Effects of Humic Acid and Suspended Solids on the Removal of Heavy Metals from Water by Adsorption onto Granular Activated Carbon
Abstract
:1. Introduction
2. Experimental Methodology
2.1. GAC
2.2. Humic Acid, Kaolinite and Chemicals
2.3. Batch Experiments
2.4. Fixed-Bed Column Experiments
3. Results and Discussion
3.1. Zeta Potential
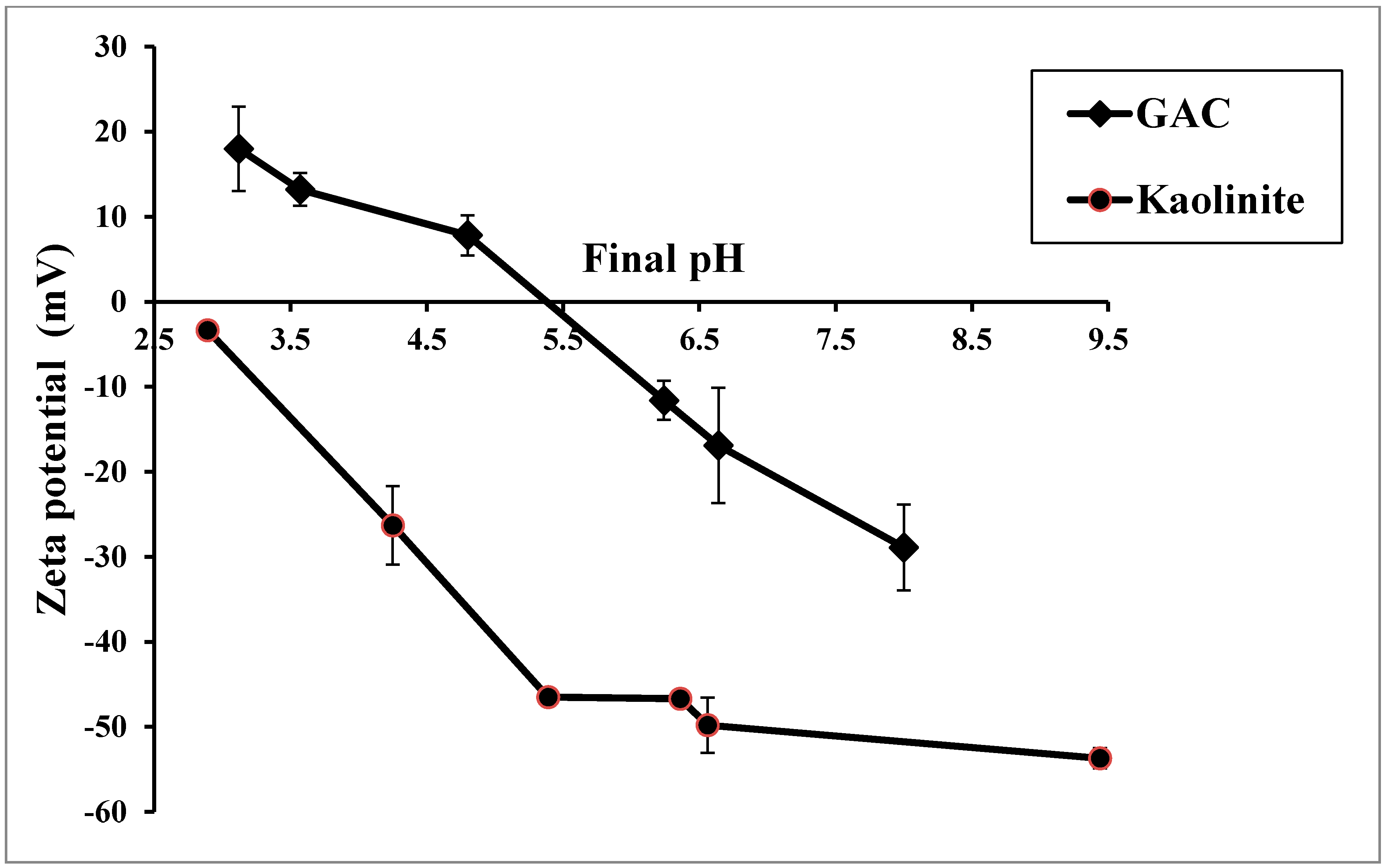
3.2. Heavy Metals Adsorption by GAC in Batch Experiments
| Metals | GAC | GAC + HA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qmax mg/g | KL L/mg | R2 | n | qe mg/g | qmax mg/g | KL L/mg | R2 | n | qe mg/g | |
| Cu | 11.8 | 42.0 | 0.99 | 6 | 11.0 | 3.7 | 67.2 | 0.99 | 6 | 3.5 |
| Pb | 11.9 | 1.8 | 0.91 | 6 | 8.1 | 7.5 | 1.7 | 0.75 | 7 | 4.0 |
| Zn | 3.3 | 6.1 | 0.94 | 5 | 2.8 | 3.2 | 5.7 | 0.96 | 7 | 2.6 |
| Ni | 1.8 | 15.3 | 0.98 | 6 | 1.3 | 2.5 | 19.9 | 0.98 | 6 | 2.0 |
| Cd | 2.0 | 4.4 | 0.95 | 7 | 0.9 | 1.6 | 8.7 | 0.99 | 6 | 0.9 |
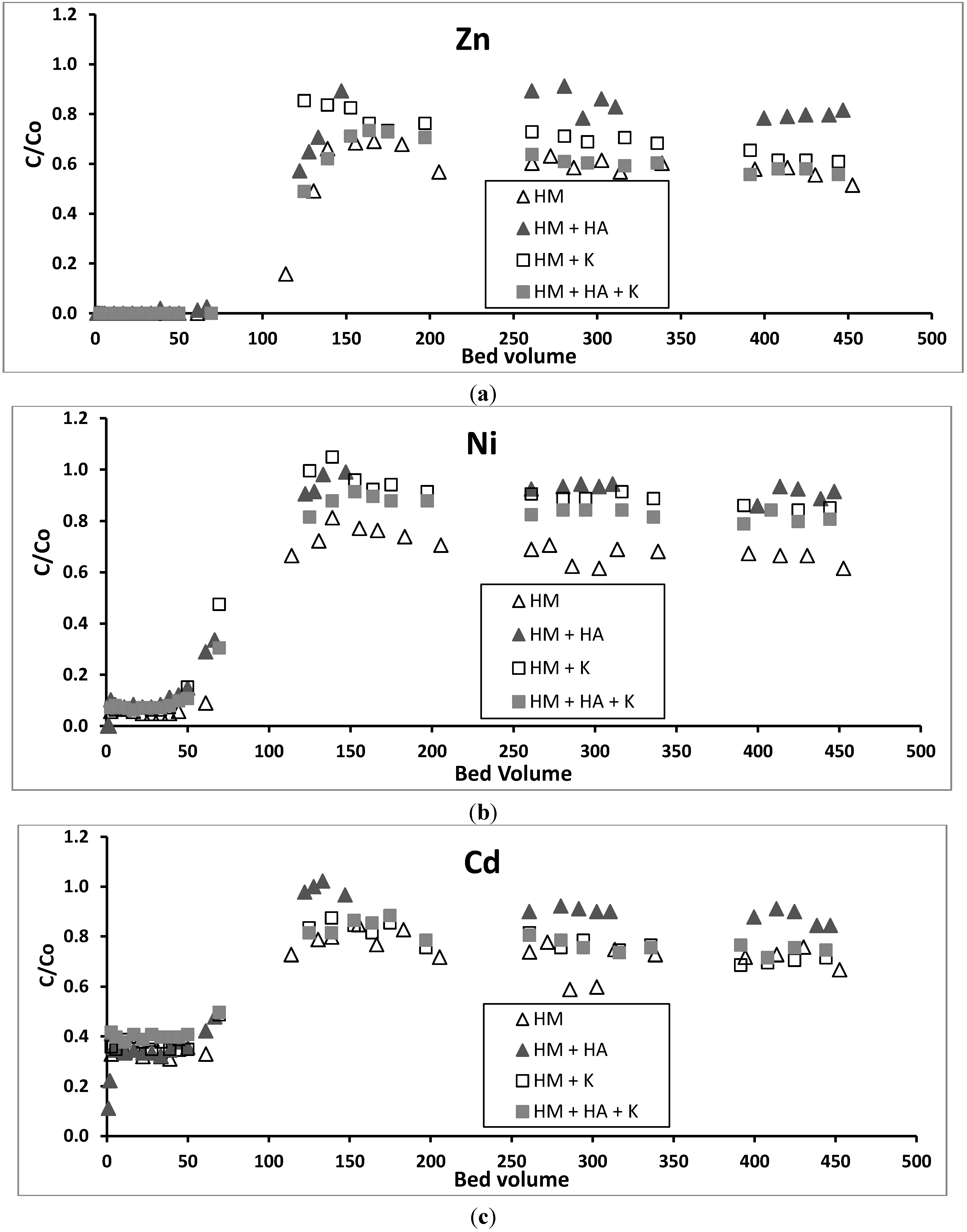
3.3. Heavy Metals Removal in Column Experiments in the Absence of HA and Kaolinite


| Zn | Cu | Cd | Ni | Pb | |
|---|---|---|---|---|---|
| HM only | 53.7 | 99.9 | 36.7 | 39.9 | 93.0 |
| HM + HA | 29.4 | 72.1 | 15.2 | 17.6 | 59.8 |
| HM + HA + K | 48.6 | 84.3 | 26.6 | 24.8 | 70.5 |
| HM + K | 39.2 | 98.0 | 28.1 | 18.1 | 86.7 |
3.4. Effect of HA on Removal of Heavy Metals in Column Experiments

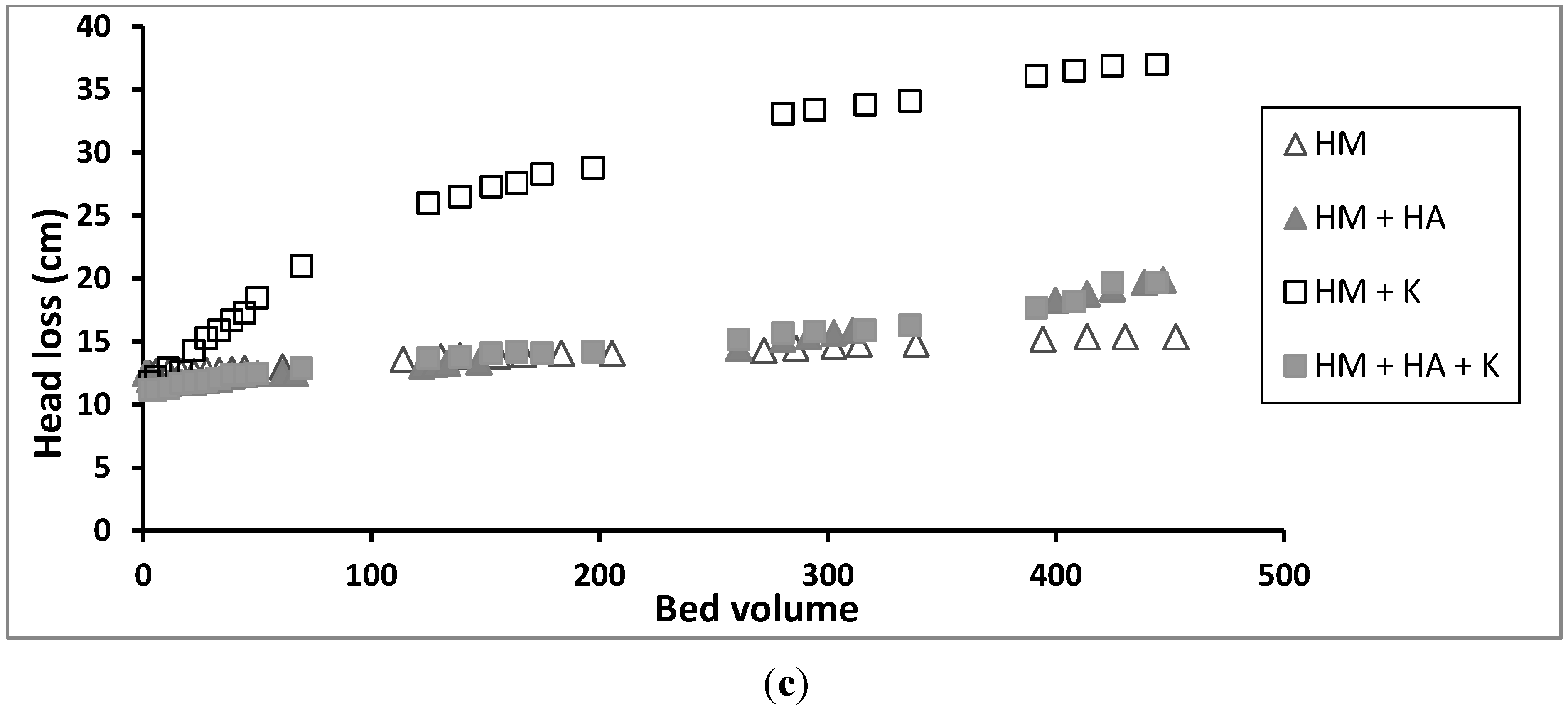
3.5. Effect of Kaolinite on Removal of Heavy Metals in Column Experiments
3.6. Effect of HA and Kaolinite together on Removal of Heavy Metals in Column Experiments
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Glover-Kerkvilet, J. Environmental assault on immunity. Environ. Health Persp. 1995, 103, 236–237. [Google Scholar] [CrossRef]
- Chen, J.P. Decontamination of Heavy Metals: Processes, Mechanisms, and Applications; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Roesner, L.A.; Charles, R.A. National storm water quality regulations and standards. J. Hydraul. Res. 1996, 34, 841–856. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I. Environmental Water: Advances in Treatment, Remediation and Recycling; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Pollard, S.J.T.; Fowler, G.D.; Sollars, C.J.; Perry, R. Low-cost adsorbents for waste and wastewater treatment: A review. Sci. Total Environ. 1992, 116, 31–52. [Google Scholar] [CrossRef]
- Yin, C.Y.; Aroua, M.K.; Daud, W.M.A.W. Review of modifications of activated carbon for enhancing contaminant uptakes from aqueous solutions. Sep. Purif. Technol. 2007, 52, 403–415. [Google Scholar] [CrossRef]
- Kim, J.; Jung, S. Soluble manganese removal by porous media filtration. Environ. Technol. 2008, 29, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Thiruvenkatachari, R.; Vigneswaran, S.; Naidu, R. Permeable reactive barrier for groundwater remediation. J. Ind. Eng. Chem. 2008, 14, 145–156. [Google Scholar] [CrossRef]
- Goel, J.; Kadirvelu, K.; Garg, V.K.; Meena, A.; Chopra, R.; Chauhan, D.; Rawat, A.; Kumar, S.; Mishra, G.K.; Rajagopal, C. A pilot scale evaluation for adsorptive removal of lead (II) using treated granular activated carbon. Environ. Technol. 2005, 26, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Sounthararajah, D.P.; Loganathan, P.; Kandasamy, J.; Vigneswaran, S. Column studies on the removal of dissolved organic carbon, turbidity and heavy metals from stormwater using granular activated carbon. Desalin. Water. Treat. 2015. [Google Scholar] [CrossRef]
- Chingombe, P.; Saha, B.; Wakeman, R.J. Surface modification and characterisation of a coal-based activated carbon. Carbon 2005, 43, 3132–3143. [Google Scholar] [CrossRef]
- Cougnaud, A.; Faur, C.; Le Cloirec, P. Removal of pesticides from aqueous solution: Quantitative relationship between activated carbon characteristics and adsorption properties. Environ. Technol. 2005, 26, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.P.; Wu, S. Simultaneous adsorption of copper ions and humic acid onto an activated carbon. J. Colloid Interface Sci. 2004, 280, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.G.; Owens, P.N. Sediments in urban river basins: A review of sediment-contaminant dynamics in an environmental system conditioned by human activities. J. Soil. Sediment. 2009, 9, 281–303. [Google Scholar] [CrossRef]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J. Road-deposited sediments pollutants: A critical review of their characteristics, source apportionment, and management. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1315–1348. [Google Scholar] [CrossRef]
- Divakaran, R.; Pillai, S.V.N. Flocculation of kaolinite suspensions in water by chitosan. Water Res. 2001, 35, 3904–3908. [Google Scholar] [CrossRef]
- Lin, E.; Page, D.; Pavelic, P.; Dillon, P.; McClure, S.; Hutson, J. Evaluation of Roughing Filtration for Pre-Treatment of Stormwater Prior to Aquifer Storage and Recovery (ASR); CSIRO Land and Water Science Report 03/06; CSIRO: Burnsite, Australia, 2006; p. 224. [Google Scholar]
- Kus, B.; Kandasamy, J.; Vigneswaran, S.; Shon, H.; Moody, G. Two stage filtration for stormwater treatment: A pilot scale study. Desalin. Water. Treat. 2012, 45, 361–369. [Google Scholar] [CrossRef]
- Mohammed, T.; Vigneswaran, S.; Loganathan, P.; Kandasamy, J.; Aryal, R. Removal of inorganic contaminants from simulated stormwater by three sorbents in columns under intermittent runoff condition. Separ. Sci. Technol. 2012, 47, 2340–2347. [Google Scholar]
- Chen, J.P.; Yoon, J.T.; Yiacoumi, S. Effects of chemical and physical properties of influent on copper sorption onto activated carbon fixed-bed columns. Carbon 2003, 41, 1635–1644. [Google Scholar] [CrossRef]
- Wong, T.H.F.; Breen, P.F.; Lloyd, S.D. Water Sensitive Road Design: Design Options for Improving Stormwater Quality of Road Runoff; CRC for Catchment Hydrology: Melburne, Australia, 2000. [Google Scholar]
- Aryal, R.; Lee, B.K. Characteristics of suspended solids and micropollutants in first-flush highway runoff. Water Air Soil Pollut. 2009, 9, 339–346. [Google Scholar] [CrossRef]
- Nie, F.; Li, T.; Yao, H.; Feng, M.; Zhang, G. Characterization of suspended solids and particle-bound heavy metals in a first flush of highway runoff. J. Zhejiang. Univ. Sci. A 2008, 9, 1567–1575. [Google Scholar] [CrossRef]
- ANZECC; ARMCANZ. Australian and New Zealand Guidelines for Fresh and Marine Water Quality; Australian and New Zealand Environment and Conservation Council and Agriculture and Resource Management Council of Australia and New Zealand: Canberra, Australia, 2000; pp. 1–103. [Google Scholar]
- Faust, S.D.; Aly, O.M. Adsorption Processes for Water Treatment; Butterworth: Boston, MA, USA, 1987. [Google Scholar]
- Nur, T.; Loganathan, P.; Nguyen, T.C.; Vigneswaran, S.; Singh, G.; Kandasamy, J. Batch and column adsorption and desorption of fluoride using hydrous ferric oxide: Solution chemistry and modeling. Chem. Eng. J. 2014, 247, 93–102. [Google Scholar] [CrossRef]
- Rao, S.R. Resource Recovery and Recycling from Metallurgical Wastes; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Walker, J.D.; Newman, M.C.; Enache, M. Fundamental QSARs for Metal Ions; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Pohlmeier, A. Metal speciation, chelation and complexing ligands in plants. In Heavy Metal Stress in Plants; Springer: Berlin, Germany, 2004; pp. 28–46. [Google Scholar]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Bolan, N.S. Removal and recovery of phosphate from water using sorption. Crit. Rev. Environ. Sci. Technol. 2014, 44, 847–907. [Google Scholar] [CrossRef]
- Sholkovitz, E.R.; Copland, D. The coagulation, solubility and adsorption properties of Fe, Mn, Cu, Ni, Cd, Co and humic acids in a river water. Geochim. Cosmochim. Acta 1981, 45, 181–189. [Google Scholar] [CrossRef]
- Pandey, A.K.; Pandey, S.D.; Misra, V. Stability constants of metal–humic acid complexes and its role in environmental detoxification. Ecotox. Environ. Safe. 2000, 47, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.P.; Wang, L. Characterization of metal adsorption kinetic properties in batch and fixed-bed reactors. Chemosphere 2004, 54, 397–404. [Google Scholar] [CrossRef]
- Reed, B.E.; Arunachalam, S.; Thomas, B. Removal of lead and cadmium from aqueous waste streams using granular activated carbon (GAC) columns. Environ. Prog. 1994, 13, 60–64. [Google Scholar] [CrossRef]
- Ghorai, S.; Pant, K.K. Investigations on the column performance of fluoride adsorption by activated alumina in a fixed-bed. Chem. Eng. J. 2004, 98, 165–173. [Google Scholar] [CrossRef]
- Akbour, R.A.; Douch, J.; Hamdani, M.; Schmitz, P. Transport of kaolinite colloids through quartz sand: Influence of humic acid, Ca2+, and trace metals. J. Colloid Interface Sci. 2002, 253, 1–8. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sounthararajah, D.P.; Loganathan, P.; Kandasamy, J.; Vigneswaran, S. Effects of Humic Acid and Suspended Solids on the Removal of Heavy Metals from Water by Adsorption onto Granular Activated Carbon. Int. J. Environ. Res. Public Health 2015, 12, 10475-10489. https://doi.org/10.3390/ijerph120910475
Sounthararajah DP, Loganathan P, Kandasamy J, Vigneswaran S. Effects of Humic Acid and Suspended Solids on the Removal of Heavy Metals from Water by Adsorption onto Granular Activated Carbon. International Journal of Environmental Research and Public Health. 2015; 12(9):10475-10489. https://doi.org/10.3390/ijerph120910475
Chicago/Turabian StyleSounthararajah, Danious P., Paripurnanda Loganathan, Jaya Kandasamy, and Saravanamuthu Vigneswaran. 2015. "Effects of Humic Acid and Suspended Solids on the Removal of Heavy Metals from Water by Adsorption onto Granular Activated Carbon" International Journal of Environmental Research and Public Health 12, no. 9: 10475-10489. https://doi.org/10.3390/ijerph120910475






