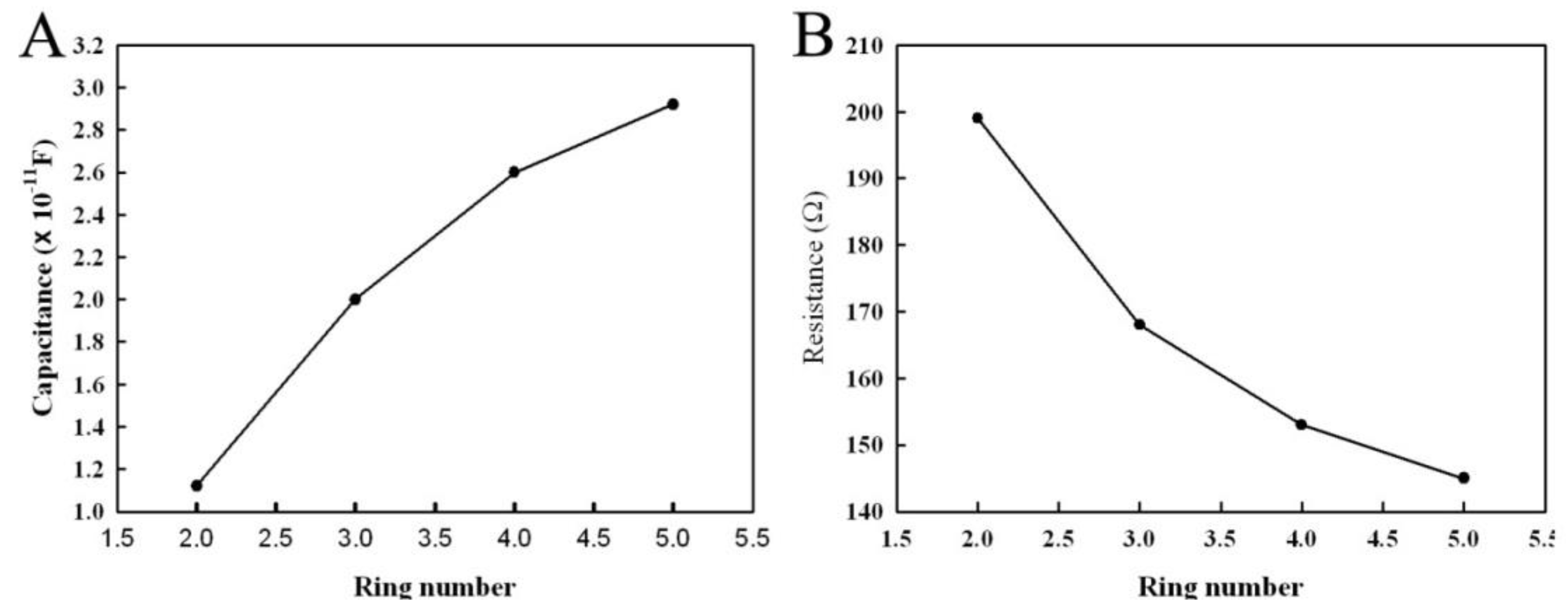
3.2. Influence of Annular Microelectrode Geometric Parameters on the Equivalent Circuit
3.3. Frequency Response of the Annular Microelectrode Piezoelectric Biosensor
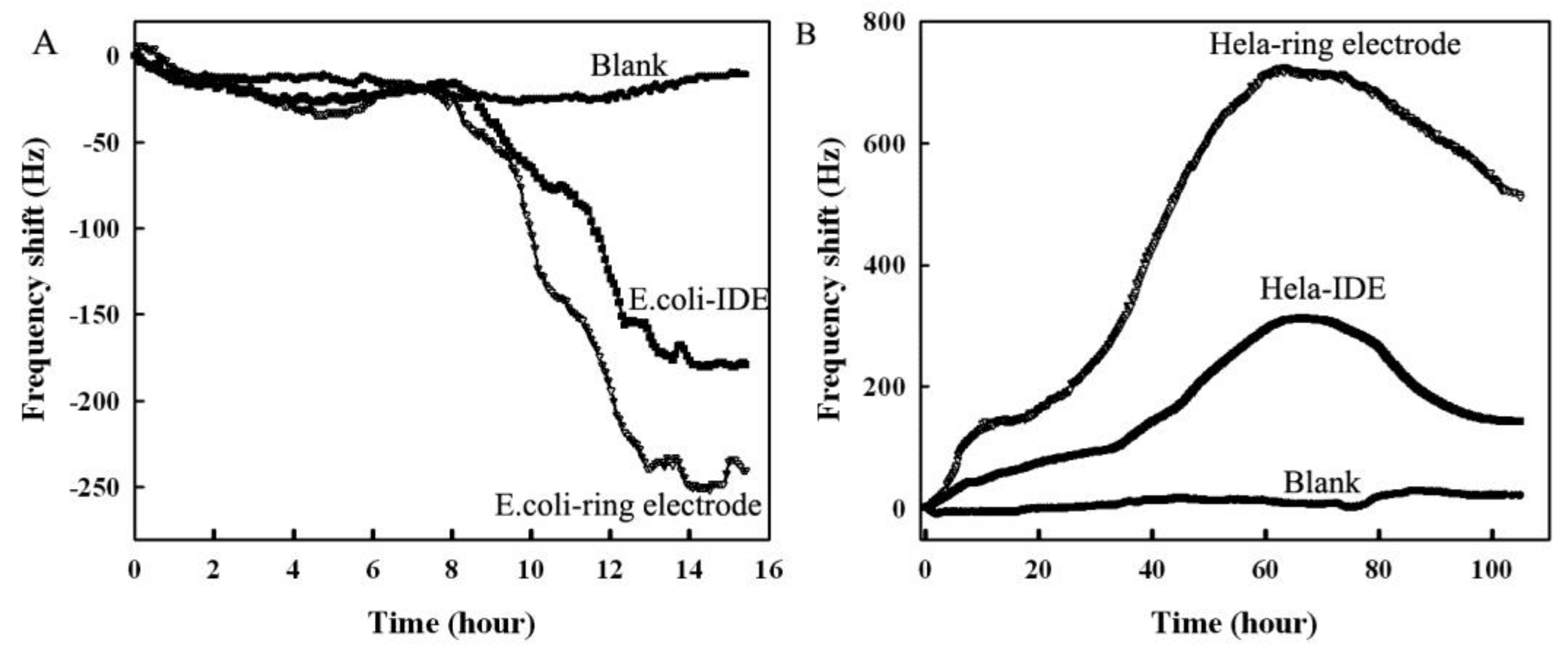
3.4. Real-Time Monitoring of the Growth of E. coli HeLa Cells Using the Annular Microelectrode Piezoelectric Biosensor
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Saitakis, M.; Gizeli, E. Acoustic sensors as a biophysical tool for probing cell attachment and cell/surface interactions. Cell. Mol. Life Sci. 2012, 69, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Fatisson, J.; Azari, F.; Tufenkji, N. Real-time QCM-D monitoring of cellular responses to different cytomorphic agents. Biosens. Bioelectron. 2011, 26, 3207–3212. [Google Scholar] [CrossRef] [PubMed]
- Bitziou, E.; Snowden, M.E.; Joseph, M.B.; Leigh, S.J.; Covington, J.A.; Macpherson, J.V.; Unwin, P.R. Dual electrode micro-channel flow cell for redox titrations: Kinetics and analysis of homogeneous ascorbic acid oxidation. J. Electroanal. Chem. 2013, 692, 72–79. [Google Scholar] [CrossRef]
- Barnes, E.O.; Compton, R.G. The rate of adsorption of nanoparticles on microelectrode surfaces. J. Electroanal. Chem. 2013, 693, 73–78. [Google Scholar] [CrossRef]
- Aoki, K.J.; Li, C.Y.; Nishiumi, T.; Chen, J.Y. Electrolysis of pure water in a thin layer cell. J. Electroanal. Chem. 2013, 695, 24–29. [Google Scholar] [CrossRef]
- Dengler, A.K.; McCarty, G.S. Microfabricated microelectrode sensor for measuring background and slowly changing dopamine concentrations. J. Electroanal. Chem. 2013, 693, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Goda, T.; Miyahara, Y. Label-free and reagent-less protein biosensing using aptamer-modified extended-gate field-effect transistors. Biosens. Bioelectron. 2013, 45, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.G.; Howell, P.D.; Stone, H.A. Time-dependent chronoamperometric response of dual inlaid disk electrodes. J. Electroanal. Chem. 2013, 689, 303–313. [Google Scholar] [CrossRef]
- Singh, R.; Sankaranarayanan, S.K.R.S.; Bhethanabotla, V.R. Enhancement of acoustic streaming induced flow on a focused surface acoustic wave device: Implications for biosensing and microfluidics. J. Appl. Phys. 2010, 107, 024503. [Google Scholar] [CrossRef]
- Alghane, M.; Fu, Y.Q.; Chen, B.X.; Li, Y.; Desmulliez, M.P.Y.; Walton, A.J. Streaming phenomena in microdroplets induced by Rayleigh surface acoustic wave. J. Appl. Phys. 2011, 109, 114901. [Google Scholar] [CrossRef]
- Khan, A. Detection and quantitation of forty eight cytokines, chemokines, growth factors and nine acute phase proteins in healthy human plasma, saliva and urine. J. Proteom. 2012, 75, 4802–4819. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Song, Z.; Wu, S.; Zhao, L.; Li, Y.; Zhang, J. Improved electron emission characteristics of ZnO nano-gap with Pd films. Appl. Surf. Sci. 2010, 256, 7323–7326. [Google Scholar] [CrossRef]
- Dickinson, J.W.; Bromley, M.; Andrieux, F.P.L.; Boxall, C. Fabrication and Characterisation of the Graphene Ring Micro Electrode (GRiME) with an Integrated, Concentric Ag/AgCl Reference Electrode. Sensors 2013, 13, 3635–3651. [Google Scholar] [CrossRef] [PubMed]
- Cachet-Vivier, C.; Keddam, M.; Vivier, V.; Yu, L.T. Development of cavity microelectrode devices and their uses in various research fields. J. Electroanal. Chem. 2013, 688, 12–19. [Google Scholar] [CrossRef]
- Li, Y.; Sella, C.; Lemaitre, F.; Collignon, M.G.; Thouin, L.; Amatore, C. Highly sensitive platinum-black coated platinum electrodes for electrochemical detection of hydrogen peroxide and nitrite in microchannel. Electroanalysis 2013, 25, 895–902. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, X.; Zhou, Z.Y.; Ye, X.Y. Controllable growth of gold nanowires and nanoactuators via high-frequency AC electrodeposition. Electrochem. Commun. 2013, 27, 133–136. [Google Scholar] [CrossRef]
- Christophe, C.; Belaidi, F.S.; Launay, J.; Gros, P.; Questel, E.; Temple-Boyer, P. Elaboration of integrated microelectrodes for the detection of antioxidant species. Sens. Actuators B Chem. 2013, 177, 350–356. [Google Scholar] [CrossRef] [Green Version]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, F.; Lian, Y.; Han, J. On-Line Monitoring the Growth of E. coli or HeLa Cells Using an Annular Microelectrode Piezoelectric Biosensor. Int. J. Environ. Res. Public Health 2016, 13, 1254. https://doi.org/10.3390/ijerph13121254
Tong F, Lian Y, Han J. On-Line Monitoring the Growth of E. coli or HeLa Cells Using an Annular Microelectrode Piezoelectric Biosensor. International Journal of Environmental Research and Public Health. 2016; 13(12):1254. https://doi.org/10.3390/ijerph13121254
Chicago/Turabian StyleTong, Feifei, Yan Lian, and Junliang Han. 2016. "On-Line Monitoring the Growth of E. coli or HeLa Cells Using an Annular Microelectrode Piezoelectric Biosensor" International Journal of Environmental Research and Public Health 13, no. 12: 1254. https://doi.org/10.3390/ijerph13121254