Universal Stress Proteins as New Targets for Environmental and Therapeutic Interventions of Schistosomiasis
Abstract
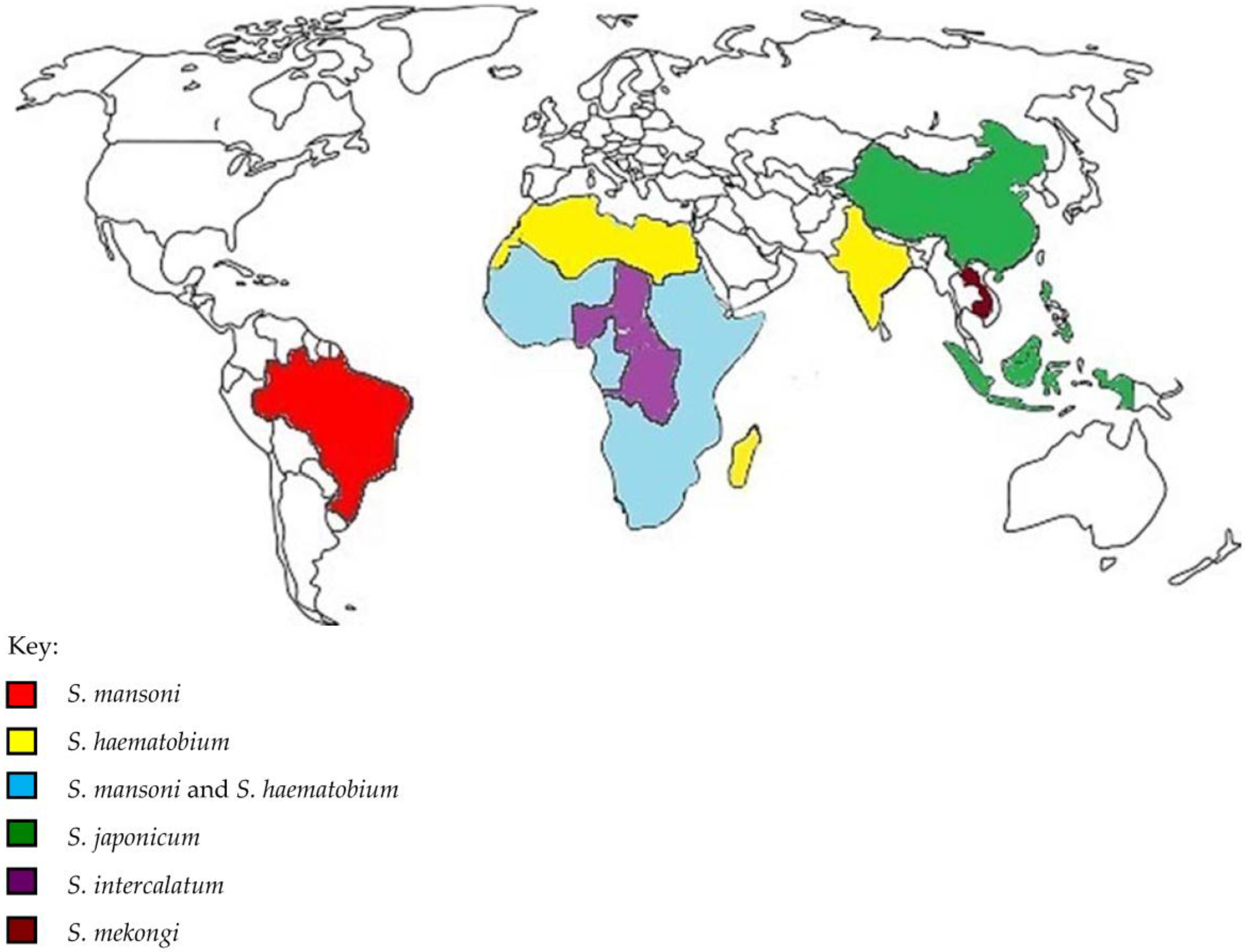
:1. Introduction
2. Anti-Schistosomal Drugs
3. Parasitic Lifecycle and Stress
3.1. Abiotic Stress
3.2. Biotic Stress
4. Universal Stress Proteins, Mode of Action and Factors that Induce and Regulate Their Activity
5. Role of USPs in Disease Progression
6. Recent Advances in Novel Promising Schistosomicides
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bergquist, R. Closing in on “perhaps the most dreadful of the remaining plagues”: An independent view of the multidisciplinary alliance to optimize schistosomiasis control in Africa. Acta Trop. 2013, 128, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Utzinger, J.N.; N’Goran, E.K.; Caffrey, C.R.; Keiser, J. From innovation to application: Social-ecological context, diagnostics, drugs and intergrated control of schistosomiasis. Acta Trop. 2011, 12, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.J. Insights into the functional biology of schistosomes. Parasites Vectors 2011, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gryseels, B.; Polman, K.; Clerinx, J.; Kestens, L. Human scistosomiasis. Lancet 2006, 368, 1106–1118. [Google Scholar] [CrossRef]
- Lambertucci, J.R. Acute Schistosomiasis mansoni: Revisited and reconsidered. Mem. Inst. Oswaldo Cruz 2010, 105, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Kamath, A. Neglected Tropical Diseases in sub-Saharan Africa: Review of their prevalence, distribution and disease burden. PLoS Negl. Trop. Dis. 2009, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- King, C.H. Parasites and poverty: The case of Schistosomiasis. Acta Trop. 2010, 113, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Bruun, B.; Aagaard-Hansen, J. The Social Context of Schistosomiasis and Its Control: An Introduction and Annotated Bibliography; WHO: Geneva, Switzerland, 2008. [Google Scholar]
- King, C.H.; Sturrock, R.F.; Kariuki, H.C.; Hamburger, J. Transmission control for schistosomiasis—Why it matters now. Trends Parasitol. 2006, 22, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human Schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef]
- Elbaz, T.; Esmat, G. Hepatic and intestinal schistosomiasis: Review. J. Adv. Res. 2013, 4, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Chitsulo, L.; Engels, D.; Montresor, A.; Savioli, L. The global status of schistosomiasis and its control. Acta Trop. 2000, 77, 41–51. [Google Scholar] [CrossRef]
- Rollinson, D.; Knopp, S.; Levitz, S.; Stothard, J.R.; Tchuenté, L.A.T.; Garba, A.; Mohammed, K.A.; Schur, N.; Person, B.; Colley, D.G.; et al. Time to set the agenda for schistosomiasis elimination. Acta Trop. 2013, 128, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, P.; Keiser, J.; Bos, R.; Tanner, M.; Utzinger, J. Schistosomiasis and water resources development: Systematic review, meta-analysis and estimates of people at risk. Lancet Infect. Dis. 2006, 6, 411–425. [Google Scholar] [CrossRef]
- Berry, A.; Moné, H.; Iriart, X.; Mouahid, G.; Aboo, O.; Boissier, J.; Fillaux, J.; Cassaing, S.; Debuisson, C.; Valentin, A.; et al. Schistosomiasis, Haematobium, Corsica, France. Emerg. Infect. Dis. 2014, 20, 1595–1597. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.; Elhelo, O.; Smith, M.; Haugen, B.; Miller, A.; Raghavan, N.; Wellman, C.; Cousin, C.; Dixon, F.; Mann, V.; et al. Susceptibility of Snails to Infection with Schistosomes is influenced by Temperature and Expression of Heat Shock Proteins. Epidemiology 2015, 5, 1–8. [Google Scholar]
- Mbah, A.N.; Mahmud, O.; Awofolu, O.R.; Isokpehi, R.D. Inferences on the biochemical and environmental regulation of universal stress proteins from Schistosomiasis parasites. Adv. Appl. Bioinform. Chem. 2013, 5, 15–27. [Google Scholar]
- Conlon, C.P. Schistosomiasis. Medicine 2005, 33, 64–67. [Google Scholar] [CrossRef]
- Coon, D.R. Schistosomiasis: Overview of the history, biology, clinicopathology and laboratory diagnosis. Clin. Microbiol. Newslett. 2005, 27, 163–169. [Google Scholar] [CrossRef]
- Aragon, A.D.; Imani, R.A.; Blockburn, V.R.; Cunningham, C. Microarray based analysis of temperature and oxidative stress induced messenger RNA in Schistosoma mansoni. Mol. Biochem. Parasitol. 2008, 162, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Bica, I.; Hamer, D.H.; Stadecker, M.J. Hepatic schistosomiasis. Infect. Dis. Clin. N. Am. 2000, 14, 583–604. [Google Scholar] [CrossRef]
- Adenowo, A.F.; Oyinloye, B.E.; Ogunyika, B.I.; Kappo, A.P. Impact of human schistososmiasis in sub-Saharan Africa. Braz. J. Infect. Dis. 2015, 422, 1–10. [Google Scholar]
- Oyinloye, B.; Adenowo, F.; Gxaba, N.; Kappo, A. The promise of antimicrobial peptides for treatment of human schistosomiasis. Curr. Drug Targets 2014, 15, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Doenhoff, M.J.; Cioli, D.; Utzinger, J. Praziquantel: Mechanisms of action, resistance and new derivatives for schistosomiasis. Curr. Opin. Infect. Dis. 2008, 21, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, F.D.; Le Clec’h, W.; Eng, N.; Rugel, A.R.; de Assis, R.R.; Oliveira, G.; Holloway, S.P.; Cao, X.; Hart, P.J.; LoVerde, P.T.; et al. Independent origins of loss-of-function mutations conferring oxamniquine resistance in a Brazilian schistosome population. Int. J. Parasitol. 2016, 46, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Aragon, A.D.; Imani, R.A.; Blackburn, V.R.; Cupit, P.M.; Melman, S.D.; Goronga, T.; Webb, T.; Loker, E.S.; Cunningham, C. Towards an understanding of the mechanism of action of Praziquantel. Mol. Biochem. Parasitol. 2009, 164, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Fallon, P.G.; Tao, L.-F.; Ismail, M.M.; Bennete, J.L. Schistosome resistance to Praziquantel: Fact or artefact? Parasitol. Today 1996, 12, 316–320. [Google Scholar] [CrossRef]
- Alsaqabi, S.M.; Lotfy, W.M. Praziquantel. J. Vet. Sci. Technol. 2014, 5, 1–8. [Google Scholar] [CrossRef]
- Cioli, D.; Pica-Mattoccia, L.; Basso, A.; Guidi, A. Schistosomiasis control: Praziquantel forever? Mol. Biochem. Parasitol. 2014, 195, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, C.R. Chemotherapy of Schistosomiasis: Present and future. Curr. Opin. Chem. Biol. 2007, 11, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, R.M. Are Ca2+ channels targets of Praziquantel action? Int. J. Parasitol. 2005, 35, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Melman, S.D.; Steinauer, M.L.; Cunningham, C.; Kubatko, L.S.; Mwangi, I.N.; Wynn, N.B.; Mutuku, M.W.; Karanja, D.M.S.; Colley, D.G.; Black, C.L.; et al. Reduced susceptibility to Praziquantel among naturally occurring Kenyan isolates of Schistosoma mansoni. PLoS Negl. Trop. Dis. 2009, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- William, S.; Botros, S.; Ismail, M.; Farghally, A.; Day, T.A.; Bennett, J.L. Praziquantel-induced tegumental damage in vitro is diminished in schistosomes derived from praziquantel-resistant infections. Parasitology 2001, 121, 63–66. [Google Scholar] [CrossRef]
- Doenhoff, M.J.; Kusel, J.R.; Coles, G.C.; Cioli, D. Resistance of Schistosoma mansoni to praziquantel: Is there a problem? Trans. R. Trop. Med. Hyg. 2002, 96, 465–469. [Google Scholar] [CrossRef]
- Coeli, R.; Baba, E.H.; Araujo, N.; Coelho, P.M.Z.; Oliveira, G. Praziquantel Treatment Decreases Schistosoma mansoni Genetic Diversity in Experimental Infections. PLoS Negl. Trop. Dis. 2012, 7, e2596. [Google Scholar]
- Couto, F.F.B.; Coelho, P.M.Z.; Araújo, N.; Kusel, J.R.; Katz, N.; Jannotti-Passos, L.K.; Mattos, A.C.A. Schistosoma mansoni: A method for inducing resistance to praziquantel using infected Biomphalaria glabrata snails. Mem. Inst. Oswaldo Cruz 2011, 106, 153–157. [Google Scholar] [CrossRef] [PubMed]
- McCreesh, N.; Boot, M. The effect of increasing water temperatures of Schistosoma mansoni transmission and Biomphalaria pfeifferi population dynamics: An agent based modellign study. PLoS ONE 2014, 9, e101462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walz, Y.; Wegmann, M.; Decg, S.; Raso, G.; Utzinger, J. Risk profiling of schistosomiasis using remote sensing: Approaches, challenges and outlook. Parasites Vectors 2015, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Upathum, E.S. Location of Biomphalaria glabrata (SAY) by Miracidia of Schistosoma mansoni Sambon in Natural Standing and Running Waters of the West Indian Island of St Lucia. Int. J. Parasitol. 1973, 3, 289–297. [Google Scholar] [CrossRef]
- Negrão-Corrêa, D.; Pereira, C.A.J.; Rosa, F.M.; Martins-Souza, R.L.; Andrade, Z.A.; Coelho, P.M.Z. Molluscan response to parasite Biomphalaria and Schistosoma mansoni interaction. ISJ 2007, 4, 101–111. [Google Scholar]
- Negrão-Corrêa, D.; Mattos, A.C.A.; Pereira, C.A.J.; Martins-Souza, R.L.; Coelho, P.M.Z. Interaction of Schistosoma mansoni Sporocysts and Hematocytes of Biomphalaria. J. Parasitol. Res. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, N.; Miller, A.N.; Gardener, M.; FitzGerald, P.C.; Kerlavage, A.R.; Johnston, D.A.; Lewis, F.A.; Knight, M. Comparative gene analysis of Biomphalaria glabrata hemocytes pre- and post-exposure to miracidia of Schistosoma mansoni. Mol. Biochem. Parasitol. 2003, 126, 181–191. [Google Scholar] [CrossRef]
- Theron, A.; Rognon, A.; Gourbal, B.; Mitta, G. Multi-parasite host susceptibility and multi-host parasite infectivity: A new approach of the Biomphalaria glabrata/Schistosoma mansoni compatibility polymorphism. Infect. Genet. Evol. 2014, 26, 80–88. [Google Scholar] [CrossRef] [PubMed]
- LoVerde, P.T. Do antioxidants play a role in Schistosome hot-parasite interactions? Parasitol. Today 1998, 14, 284–289. [Google Scholar] [CrossRef]
- Pearce, E.J.; MacDonald, A.S. The Immunobiology of Schistosomes. Nat. Rev. Immunol. 2002, 2, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.J.; Hewitson, J.P.; Jenkins, G.R.; Mountford, A.P. Modulation of the host’s immune response by schistosome larvae. Parasite Immunol. 2005, 27, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Colley, D.G.; Secor, W.E. Immunology of human schistosomiasis. Parasite Immunol. 2014, 36, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Kvint, K.; Nachin, L.; Diez, A.; Nyström, T. The bacterial universal stress protein: Function and regulation. Curr. Opin. Microbiol. 2003, 6, 140–145. [Google Scholar] [CrossRef]
- Forêt, S.; Seneca, F.; de Jong, O.; Bieller, A.; Hemmich, G.; Augustin, R.; Hayward, D.C.; Ball, E.E.; Bosch, T.C.G.; Agata, K.; et al. Phylogenomics reveals an anomalous distribution of USP genes in metazoans. Mol. Biol. Evol. 2011, 28, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.C.; McKay, D.B. Structure of the universal stress protein of Haemophilus influenzae. Structure 2001, 9, 1135–1141. [Google Scholar] [CrossRef]
- Drumm, J.E.; Mi, K.; Bilder, P.; Sun, M.; Lim, J.; Bielefeldt-Ohmann, H.; Basaraba, R.; Therefore, M.; Zhu, G.; Tufariello, J.M.; et al. Mycobacterium tuberculosis universal stress protein Rv2623 regulates bacillary growth by ATP-Binding: Requirement for establishing chronic persistent infection. PLoS Pathog. 2009, 5, e1000460. [Google Scholar] [CrossRef]
- Tkaczuk, K.L.; Shumilin, A.; Chruszcz, I.; Evdokimova, E.; Savchenko, A.; Minor, W. Structural and functional insight into the universal stress protein family. Evol. Appl. 2013, 6, 434–449. [Google Scholar] [CrossRef] [PubMed]
- Bangera, M.; Panigrahi, R.; Sagurthi, R.; Savithri, H.S.; Murthy, M.R.N. Structural and functional analysis of two universal stress proteins YdaA and YnaF from Salmonella typhimurium: Possible roles in microbial stress tolerance. J. Struct. Biol. 2015, 189, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Lino, H.; Shimizu, N.; Goto, M.; Ebihara, A.; Fukui, K.; Hirotsu, K.; Kuramitsu, S. Crystal structure of the tandem-type universal stress protein TTHA0350 from Thermus thermophiles HB8. J. Biochem. 2011, 150, 295–302. [Google Scholar]
- Schweikhard, E.S.; Kuhlmann, S.I.; Kunte, H.J.; Grammann, K.; Ziegler, C.M. Structure and function of the universal stress protein TeaD and its role in regulating the ectoine transporter TeaABC of Halomonas elongata DSM2581T. Biochemistry 2010, 49, 2194–2204. [Google Scholar] [CrossRef] [PubMed]
- Zarembinski, T.I.; Hung, L.W.; Mueller-Dieckmann, H.J.; Kim, K.K.; Yokota, H.; Kim, R.; Kim, S.H. Structure-based assignment of the biochemical function of a hypothetical protein: A test case of structural genomics. Proc. Natl. Acad. Sci. USA 1998, 95, 15189–15193. [Google Scholar] [CrossRef] [PubMed]
- Loukehaich, R.; Wang, T.; Ouyang, B.; Ziaf, K.; Li, H.; Zhang, J.; Lu, Y.; Ye, Z. SpUSP, an annexin-interacting universal stress protein, enhances drought tolerance in tomato. J. Exp. Bot. 2012, 63, 5593–5606. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, A.; Zahur, M.; Husnain, T.; Riazuddin, S. GUSP1 and GUSP2, two drought-responsive genes in Gossypium arboreum have homology to universal stress proteins. Plant Mol. Biol. 2009, 27, 109–114. [Google Scholar] [CrossRef]
- Kim, D.J.; Bitto, E.; Bingman, C.A.; Kim, H.J.; Han, B.W.; Phillips, G.N. Crystal structure of the protein At3g01520, a eukaryotic universal stress protein-like protein from Arabidopsis thaliana in complex with AMP. Proteins 2015, 83, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Isokpehi, R.D.; Mahmud, O.; Mbah, A.N.; Simmons, S.S.; Avelar, L.; Rajnarayanan, R.V.; Udensi, U.K.; Ayensu, W.K.; Cohly, H.H.; Brown, S.D.; et al. Developmental regulation of genes encoding universal stress proteins in Schistosoma mansoni. Gene Regul. Syst. Biol. 2011, 5, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Jolly, E.R.; Chin, C.; Miller, S.; Bahgat, M.M.; Lim, K.C.; DeRisi, J.; McKerrow, J.H. Gene expression patterns during adaptation of a helminth parasite to different environmental niches. Genome Biol. 2007, 8, R65. [Google Scholar] [CrossRef] [PubMed]
- Nachin, L.; Nannmark, U.; Nystrom, T. Differential roles of the universal stress proteins of Escherichia coli in oxidative stress resistance, adhesion and motility. J. Bacteriol. 2005, 187, 6265–6272. [Google Scholar] [CrossRef] [PubMed]
- Chiumiento, L.; Bruschi, F. Enzymatic antioxidant systems in helminth parasites. Parasitol. Res. 2009, 105, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-dos-Santos, G.; Verjovski-Ameida, S.; Leite, L.C.C. Schistosomiasis-a century searching for chemotherapeutic drugs. Parasitol. Res. 2006, 99, 505–521. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Singh, R. A review on anthelminthic drugs and their future scope. Int. J. Pharm. Pharm. Sci. 2011, 3, 17–21. [Google Scholar]
- Ali, S.A. Natural products as therapeutic agents for schistosomiasis. Res. J. Med. Plants 2011, 5, 1–20. [Google Scholar]


| Organism/Kingdom | Specie | USP Name | ATP-Binding Potential | References |
|---|---|---|---|---|
| Bacteria | Escherichia coli | UspA | Non ATP-binding | [48,49] |
| UspC, UspD | ATP-binding? | |||
| UspE, UspF, UspG | ATP-binding | |||
| Heamophilus influenza | H10815 | Non ATP-binding | [50] | |
| Mycobacterium tuberculosis | RV2623 | ATP-binding | [51] | |
| Nitrosomonas europea | NE1028 | Non ATP-binding | [52] | |
| Salmonella typhimurium | YdaA | Assumed to bind ATP | [53] | |
| YnaF | ATP-binding | |||
| Thermos thermophiles | TTHA0350 | ATP-binding | [54] | |
| Halomonas elongate | HELO1754 | ATP-binding | [55] | |
| Archaea | Methanococcus jannaschii | MJ0577 | ATP-binding | [56] |
| Archeaoglobus fulgidus | AF0836 | Non ATP-binding | [52] | |
| Plants | Solanum pennellii | SpUSP | ATP-binding region | [57] |
| Grosspyiumarbo-retum | GUSP1 | Non ATP-binding | [58] | |
| GUSP2 | ||||
| Arabidopsis thaliana | At3g01520 | Suggested to be ATP-binding | [59] | |
| Schistosomes | S. japonicum | Q86DX1, Q5DDH7 | ATP-binding | [17] |
| Q5DED2, Q5DHK1, Q86DW2, Q5DG19, Q5DH64, Q5D136 | ||||
| S. mansoni | G4V552, G4VPM6, G4LZI3, C1MOQ2, G4VIW9 | ATP-binding | [17] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masamba, P.; Adenowo, A.F.; Oyinloye, B.E.; Kappo, A.P. Universal Stress Proteins as New Targets for Environmental and Therapeutic Interventions of Schistosomiasis. Int. J. Environ. Res. Public Health 2016, 13, 972. https://doi.org/10.3390/ijerph13100972
Masamba P, Adenowo AF, Oyinloye BE, Kappo AP. Universal Stress Proteins as New Targets for Environmental and Therapeutic Interventions of Schistosomiasis. International Journal of Environmental Research and Public Health. 2016; 13(10):972. https://doi.org/10.3390/ijerph13100972
Chicago/Turabian StyleMasamba, Priscilla, Abiola Fatimah Adenowo, Babatunji Emmanuel Oyinloye, and Abidemi Paul Kappo. 2016. "Universal Stress Proteins as New Targets for Environmental and Therapeutic Interventions of Schistosomiasis" International Journal of Environmental Research and Public Health 13, no. 10: 972. https://doi.org/10.3390/ijerph13100972






