Effective Strategies for Monitoring and Regulating Chemical Mixtures and Contaminants Sharing Pathways of Toxicity
Abstract
:1. Introduction
2. Organohalogen Flame Retardants and Current Regulations
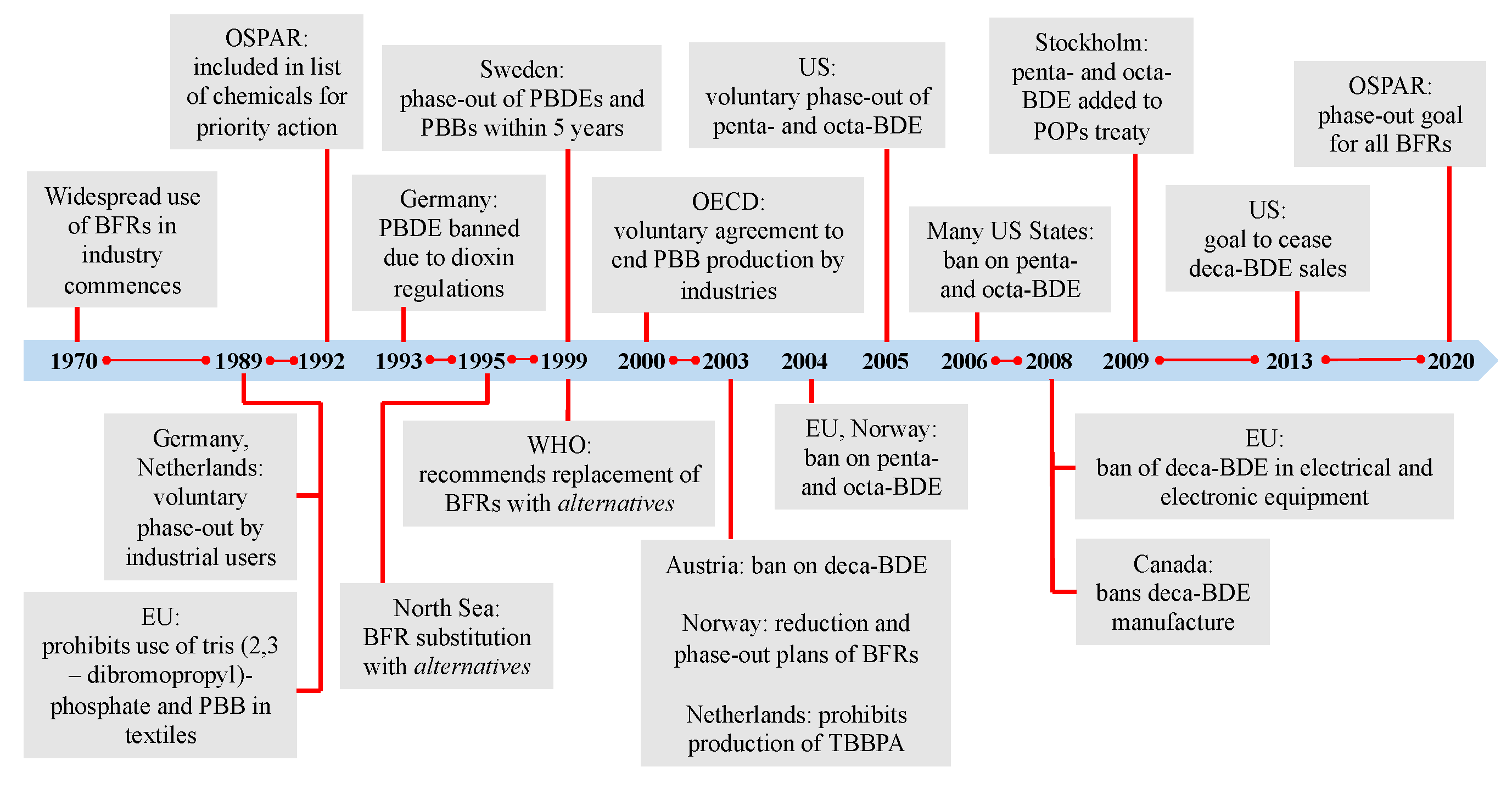
- Avoiding the use of flame retardants in consumer products that do not pose a significant fire hazard in the first place;
- Avoiding the use of flame retardants that resemble in molecular size and structure their harmful predecessors;
- Developing for use in products that absolutely require them, next-generation flame retardants featuring the following characteristics while still delivering the desired flame retardation qualities:
- ○
- covalently bound to the consumer products to minimize release and exposure, wherever possible and practical;
- ○
- large molecular size (e.g., polymers) that limits and ideally precludes uptake by biota following environmental release [18];
- ○
- absence of size and shape characteristics contributing to affinity and binding to human and animal receptors, thereby reducing the risk of toxicity, for example, of endocrine disruption via binding to hormone receptors.
3. Future Monitoring Strategies
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Carpenter, D.O.; Arcaro, K.; Spink, D.C. Understanding the human health effects of chemical mixtures. Environ. Health Perspect. 2002, 110 (Supplement 1), 25–42. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. TSCA Chemical Substance Inventory: Basic Information. Available online: http://www.epa.gov/oppt/existingchemicals/pubs/tscainventory/basic.html (accessed on 25 August 2015).
- Morrison, H.; Villeneuve, P.; Lubin, J.; Schaubel, D. Radon-progeny exposure and lung cancer risk in a cohort of Newfoundland fluorspar miners. Radiat. Res. 1998, 150, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Erren, T.C.; Jacobsen, M.; Piekarski, C. Synergy between asbestos and smoking on lung: Cancer risks. Epidemiology 1999, 10, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Backhaus, T.; Faust, M. Predictive environmental risk assessment of chemical mixtures: A conceptual framework. Environ. Sci. Technol. 2012, 46, 2564–2573. [Google Scholar] [CrossRef] [PubMed]
- Monosson, E. Chemical Mixtures: Considering the evolution of toxicology and chemical assessment. Environ. Health Perspect. 2005, 113, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Wölfle, D. Interactions between 2,3,7,8-TCDD and PCBs as Tumor Promoters: Limitations of TEFs. Teratog. Carcinog. Mutagen. 1997, 17, 217–224. [Google Scholar] [CrossRef]
- Birnbaum, L.S.; Staskal, D.F. Brominated Flame Retardants: Cause for Concern? Environ. Health Perspect. 2004, 112, 9–17. [Google Scholar] [CrossRef]
- Cox, P.; Efthymiou, P. Directive 2003/11/EC of the European Parliament and of the Council of February 6 2003 Amending for the 24th Time Council Directive 76/669/EEC Relating to Restrictions on the Marketing and use of Certain Dangerous Substances and Preparations (Pentabromodiphenyl Ether, Octabromodiphenyl Ether). Off. J. Eur. Union. OJ. L. 2003, 42, 45–46. [Google Scholar]
- Covaci, A.; Harrad, S.; Abdallah, M.A.; Ali, N.; Law, R.J.; Herzke, D.; de Wit, C.A. Novel brominated flame retardants: A review of their analysis, environmental fate and behaviour. Environ. Int. 2011, 37, 532–556. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Guan, Y.; Zhang, Y.; Luo, X.; Zhi, H.; Chen, S.; Mai, B. Several current-use, non-PBDE brominated flame retardants are highly bioaccumulative: Evidence from field determined bioaccumulation factors. Environ. Int. 2011, 37, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Breivik, K.; Sweetman, A.; Pacyna, J.M.; Jones, K.C. Towards a global historical emission inventory for selected PCB congeners—A mass balance approach: 3. An update. Sci. Total Environ. 2007, 377, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, M.; Vorkamp, K.; Thomsen, M.; Knudsen, L.E. Human internal and external exposure to PBDEs—A review of levels and sources. Int. J. Hyg. Environ. Health 2009, 212, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Meerts, I.A.; Letcher, R.J.; Hoving, S.; Marsh, G.; Bergman, A.; Lemmen, J.G.; van der Burg, B.; Brouwer, A. In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PDBEs, and polybrominated bisphenol a compounds. Environ. Health Perspect. 2001, 109, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Dingemans, M.M.; van den Berg, M.; Westerink, R.H. Neurotoxicity of brominated flame retardants: (In) direct effects of parent and hydroxylated polybrominated diphenyl ethers on the (developing) nervous system. Environ. Health Perspect. 2011, 119, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, S.B.; Wan, Y.; Chang, H.; Zhang, X.; Hecker, M.; Jones, P.D.; Giesy, J.P. Polybrominated diphenyl ethers and their hydroxylated/methoxylated analogs: Environmental sources, metabolic relationships, and relative toxicities. Mar. Pollut. Bull. 2011, 63, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Hanari, N.; Kannan, K.; Miyake, Y.; Okazawa, T.; Kodavanti, P.R.S.; Aldous, K.M.; Yamashita, N. Occurrence of polybrominated biphenyls, polybrominated dibenzo-p-dioxins, and polybrominated dibenzofurans as impurities in commercial polybrominated diphenyl ether mixtures. Environ. Sci. Technol. 2006, 40, 4400–4405. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. Draft: An Alternatives Assessment for the Flame Retardant Decabromodiphenyl Ether (DecaBDE). Available online: http://www.epa.gov/oppt/existingchemicals/pubs/actionplans/aa-for-deca-full-version.pdf (accessed on 25 August 2015).
- Venkatesan, A.K.; Halden, R.U. Wastewater treatment plants as chemical observatories to forecast ecological and human health risks of manmade chemicals. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Manahan, S.E. Green Chemistry and the Ten Commandments of Sustainability; ChemChar Research: Columbia, Missouri, MO, USA, 2011. [Google Scholar]
- Burgess, R.M.; Ho, K.T.; Brack, W.; Lamoree, M. Effects-directed analysis (EDA) and toxicity identification evaluation (TIE): Complementary but different approaches for diagnosing causes of environmental toxicity. Environ. Toxicol. Chem. 2013, 32, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Hartung, T. From Alternative Methods to a New Toxicology. Eur. J. Pharm. Biopharm. 2011, 77, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, L.M.; Marvin, C.H. Analytical Methods in environmental effects-directed investigations of effluents. Mutat Res Rev Mutat Res. 2005, 589, 208–232. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. Chemical Testing & Data Collection. Available online: http://www.epa.gov/oppt/chemtest/ (accessed on 25 August 2015).
- Kavlock, R.; Chandler, K.; Houck, K.; Hunter, S.; Judson, R.; Kleinstreuer, N.; Knudsen, T.; Martin, M.; Padilla, S.; Reif, D. Update on EPA’s ToxCast program: Providing high throughput decision support tools for chemical risk management. Chem. Res. Toxicol. 2012, 25, 1287–1302. [Google Scholar] [CrossRef] [PubMed]
- Macova, M.; Toze, S.; Hodgers, L.; Mueller, J.F.; Bartkow, M.; Escher, B.I. Bioanalytical tools for the evaluation of organic micropollutants during sewage treatment, water recycling and drinking water generation. Water Res. 2011, 45, 4238–4247. [Google Scholar] [CrossRef] [PubMed]
- Escher, B.I.; Leusch, F. Bioanalytical Tools in Water Quality Assessment; IWA Publishing: London, UK, 2012. [Google Scholar]
- Escher, B.I.; Allinson, M.; Altenburger, R.; Bain, P.A.; Balaguer, P.; Busch, W.; Crago, J.; Denslow, N.D.; Dopp, E.; Hilscherova, K. Benchmarking organic micropollutants in wastewater, recycled water and drinking water with in vitro bioassays. Environ. Sci. Technol. 2013, 48, 1940–1956. [Google Scholar] [CrossRef] [PubMed]
- Krauss, M.; Singer, H.; Hollender, J. LC-high resolution MS in environmental analysis: From target screening to the identification of unknowns. Anal. Bioanal. Chem. 2010, 397, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Altenburger, R.; Nendza, M.; Schüürmann, G. Mixture Toxicity and its Modeling by Quantitative Structure-Activity Relationships. Environ. Toxicol. Chem. 2003, 22, 1900–1915. [Google Scholar] [CrossRef] [PubMed]
- Kostal, J.; Voutchkova-Kostal, A.; Anastas, P.T.; Zimmerman, J.B. Identifying and designing chemicals with minimal acute aquatic toxicity. Proc. Natl. Acad. Sci. 2015, 112, 6289–6294. [Google Scholar] [CrossRef] [PubMed]
- Halden, R.U. Epistemology of Contaminants of Emerging Concern and Literature Meta-Analysis. J. Hazard. Mater. 2015, 282, 2–9. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatesan, A.K.; Halden, R.U. Effective Strategies for Monitoring and Regulating Chemical Mixtures and Contaminants Sharing Pathways of Toxicity. Int. J. Environ. Res. Public Health 2015, 12, 10549-10557. https://doi.org/10.3390/ijerph120910549
Venkatesan AK, Halden RU. Effective Strategies for Monitoring and Regulating Chemical Mixtures and Contaminants Sharing Pathways of Toxicity. International Journal of Environmental Research and Public Health. 2015; 12(9):10549-10557. https://doi.org/10.3390/ijerph120910549
Chicago/Turabian StyleVenkatesan, Arjun K., and Rolf U. Halden. 2015. "Effective Strategies for Monitoring and Regulating Chemical Mixtures and Contaminants Sharing Pathways of Toxicity" International Journal of Environmental Research and Public Health 12, no. 9: 10549-10557. https://doi.org/10.3390/ijerph120910549