Mercury in Hair Is Inversely Related to Disease Associated Damage in Systemic Lupus Erythematosus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Clinical Assessment
Serological Measures
2.3. Urine and Hair Collection
2.4. Anthropometric Assessment
2.5. Urinary and Hair Mercury Assessment
2.6. Quantification of Dental Amalgams
2.7. Statistical Analysis
3. Results
3.1. Study Population
| Mean (±SD) | |
|---|---|
| N = | 52 |
| Age | 48 (±13.19) |
| Gender—Female (%) | 50 (96.2%) |
| BMI | 27 (±4.96) |
| Smoker, n (%) | 9 (17.3%) |
| Taking immunosuppresants, n (%) | 7 (13.5%) |
| Taking steroids, n (%) | 12 (23.1%) |
| Anti-DsDNA antibody iu/mL | 23.2 (±42.7) |
| Anti-DsDNA positive, n (%) | 16 (30.8%) |
| ESR mm/h | 15.2 (±18.04) |
| C3 g/L | 1.09 (±0.31) |
| C4 g/L | 0.21 (±0.14) |
| SLAM | 5.66 (±2.39) |
| SELENA SLEDAI | 4.46 (±4) |
| BILAG | 6.1 (±6.29) |
| SLICC/ACR | 0.96 (±1.12) |
| Neurological involvement, n (%) | 5 (9.6%) |
| Renal involvement, n (%) | 4 (7.7%) |
| Urine Hg ng/g creatinine | 1.1 (±1.24) |
| Hair Hg ppm | 1.5 (±1.55) |
| Dental amalgams | 4.9 (±4.6) |
3.2. Univariate Correlations
3.3. Multiple Regression Analysis
| Markers of Hg Exposure | BILAG | SLAM | SELENA SLEDAI | SLICC ACR | ||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| Hair Hg | −0.323 | 0.029 * | −0.251 | 0.093 | 0.105 | 0.843 | −0.377 | 0.038 * |
| Urine Hg | −0.081 | 0.592 | −0.078 | 0.605 | −0.023 | 0.895 | 0.182 | 0.226 |
| Dental Amalgams | 0.222 | 0.274 | 0.253 | 0.086 | −0.218 | 0.136 | −0.032 | 0.830 |
| Model | Predictor Variables | BILAG | SLAM | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Model R2 | p Value | (95% CI) | Beta | Model R2 | p Value | (95% CI) | Beta | ||
| 1 | Hair Hg | 0.111 | 0.062 | −0.189, 0.105 | −0.088 | 0.065 | 0.096 | −3.250, 0.273 | −0.088 |
| Age | 0.569 | −0.189, 0.105 | −0.088 | 0.752 | −0.048, 0.067 | 0.05 | |||
| 2 | Hair Hg | 0.111 | 0.066 | −.884, 0.293 | −0.294 | 0.127 | 0.131 | −3.057, 0.411 | −0.238 |
| Age | 0.571 | −0.191, 0.107 | −0.088 | 0.697 | −0.45, 0.067 | 0.06 | |||
| BMI | 0.928 | −0.378, 0.345 | −0.013 | 0.091 | −0.020, 0.253 | 0.252 | |||
| 1a | Urine Hg | 0.02 | 0.621 | −6.250, 3.774 | −0.188 | 0.007 | 0.614 | −2.398, 1.434 | −0.12 |
| Age | 0.449 | −0.200, 0.090 | −0.116 | 0.89 | −0.59, 0.052 | −0.12 | |||
| 2a | Urine Hg | 0.02 | 0.629 | −6.399, 3.910 | −0.076 | 0.057 | 0.808 | −2.152, 1.687 | −0.037 |
| Age | 0.323 | −0.203, 0.092 | −0.116 | 0.974 | −0.056, 0.054 | −0.021 | |||
| BMI | 0.988 | −0.401, 0.395 | −0.002 | 0.141 | −0.038, 0.258 | 0.229 | |||
| SELENA SLEDAI | SLICC ACR | ||||||||
| 1 | Hair Hg | 0.031 | 0.586 | −2.242, 4.078 | −0.267 | 0.021 | 0.019 * | −1.769, −0.155 | −0.366 |
| Age | 0.258 | −0.159, 0.044 | −0.186 | 0.263 | −0.012, 0.041 | 0.173 | |||
| 2 | Hair Hg | 0.057 | 0.506 | −2.115, 4.223 | −0.238 | 0.147 | 0.015 * | −1.823, −0.204 | −0.385 |
| Age | 0.28 | −0.157, 0.047 | 0.177 | 0.281 | −0.012, 0.040 | 0.166 | |||
| BMI | 0.301 | −0.120, 0.378 | 0.16 | 0.256 | −0.100, 0.027 | −0.165 | |||
| 1a | Urine Hg | 0.264 | 0.821 | −1.126, 1.412 | 0.33 | 0.182 | 0.234 | −0.351, 1.4 | 0.184 |
| Age | 0.081 | −0.069, 0.004 | −0.263 | 0.939 | −0.024, 0.026 | 0.012 | |||
| 2a | Urine Hg | 0.266 | 0.791 | −1.132, 1.477 | 0.04 | 0.203 | 0.288 | −0.418, 1.375 | 0.165 |
| Age | 0.088 | −0.070, 0.005 | −0.26 | 0.973 | −0.25, 0.026 | 0.005 | |||
| BMI | 0.797 | −0.088, 0.114 | 0.039 | 0.558 | −0.089, 0.049 | −0.091 | |||
3.4. Hg Concentrations in Those with Active Disease versus Inactive
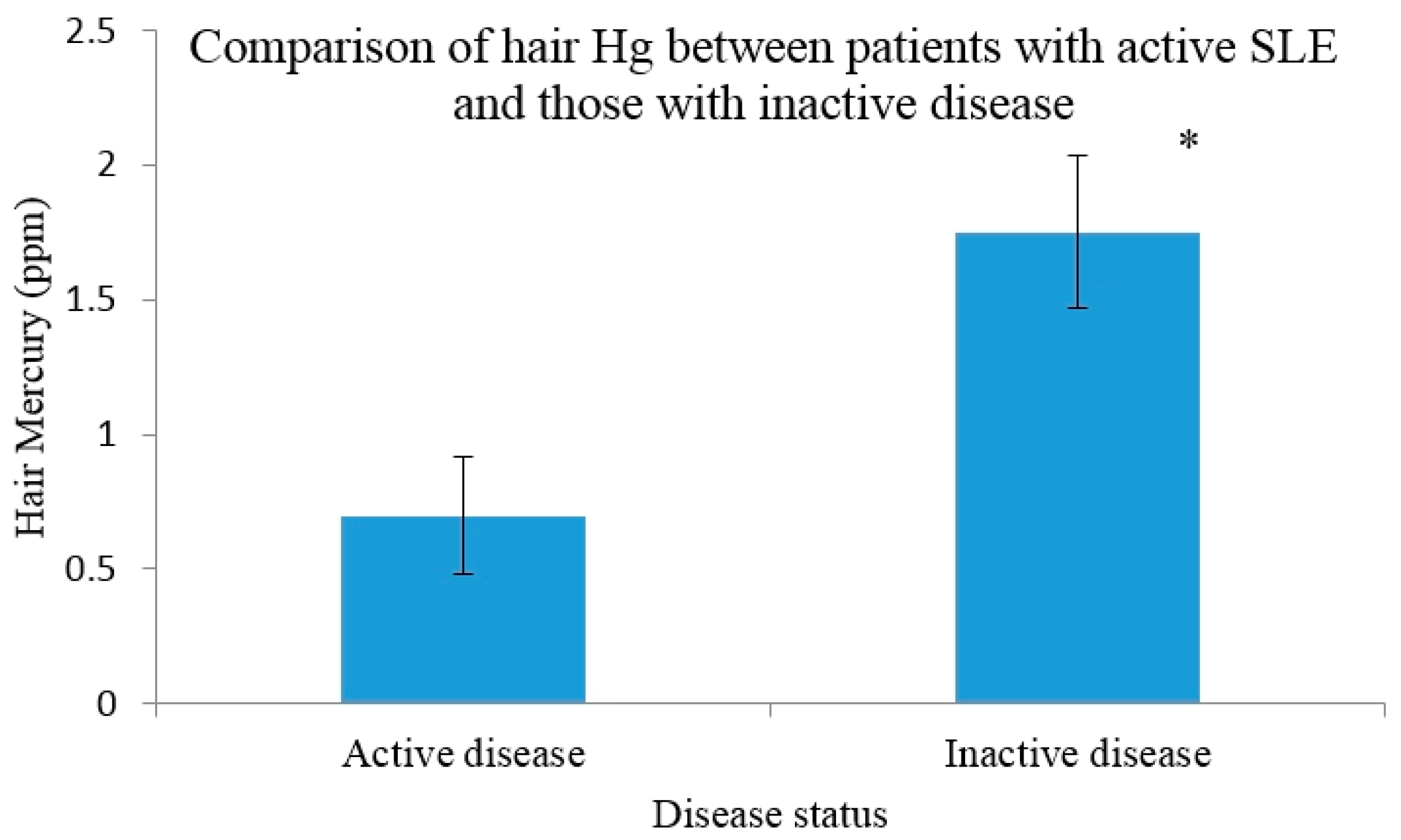
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zandman-Goddard, G.; Solomon, M.; Rosman, Z.; Peeva, E.; Shoenfeld, Y. Environment and lupus-related diseases. Lupus 2012, 21, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, J.; Takhar, H.; Anderson-Mahoney, P.; Kotlerman, J.; Tarr, J.; Warshaw, R. Cluster of systemic lupus erythematosus (SLE) associated with an oil field waste site: A cross sectional study. Environ. Health 2007, 6. [Google Scholar] [CrossRef]
- Havarinasab, S.; Hultman, P. Organic mercury compounds and autoimmunity. Autoimmun. Rev. 2005, 4, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Park, J.D.; Zheng, W. Human exposure and health effects of inorganic and elemental mercury. J. Prev. Med. Public Health 2012, 45, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry (ATSDR). Public Health Statement Mercury, 1999. Available online: http://www.atsdr.cdc.gov/ToxProfiles/tp46-c1-b.pdf (accessed on 3 April 2015).
- Environmental Protection Agency (EPA). United States. Available online: http://www.epa.gov/mercury/exposure.htm (accessed on 21 August 2015).
- McDowell, M.A.; Dillon, C.F.; Osterloh, J.; Bolger, P.M.; Pellizari, E.; Fernando, R.; Montes de Oca, R.; Schober, S.E.; Sinks, T.; Jones, R.L.; et al. Hair mercury levels in US children and women of childbearing age. Reference range data from NHANES 1999–2000. Environ. Health Perspect. 2004, 112, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Kingman, A.; Albertini, T.; Brown, L.J. Mercury concentrations in urine and whole blood associated with amalgam exposure in a US military population. J. Dent. Res. 1998, 77, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Harada, M. Minamata disease: Methylmercury poisoning in Japan caused by environmental pollution. Crit. Rev. Toxicol. 1995, 25, 1–14. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Environmental Health Criteria 101: Methylmercury; WHO: Geneva, Switzerland, 1990. [Google Scholar]
- Shenker, B.J.; Guo, T.L.; Shapiro, I.M. Mercury-induced apoptosis in human lymphoid cells: Evidence that the apoptotic pathway is mercurial species dependent. Environ. Res. 2000, 84, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.A.; Graber, J.; Nyland, J.F.; Silbergeld, E.K. In vitro HgCl2 exposure of immune cells at different stages of maturation: Effects on phenotype and function. Environ. Res. 2005, 98, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.M.; Nyland, J.F.; Evans, S.L.; Wang, S.B.; Doyle, K.M.; Crainiceanu, C.M.; Silbergeld, E.K. Mercury induces an unopposed inflammatory response in human peripheral blood mononuclear cells in vitro. Environ. Health Perspect. 2009, 117, 1932–1938. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.M.; Nyland, J.F.; Silbergeld, E.K. Differential immunotoxic effects of inorganic and organic mercury species in vitro. Toxicol. Lett. 2010, 198, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Kakuschke, A.; Valentine-Thon, E.; Fonfara, S.; Kramer, K.; Prange, A. Effects of methyl-, phenyl-, ethylmercury and mercurychloride on immune cells of harbor seals (Phoca vitulina). J. Environ. Sci. 2009, 21, 1716–1721. [Google Scholar] [CrossRef]
- Bagenstose, L.M.; Salgame, P.; Monestier, M. Cytokine regulation of a rodent model of mercuric chloride-induced autoimmunity. Environ. Health Perspect. 1999, 107, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Häggqvist, B.; Hultman, P. Murine metal-induced systemic autoimmunity: Baseline and stimulated cytokine mRNA expression in genetically susceptible and resistant strains. Clin. Exp. Immunol. 2001, 126, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Hultman, P.; Nielsen, J.B. The effect of dose, gender, and non-H-2 genes in murine mercury-induced autoimmunity. J. Autoimmun. 2001, 17, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Hultman, P.; Bell, L.J.; Eneström, S.; Pollard, K.M. Murine susceptibility to mercury: II. Autoantibody profiles and renal immune deposits in hybrid, backcross, and H-2d congenic mice. Clin. Immunol. Immunopathol. 1993, 68, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Häggqvist, B.; Havarinasab, S.; Björn, E.; Hultman, P. The immunosuppressive effect of methylmercury does not preclude development of autoimmunity in genetically susceptible mice. Toxicology 2005, 208, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Havarinasab, S.; Björn, E.; Nielsen, J.B.; Hultman, P. Mercury species in lymphoid and non-lymphoid tissues after exposure to methyl mercury: Correlation with autoimmune parameters during and after treatment in susceptible mice. Toxicol. Appl. Pharmacol. 2007, 221, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Pollard, K.M.; Pearson, D.L.; Hultman, P.; Deane, T.N.; Lindh, U.; Kono, D.H. Xenobiotic acceleration of idiopathic systemic autoimmunity in lupus-prone bxsb mice. Environ. Health Perspect. 2001, 109, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Havarinasab, S.; Hultman, P. Alteration of the spontaneous systemic autoimmune disease in (NZB x NZW) F1 mice by treatment with thimerosal (ethyl mercury), National Library of Medicine. Toxicol. Appl. Pharmacol. 2006, 214, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Kardestuncer, T.; Frumkin, H. Systemic lupus erythematosus in relation to environmental pollution: An investigation in an African-American community in North Georgia. Arch. Environ. Health 1997, 52, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Balluz, L.; Philen, R.; Ortega, L.; Rosqles, C.; Brock, J.; Barr, D.; Kieszak, S. Investigation of systemic lupus erythematosus in Nogales, Arizona. Am. J. Epidemiol. 2001, 154, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.S.; Parks, C.G. Occupational and environmental exposures as risk factors for systemic lupus erythematosus. Curr. Rheumatol. Rep. 2004, 6, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Arnett, F.C.; Fritzler, M.J.; Ahn, C.; Holian, A. Urinary mercury levels in patients with autoantibodies to U3-RNP (fibrillarin). J. Rheumatol. 2000, 27, 405–410. [Google Scholar] [PubMed]
- Alves, M.F.A.; Fraiji, N.A.; Barbosa, A.C.; de Limaa, D.S.N.; Souzab, J.R.; Dóreac, J.G.; Cordeiro, G.W. Fish consumption, mercury exposure and serum antinuclear antibody in Amazonians. Int. J. Environ. Health Res. 2006, 16, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Silbergeld, E.K.; Silva, I.A.; Nyland, J.F. Mercury and autoimmunity: Implications for occupational and environmental health. Toxicol. Appl. Pharmacol. 2005, 207, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Barregård, L.; Eneström, S.; Ljunghusen, O.; Wieslander, J.; Hultman, P. A study of autoantibodies and circulating immune complexes in mercury-exposed chloralkali workers. Int. Arch. Occup. Environ. Health 1997, 70, 101–106. [Google Scholar] [PubMed]
- Ellingsen, D.G.; Barregård, L.; Gaarder, P.I.; Hultberg, B.; Kjuus, H. Assessment of renal dysfunction in workers previously exposed to mercury vapour at a chloralkali plant. Br. J. Ind. Med. 1993, 50, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Nyland, J.F.; Fillion, M.; Barbosa, J.F.; Shirley, D.L.; Chine, C.; Lemire, M.; Mergler, D.; Silbergeld, E.K. Biomarkers of methylmercury exposure immunotoxicity among fish consumers in Amazonian Brazil. Environ. Health Perspect. 2011, 119, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Shoenfeld, Y. “ASIA”—Autoimmune/inflammatory syndrome induced by adjuvants. J. Autoimmun. 2011, 36, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, V. Mercury-Induced Inflammation: Yet Another Example of ASIA Syndrome. IMAJ 2013, 15, 714–715. [Google Scholar] [PubMed]
- Breslin, L.C. An Investigation of Vitamin D Status in Systemic Lupus Erythematosus—Its Relationship with Disease Activity, Bone Mineral Density and Quality of Life. Ph.D. Thesis, Ulster University, Northern Ireland, UK, 2012. [Google Scholar]
- Tan, E.M.; Cohen, A.S.; Fries, J.F.; Masi, A.T.; McShane, D.J.; Rothfeld, N.F.; Schaller, J.G.; Talal, N.; Winchester, R.J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheumatol. 1982, 25, 1271–1277. [Google Scholar] [CrossRef]
- Yee, C.S.; Cresswell, L.; Farewell, V.; Rahman, A.; The, L.S.; Griffiths, B.; Bruce, I.N.; Ahmad, Y.; Prabu, A.; Akil, M.; et al. Numerical scoring for the BILAG-2004 index. Rheumatology 2010, 49, 1665–1669. [Google Scholar] [CrossRef] [PubMed]
- Gladman, D.; Ginzler, E.; Goldsmith, C.; Fortin, P.; Liang, M.; Urowitz, M.; Bacon, P.; Bombardieri, S.; Hanly, J.; Hay, E.; et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheumatol. 1996, 39, 363–369. [Google Scholar] [CrossRef]
- Liang, M.H.; Socher, S.A.; Larson, M.G.; Schur, P.H. Reliability and validity of six systems for the clinical assessment of disease activity in systemic lupus erythematosus. Arthritis Rheumatol. 1989, 32, 1107–1118. [Google Scholar] [CrossRef]
- Lam, G.K.W.; Petri, M. Assessment of systemic lupus erythematosus. Clin. Exp. Rheumatol. 2005, 23, 120–132. [Google Scholar]
- Gladman, D.D.; Urowitz, M.B.; Kagal, A.L.; Hallett, D.A. Accurately describing changes in disease activity in Systemic Lupus Erythematosus. J. Rheumatol. 2000, 27, 377–379. [Google Scholar] [PubMed]
- Magos, L. Selective atomic-absorption determination of inorganic mercury and methylmercury in undigested biological samples. Analyst 1971, 96, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Glass, D.C.; Gray, C.N. Estimating mean exposures from censored data: Exposure to benzene in the Australian petroleum industry. Ann. Occup. Hyg. 2001, 45, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Pichichero, M.E.; Gentile, A.; Giglio, N.; Alonso, M.M.; Fernandez Mentaberri, M.V.; Zareba, G.; Clarkson, T.; Gotelli, C.; Gotelli, M.; Yan, L.; et al. Mercury levels in premature and low birth weight newborn infants after receipt of thimerosal-containing vaccines. J. Pediatr. 2009, 155, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Esteban, M.; Schindler, B.K.; Jiménez, J.A.; Koch, H.M.; Angerer, J.; Rosado, M. Mercury analysis in hair: Comparability and quality assessment within the transnational COPHES/DEMOCOPHES project. Environ. Res. 2015, 141, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.M.; Nyland, J.F.; Silva, I.A.; Venturac, A.M.; de Souzac, J.M.; Silbergeld, E.K. Mercury exposure, serum antinuclear/antinucleolar antibodies, and serum cytokine levels in mining populations in Amazonian Brazil: A cross-sectional study. Environ. Res. 2010, 110, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Duffy, E.M.; Meenagh, G.K.; McMillan, S.A.; Strain, J.J.; Hannigan, B.M.; Bell, A.L. The clinical effect of dietary supplementation with omega-3 fish oils and/or copper in systemic lupus erythematosus. J. Rheumatol. 2004, 31, 1551–1556. [Google Scholar] [PubMed]
- Wright, S.; O’Prey, F.M.; McHenry, M.T.; Leahey, W.J.; Devine, A.B.; McVeigh, G.E.; Duffy, E.M.; Johnston, D.G.; Finch, M.B.; Bell, A.L.; et al. A randomised interventional trial of omega-3-polyunsaturated fatty acids on endothelial function and disease activity in systemic lupus erythematosus. Ann. Rheum. Dis. 2008, 67, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Davidson, P.W.; Myers, G.J.; Cox, C.; Axtell, C.; Shamlaye, C.; Sloane-Reeves, J.; Cernichiari, E.; Needham, L.; Choi, A.; Wang, Y.; et al. Effects of prenatal and postnatal methylmercury exposure from fish consumption on neurodevelopment: Outcomes at 66 months of age in the Seychelles Child Development Study. JAMA 1998, 280, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Strain, J.J.; Davidson, P.W.; Thurston, S.W.; Mulhern, M.S.; McAfee, A.J.; Wijngaarden, E.V.; van Wijngaarden, E.; Shamlaye, C.F.; Henderson, J.; Watson, G.E.; et al. Maternal PUFA status but not prenatal methylmercury exposure is associated with children’s language functions at age five years in the Seychelles. J. Nutr. 2012, 142, 1943–1949. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. N-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505–1519. [Google Scholar]
- Brown, K.M.; Arthur, J.R. Selenium, selenoproteins and human health: A review. Public Health Nutr. 2001, 4, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Somers, E.C.; Ganser, M.A.; Warren, J.S.; Basu, N.; Wang, L.; Zick, S.M.; Park, S.K. Mercury Exposure and Antinuclear Antibodies among Females of Reproductive Age in the United States: NHANES. Environ. Health Perspect. 2015, 123, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Kenow, K.P.; Grasman, K.A.; Hines, R.K.; Meyer, M.W.; Gendron-Fitzpatrick, A.; Spalding, M.G.; Gray, B.R. Effects of methylmercury exposure on the immune function of juvenile common loons (Gavia immer). Environ. Toxicol. Chem. 2007, 26, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Day, R.D.; Segars, A.L.; Arendt, M.D.; Lee, A.M.; Peden-Adams, M.M. Relationship of blood mercury levels to health parameters in the loggerhead sea turtle (Caretta caretta). Environ. Health Perspect. 2007, 115, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Prochazkova, J.; Sterzl, I.; Kucerova, H.; Bartova, J.; Stejskal, V.D. The beneficial effect of amalgam replacement on health in patients with autoimmunity. Neuroendocrinol. Lett. 2004, 25, 211–218. [Google Scholar] [PubMed]
- Cederbrant, K.; Gunnarsson, L.G.; Hultman, P.; Norda, R.; Tibbling-Grahn, L. In vitro lymphoproliferative assays with HgCl2 cannot identify patients with systemic symptoms attributed to dental amalgam. J. Dent. Res. 1999, 78, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Langworth, S.; Sällsten, G.; Barregård, L.; Cynkier, I.; Lind, M.L.; Söderman, E. Exposure to mercury vapor and impact on health in the dental profession in Sweden. J. Dent. Res. 1997, 76, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Dórea, J.G.; Bezerra, V.L.; Fajon, V.; Horvat, M. Speciation of methyl- and ethyl-mercury in hair of breastfed infants acutely exposed to thimerosal-containing vaccines. Clin. Chim. Acta 2011, 412, 1563–1566. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crowe, W.; Doherty, L.; Watson, G.; Armstrong, D.; Ball, E.; Magee, P.; Allsopp, P.; Bell, A.; Strain, J.J.; McSorley, E. Mercury in Hair Is Inversely Related to Disease Associated Damage in Systemic Lupus Erythematosus. Int. J. Environ. Res. Public Health 2016, 13, 75. https://doi.org/10.3390/ijerph13010075
Crowe W, Doherty L, Watson G, Armstrong D, Ball E, Magee P, Allsopp P, Bell A, Strain JJ, McSorley E. Mercury in Hair Is Inversely Related to Disease Associated Damage in Systemic Lupus Erythematosus. International Journal of Environmental Research and Public Health. 2016; 13(1):75. https://doi.org/10.3390/ijerph13010075
Chicago/Turabian StyleCrowe, William, Leanne Doherty, Gene Watson, David Armstrong, Elisabeth Ball, Pamela Magee, Philip Allsopp, Aubrey Bell, J. J. Strain, and Emeir McSorley. 2016. "Mercury in Hair Is Inversely Related to Disease Associated Damage in Systemic Lupus Erythematosus" International Journal of Environmental Research and Public Health 13, no. 1: 75. https://doi.org/10.3390/ijerph13010075






