Treatment of Ammonia Nitrogen Wastewater in Low Concentration by Two-Stage Ozonization
Abstract
:1. Introduction
2. Experimental Section
2.1. Experimental Reagents and Apparatus
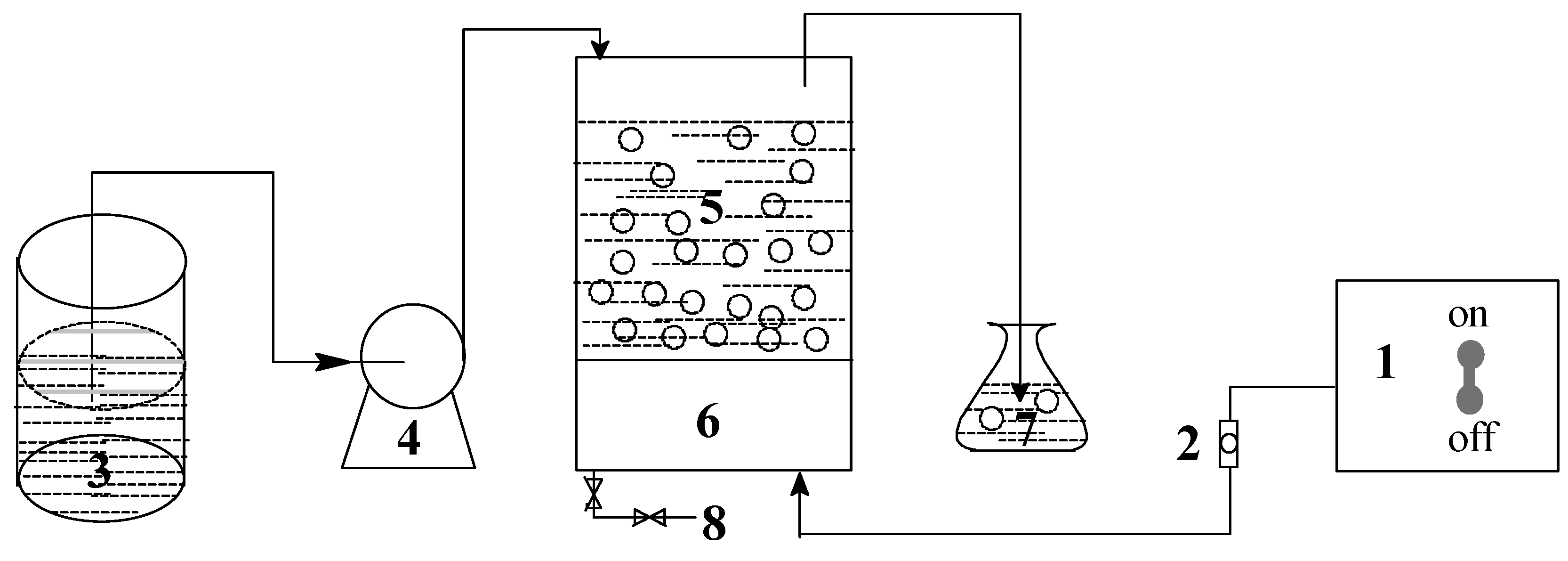
2.2. Analysis Method
| Project Name | Determination | Standard Number | Reference |
|---|---|---|---|
| Ammonia nitrogen | Nessler’s reagent spectrophotometry | HJ 535-2009 | [19] |
| Nitrite | N-(1-naphthyl)-ethylenediamine spectrophotometry | GB 7493-87 | [20] |
| Nitrate | Phenol disulfonic acid spectrophotometry | GB 7480-87 | [21] |
| Total nitrogen | Alkaline potassium persulfate digestion spectrophotometry | HJ 636-2012 | [22] |
3. Results and Discussion
3.1. Blank Stripping
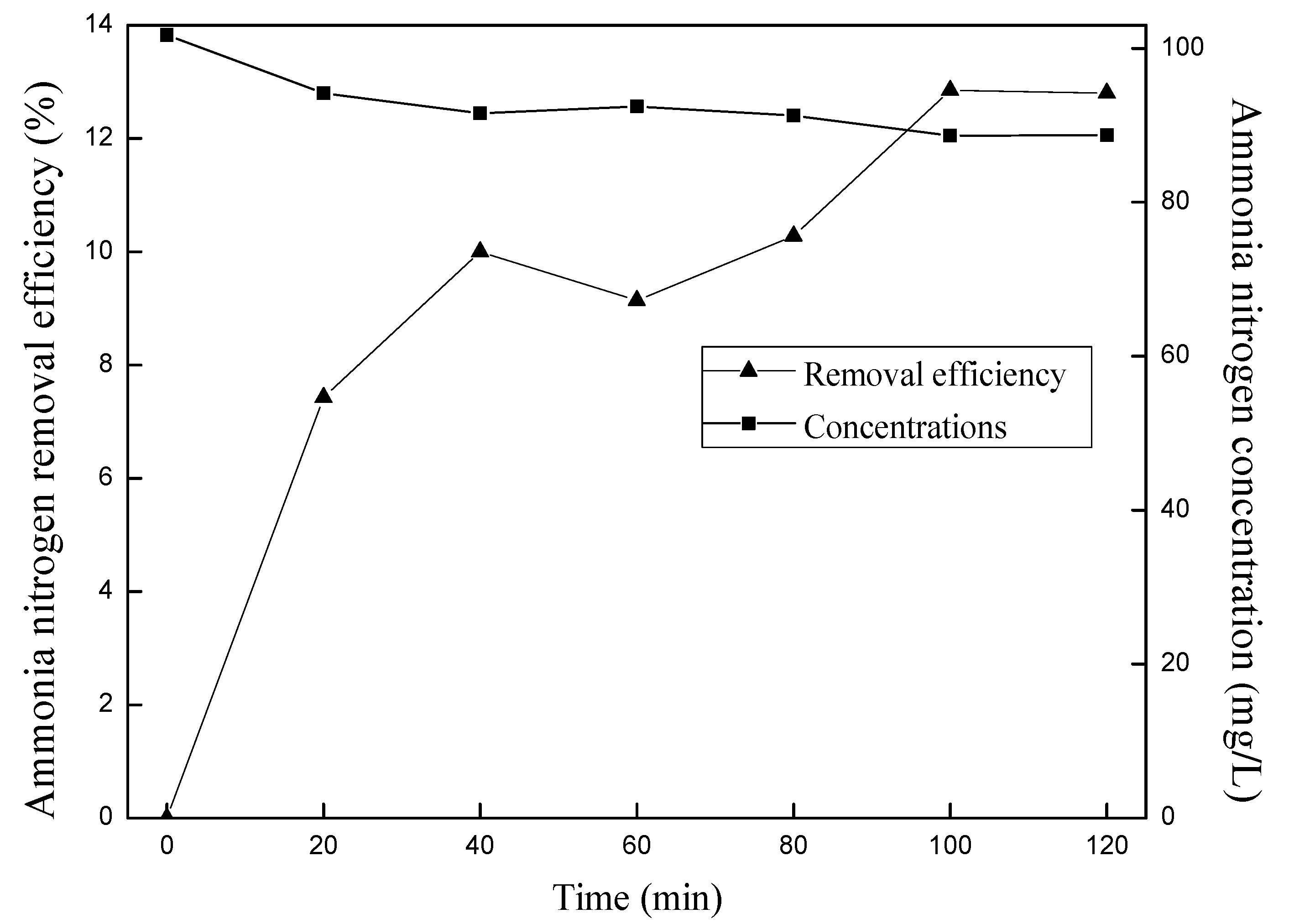
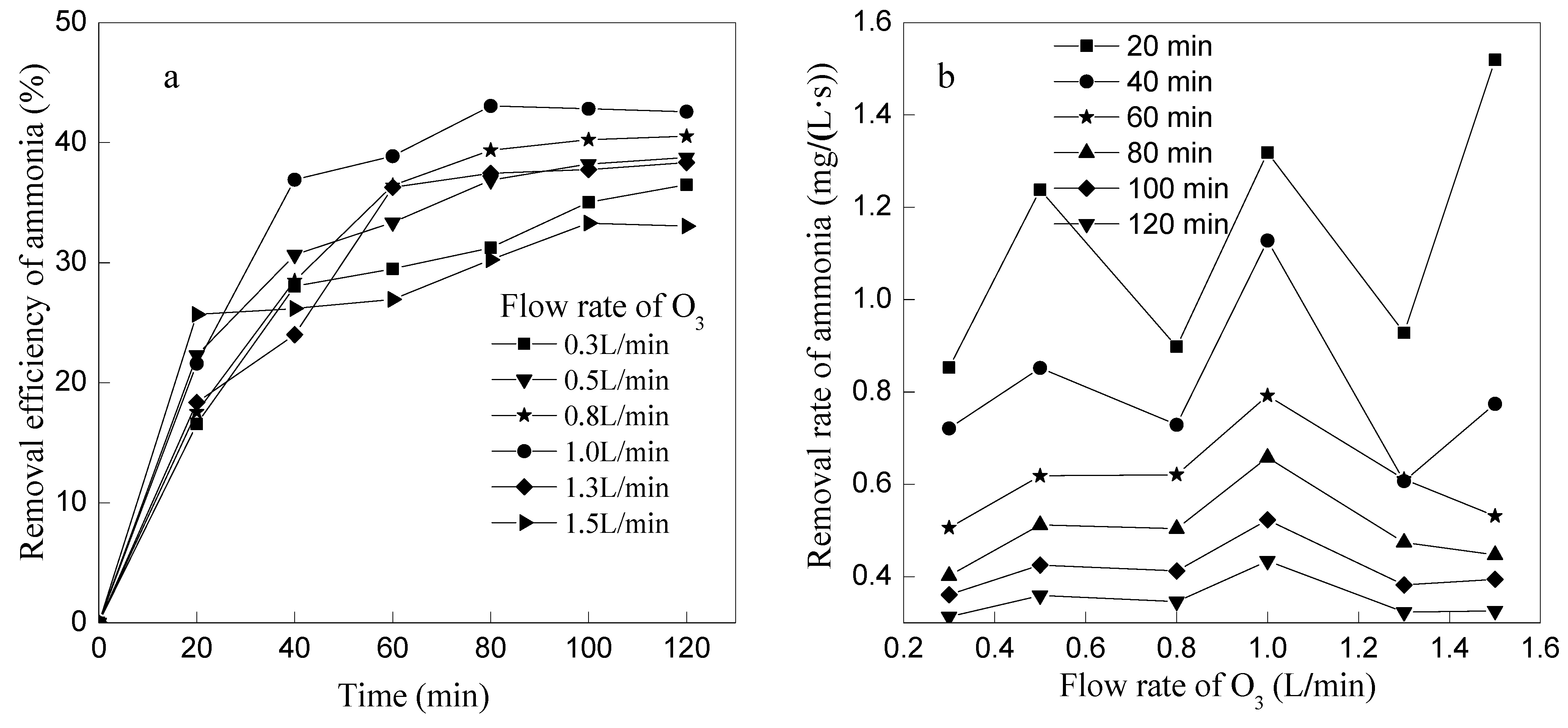
3.2. Effects of Ozone Concentrations on Ammonia Removal
| Ozone Flow Rate (L/min) | 0 | 0.3 | 0.5 | 0.8 | 1.0 | 1.3 |
| Ozone Concentration (mg/L) | 0 | 25.83 | 30.24 | 51 | 58.14 | 71.82 |

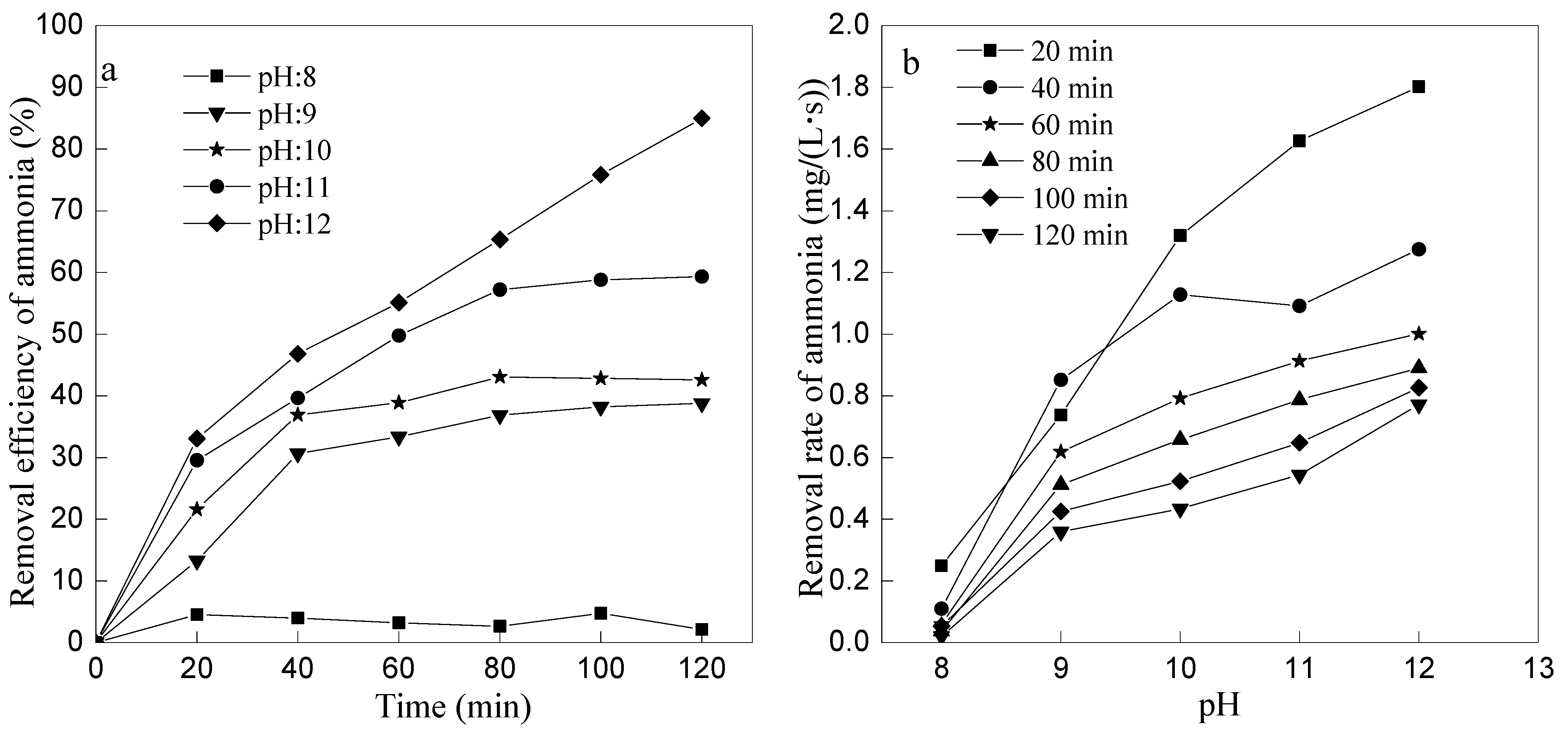
3.3. Effect of Initial pH on Ammonia Removal by Ozone Oxidation
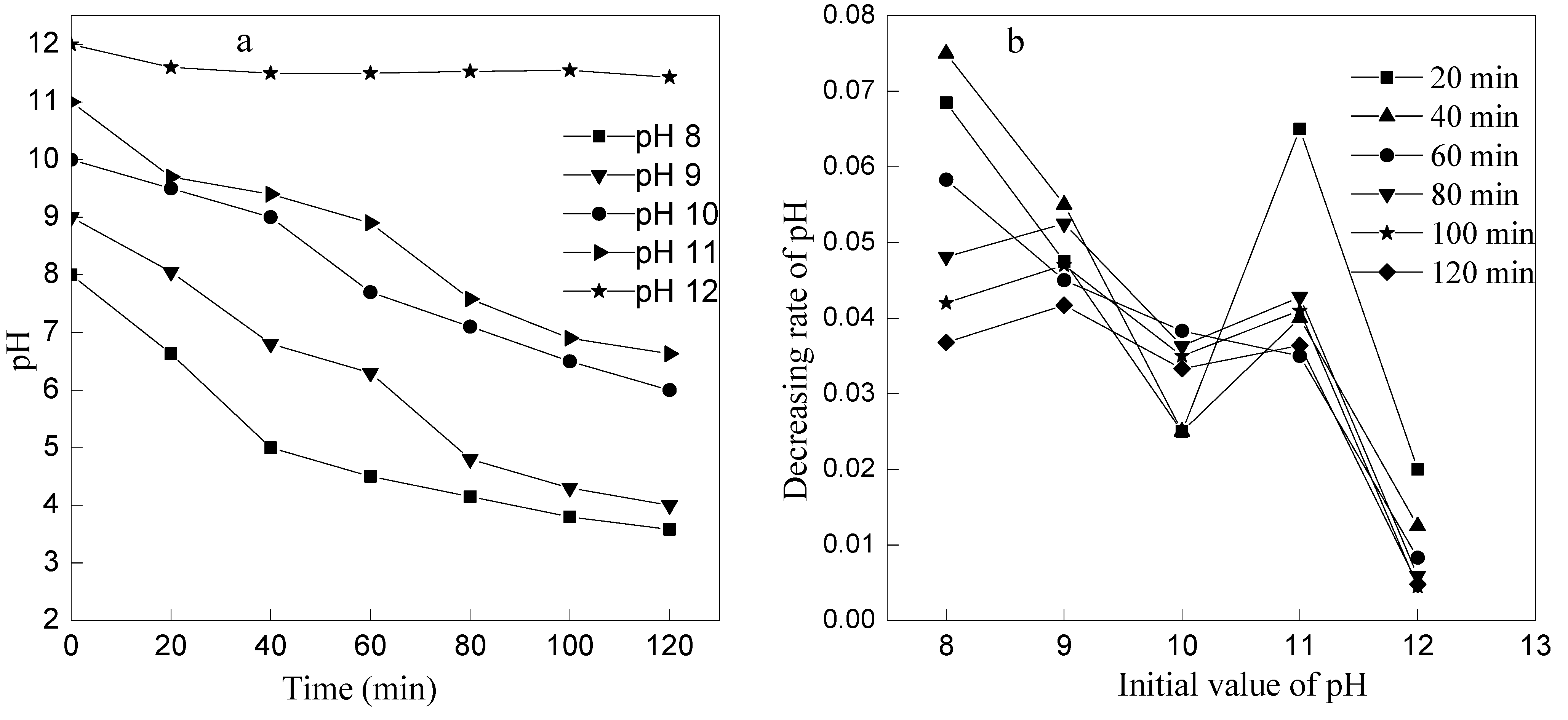
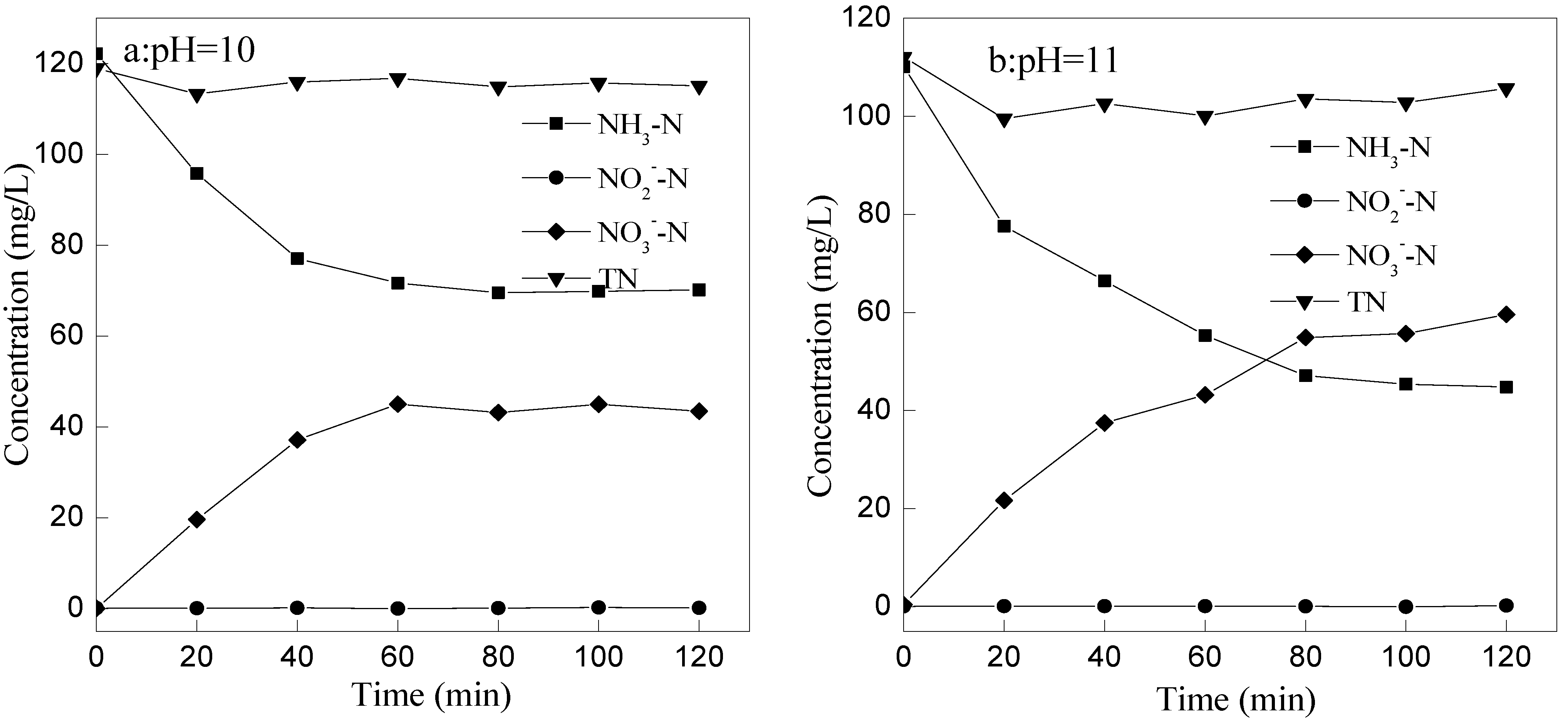
3.4. Mechanism Analysis
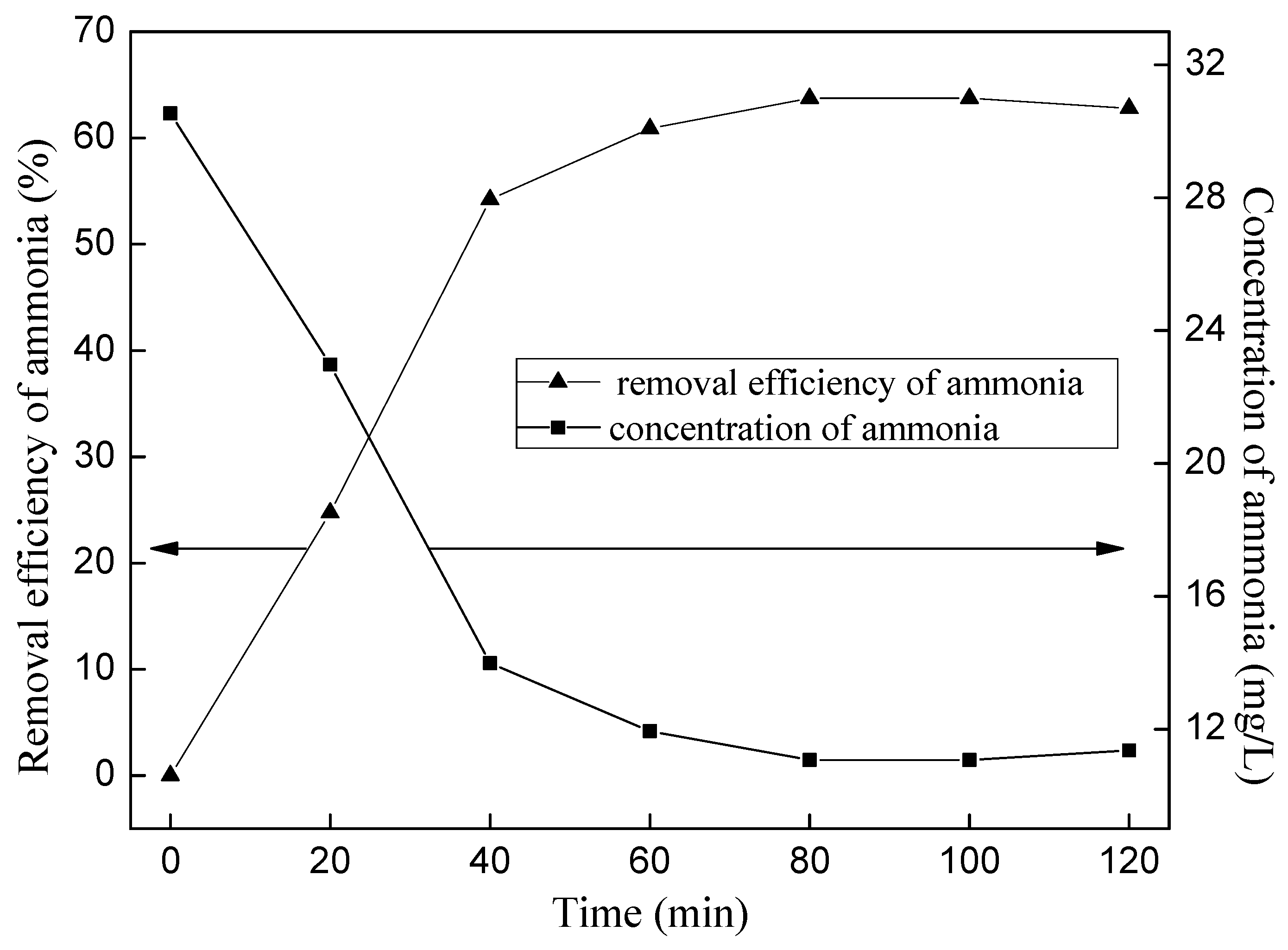
4. The Second Stage Treatment of Ammonia Nitrogen Wastewater
5. Conclusions
- (1)
- Ammonia removal efficiency increased with the ozone flow rate increased when ozone flow rate was ≤1 L/min, while the ammonia removal efficiency decreased with increasing of ozone flow rate when ozone flow rate was ≥1 L/min; the higher of the initial pH, the higher of the ammonia nitrogen removal efficiency. However, too much acid would be needed to neutralize the excessive alkalis if the initial pH > 11; at the optimum conditions of 1 L/min ozone flow rate and initial pH 11, ammonia removal efficiency could reach 59.32% and pH reduced to 6.63 after 120 min by the primary ozone oxidation, and the efficiency could reach 85% after the second stage of ozone oxidation, which would meet the discharge standard of China.
- (2)
- During the ozonization of ammonia nitrogen, there is a certain contribution to ammonia removal by the flow stripping, but ozone oxidation predominates the removal reaction.
- (3)
- The removal mechanism of ammonia nitrogen might be that ammonia nitrogen was mainly transformed into NO3−-N, less into NO2−-N, not into N2.
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xiao, Y.F.; Feng, Z.Y.; Hu, G.H.; Huang, L.; Huang, X.W.; Chen, Y.Y.; Li, M.L. Leaching and mass transfer characteristics of elements from ion-adsorption type rare earth ore. Rare Met. 2015, 34, 357–365. [Google Scholar] [CrossRef]
- Juan, M.G.; Lorna, G.; Ramón, M.; Juan, M.L. Nitrification of waste waters from fish-meal factories. Water SA 1998, 24, 245–249. [Google Scholar]
- Udeh, P.J. A Guide to Healthy Drinking Water; iUniverse: Bloomington, IN, USA, 2004. [Google Scholar]
- Graham, D.W.; Knapp, C.W.; Vleck, E.S.; Bloor, K.; Lane, T.B.; Graham, C.E. Experimental demonstration of chaotic instability in biological nitrification. ISME J. 2007, 1, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Mahamudur, I.; Rajkishore, P. Synthesis and physicochemical characterization of Zn/Al chloride layered double hydroxide and evaluation of its nitrate removal efficiency. Desalination 2010, 256, 120–128. [Google Scholar]
- Yang, H.; Cheng, H. Controlling nitrite level in drinking water by chlorination and chloramination. Sep. Purif. Technol. 2007, 56, 392–396. [Google Scholar] [CrossRef]
- Guo, Z.; Zheng, Z.; Gu, C.; Zheng, Y. Gamma irradiation-induced removal of low-concentration nitrite in aqueous solution. Radiat. Phys. Chem. 2008, 77, 702–707. [Google Scholar] [CrossRef]
- Markou, G.; Vandamme, D.; Muylaert, K. Using natural zeolite for ammonia sorption from wastewater and as nitrogen releaser for the cultivation of Arthrospira platensis. Bioresour. Tchnol. 2014, 155, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, K.G. The research development on the treatment of ammonia-nitrogen wastewater. Ind. Saf. Environ. Prot. 2006, 32, 7–9. (In Chinese) [Google Scholar]
- Li, B.; Zhao, J.Y.; Lu, J.F. Numerical study of the simultaneous oxidation of NO and SO2 by ozone. Int. J. Environ. Res. Public Health 2015, 12, 1595–1611. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, Y.; Tokumura, M.; Mizukoshi, A.; Miyuki Noguchi, M.; Yanagisawa, Y. Measurement of Secondary Products during oxidation reactions of terpenes and ozone based on the Prt-MS analysis: Effects of coexistent carbonyl compounds. Int. J. Environ. Res. Public Health 2010, 7, 3853–3870. [Google Scholar] [CrossRef] [PubMed]
- Turhan, K.; Turgut, Z. Decolorization of direct dye in textile wastewater by ozonization in a semi-batch bubble column reactor. Desalination 2009, 242, 256–263. [Google Scholar] [CrossRef]
- Domeno, C.; Rodriguex-Lafuente, A.; Martos, J.M.; Bilbao, R. VOC removal and deodorization of effluent gases from an industrial plant by photo-oxidation, chemical oxidation, and ozonization. Environ. Sci. Technol. 2010, 44, 2585–2591. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C. The removal of tin from ITO-scrap via ozonization. Bull. Korean Chem. Soc. 2009, 30, 3141–3142. [Google Scholar] [CrossRef]
- Khuntia, S.; Majumder, S.K.; Ghosh, P. Removal of ammonia from water by ozone microbubbles. Ind. Eng. Chem. Res. 2012, 52, 318–326. [Google Scholar] [CrossRef]
- Tanaka, J.; Matsumura, M. Application of ozone treatment for ammonia removal in spent brine. Adv. Environ. Res. 2003, 7, 835–845. [Google Scholar] [CrossRef]
- Schroeder, J.P.; Croot, P.L.; von Dewitz, B.; Waller, U.; Hanel, R. Potential and limitations of ozone for the removal of ammonia, nitrite, and yellow substances in marine recirculating aquaculture systems. Aquac. Eng. 2011, 45, 35–41. [Google Scholar] [CrossRef]
- Bogoczek, R.; Kociołek-Balawejder, E.; Stanisławska, E. A macromolecular oxidant, the N,N-dichlorosulfonamide for removal of residual nitrites from aqueous media. React. Funct. Polym. 2006, 66, 609–617. [Google Scholar] [CrossRef]
- Ministry of Environmental Protection. Water Quality—Determination of Ammonia Nitrogen—Nessler’s Reagent Spectrophotometry, 1st ed.; China Environmental Science Press: Beijing, China, 2010; pp. 1–4. (In Chinese) [Google Scholar]
- State Environmental Protection Administration. Water Quality-Determination of Nitrogen (Nitrite)-Spectrophotometric Method, 1st ed.; China Standard Press: Beijing, China, 1987; pp. 1–4. (In Chinese) [Google Scholar]
- State Environmental Protection Administration. Water Quality-Determination of Nitrate-Spectrophotometric Method with Phenol Disulfonic Acid, 1st ed.; China Standard Press: Beijing, China, 1987; pp. 1–4. (In Chinese) [Google Scholar]
- Ministry of Environmental Protection. Water Quality-Determination of Total Nitrogen-Alkaline Potassium Persulfate Digestion UV Spectrophotometric Method, 1st ed.; China Environmental Science Press: Beijing, China, 2012; pp. 1–8. (In Chinese) [Google Scholar]
- Ministry of Housing and Urban-Rural Development of People’s Republic of China. Ozone Generator for Water and Wastewater Treatment, 1st ed.; China Standard Press: Beijing, China, 2010; p. 8. (In Chinese) [Google Scholar]
- Sun, D.C. Research on Disposing Wastewater with High Concentration Ammonia Nitrogen Using Ozone. Master’s Thesis, Qingdao Technological University, Qingdao, China, 2009. [Google Scholar]
- Hoigné, J.; Bader, H.; Haag, W.R.; Staehelin, J. Rate constants of reactions of ozone with organic and inorganic compounds in water-III: Inorganic compounds and radicals. Water Res. 1985, 19, 993–1004. [Google Scholar] [CrossRef]
- Wu, X.G. Degradation of Some Organic Pollutants Using a Combination Teehnique of Ozone-Activated Carbon and Advanced Oxidation. Ph.D. Thesis, Graduate University of Chinese Academy of Science, Beijing, China, 2007. [Google Scholar]
- Zhong, L.; Kuo, C.H. Kinetics and mechanism of ozone with hydrogen peroxide. J. South China Univ. Technol. (Nat. Sci.) 1997, 25, 50–54. (In Chinese) [Google Scholar]
- Zhong, L.; Kuo, C.H. Investigation on wet-ozonization degradation of ammonia nitrogen. China Water Wastewater 2000, 16, 15–16. (In Chinese) [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Yan, Q.; Wang, C.; Luo, C.; Zhou, N.; Jian, C. Treatment of Ammonia Nitrogen Wastewater in Low Concentration by Two-Stage Ozonization. Int. J. Environ. Res. Public Health 2015, 12, 11975-11987. https://doi.org/10.3390/ijerph120911975
Luo X, Yan Q, Wang C, Luo C, Zhou N, Jian C. Treatment of Ammonia Nitrogen Wastewater in Low Concentration by Two-Stage Ozonization. International Journal of Environmental Research and Public Health. 2015; 12(9):11975-11987. https://doi.org/10.3390/ijerph120911975
Chicago/Turabian StyleLuo, Xianping, Qun Yan, Chunying Wang, Caigui Luo, Nana Zhou, and Chensheng Jian. 2015. "Treatment of Ammonia Nitrogen Wastewater in Low Concentration by Two-Stage Ozonization" International Journal of Environmental Research and Public Health 12, no. 9: 11975-11987. https://doi.org/10.3390/ijerph120911975






