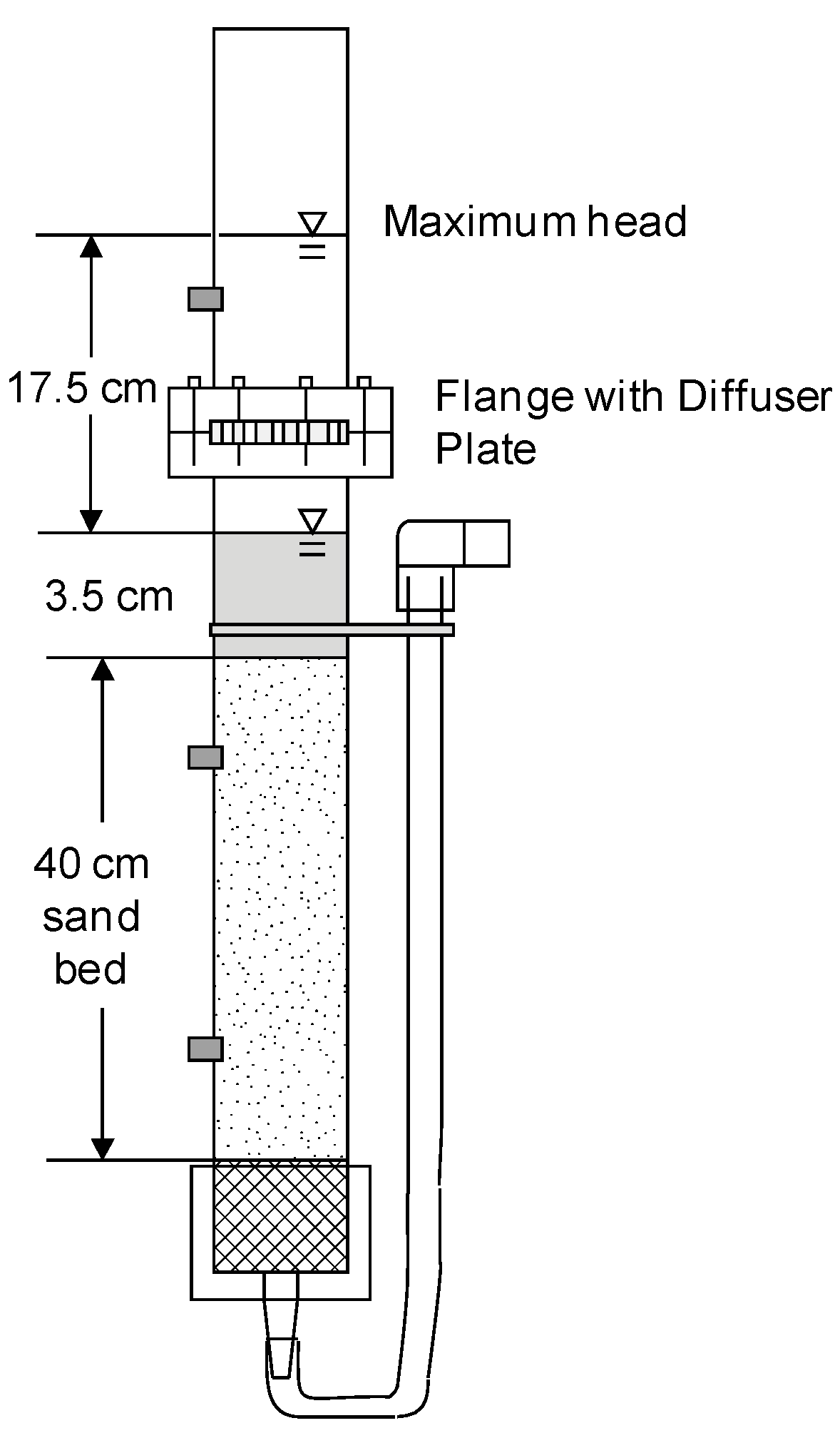
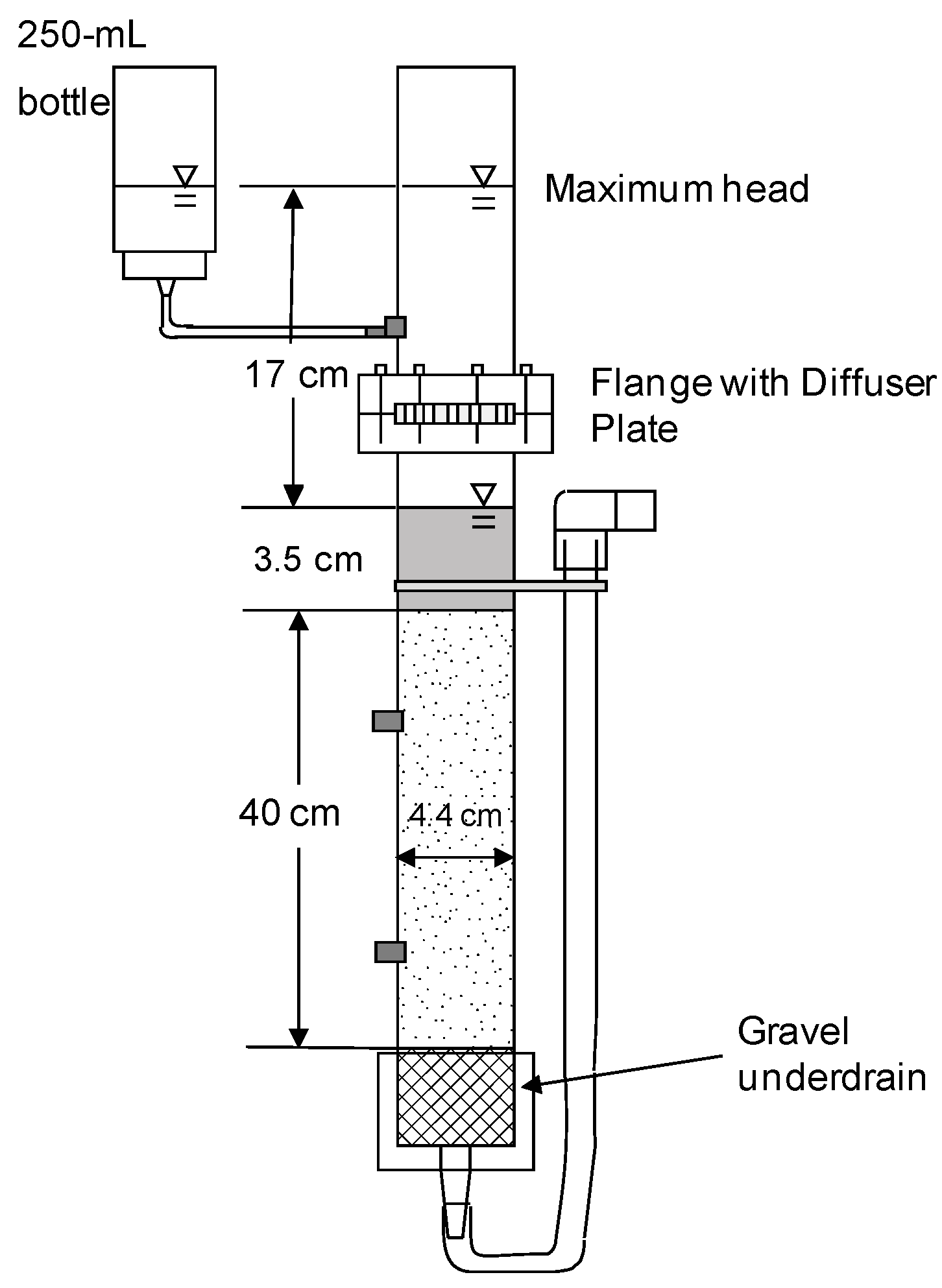
3.1. Comparison of Filter Media Characteristics
The inorganic composition of the granite and Accusand filter media is presented in
Table 4. Concentrations (mg/kg) of metals (Ca, Mg, Mn, Al, and Fe) were two orders of magnitude higher on granite than on Accusand. Granite is generally composed of only 72% SiO
2, with substantial quantities of Al
2O
3 (14.4%), CaO (1.82%), FeO (1.68%), Fe
2O
3 (1.22%), MgO (0.71%) and MnO (0.05%) [
40].
The large difference in media composition, especially of Al and Fe, could have implications for virus attachment. Aluminum and ferric oxyhydroxide surfaces tend to carry a net positive charge at near-neutral pH conditions and thus adsorb viruses that are negatively charged. The region of pH where surface charge is positive is quantified by the point of zero charge (PZC) value. The PZC is about 2.0 for SiO
2 in contrast to 9.1 for Al oxides (α-Al
2O
3), 6.7 for γ-Fe
2O
3 and 8.5 for amorphous Fe(OH)
3 [
41]. These differences in PZC would suggest less reduction of viruses in filtration through pure SiO
2 (Accusand) than granite. Whether elemental composition influences microbial reduction is discussed in
Section 3.3 and
Section 3.4.
Table 4.
Elemental analysis of the two media used in filter tests.
Table 4.
Elemental analysis of the two media used in filter tests.
| | Granite (mg/kg) | Accusand (mg/kg) |
|---|
| Calcium (Ca) | 12,270 | 90 |
| Magnesium (Mg) | 14,875 | 60 |
| Manganese (Mn) | 920 | 0 |
| Iron (Fe) | 23,250 | 55 |
| Aluminum (Al) | 17.03 | 90 |
3.2. Effects of Backwashing on Grain Size Distribution and Hydraulic Characteristics
Visual inspection of the media packing during Column Test No. 1 revealed considerable variation in the grain size composition of the topmost layer among those columns filled with granite. Differences in grain size were not a major concern from the standpoint of hydraulic performance given that clean-bed head loss and starting filtration rate were similar in all columns. Nevertheless, these factors could reduce replicability among columns loaded with granite media and could affect particle trapping and microbial attachment near the media surface, potentially leading to variability in the rate of schmutzdecke development. Therefore, all six columns were backwashed prior to Column Test No. 2 and before measurement of the grain size distributions. The backwashing procedure consisted of 20%–30% expansion for 20 min. Visual observation showed removal of a substantial amount of very fine silty material from the granite loaded columns. The mass of the silty material that was backwashed out could not be quantified directly. However, a sieve analysis of pre-backwashed granite media showed that silt (grains <0.0625 mm) constituted <1.4% of the total granite media mass (following the washing steps described in [
25]).
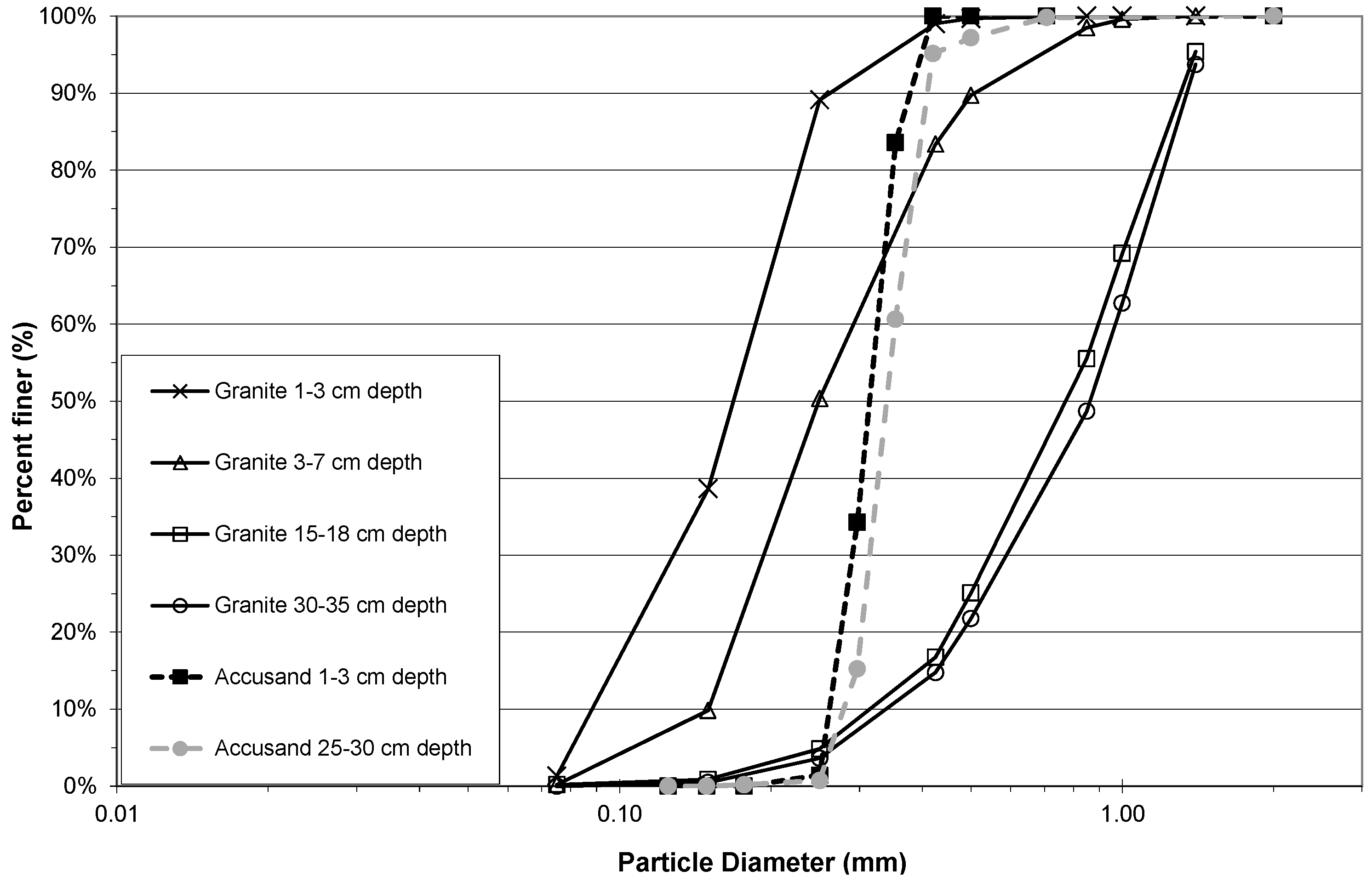
The Column Test No. 2 grain size distributions of granite and Accusand media at different filter depths after backwashing are compared in
Figure 3. The effect of backwashing of the columns filled with granite medium was to cause substantial depth stratification of grain sizes. The cumulative distribution showed that 90% of those near the top of the media were <0.2 mm in diameter compared with <1.3 mm near the bottom of the media. A large difference in grain size distributions between granite and Accusand media was still apparent (
Figure 3) even though the backwash procedure had removed much of the silty fraction from the granite loaded columns. This was to be expected because the granite was crushed and then sieved following the crude procedure that was recommended at that time for field installation of BSF [
25]. In contrast, the Accusand was carefully sieved by Unimin Corp. into narrow size fractions that were then blended together by the manufacturer [
27].
Besides removing silty fines, backwashing of the granite media changed the hydraulic characteristics as shown by comparison of MDI values in
Table 5. In Column Test No. 1, the MDI was close to 1.0 in both granite-filled and Accusand-filled columns and this indicates conditions very close to perfect plug flow (
i.e., very little longitudinal dispersion). The same was observed from tracer tests on full-scale BSFs [
10]. However, the MDI obtained for the granite-filled columns after backwashing increased from 1.3 to greater than 2.2 while it remained the same for the Accusand filled columns. The increase in MDI was most likely due to the development of preferential flow paths as the packing configuration shifted from unstratified (not shown) to stratified (
Figure 3).
Figure 3.
Sieve analysis of backwashed Accusand and granite media from Column Test No. 2.
Figure 3.
Sieve analysis of backwashed Accusand and granite media from Column Test No. 2.
Table 5.
Morrill Dispersion Index (MDI) for tracer tests conducted prior to Column Tests No. 1 and No. 2. MDI of 1.0 corresponds to perfect plug flow; <2.0 is effective plug flow according to EPA (1996).
Table 5.
Morrill Dispersion Index (MDI) for tracer tests conducted prior to Column Tests No. 1 and No. 2. MDI of 1.0 corresponds to perfect plug flow; <2.0 is effective plug flow according to EPA (1996).
| Replicate | | |
|---|
| Columns in | Column | Column |
| Each Test | Test No. 1 | Test No. 2 |
| Granite #1 | 1.31 | 2.24 |
| Granite #2 | 1.31 | 2.66 |
| Granite #3 | 1.29 | 3.10 |
| Accusand #1 | 1.16 | 1.19 |
| Accusand #2 | 1.22 | 1.36 |
| Accusand #3 | 1.16 | 1.25 |
Backwashing was intended to improve the replicability between parallel columns; it made no discernable difference in replicability and brought a number of other disadvantages. Backwashing caused preferential flow paths through the granite filter bed, causing the filters to operate at hydraulic conditions much further from plug flow than conventionally loaded BSFs. Much of the finest fraction of media was eliminated by backwashing the granite columns; this may have adversely impacted virus reductions as described below. Additionally, stratification of media is not recommended in the current guidance on BSF construction. Backwashing is not recommended for BSF laboratory studies.
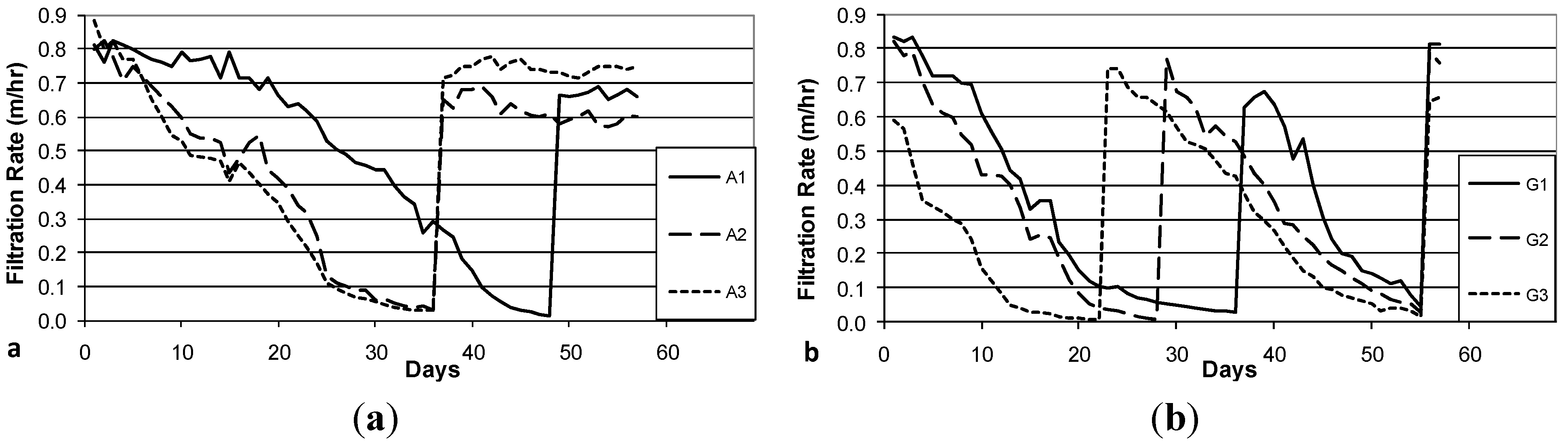
3.3. Decline in Filtration Rate with Filter Operation
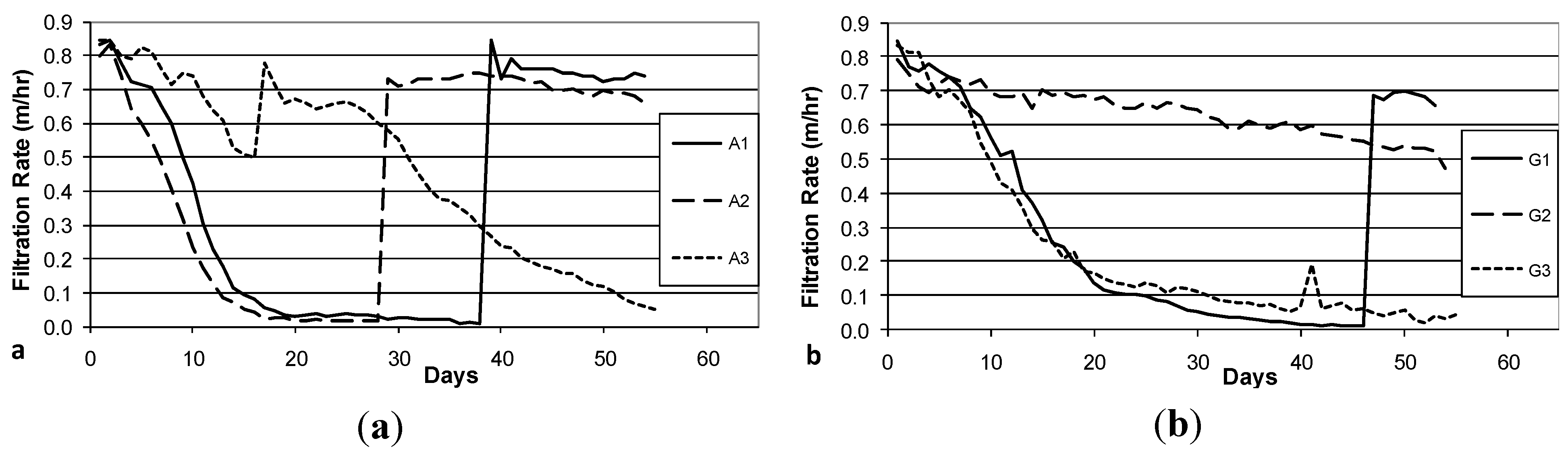
The decline in filtration rate during Column Test No. 1 is presented in
Figure 4a (Accusand media) and 4b (granite media). Filter maturation due to growth of the schmutzdecke and particle trapping increases head loss and thus decreases filtration rate. However, head loss did not develop at the same rates in each of the three parallel columns with the same media: the decline was far more rapid in Columns A1 and A2 than in Column A3 and in Columns G1 and G3 than in Column G2. In fact, the filtration rate in Columns A3 and G3 increased briefly (Days 16 and 41, respectively) due to unintentional disturbance of the schmutzdecke during filter charging (this disturbance may have been caused by the shallow depth of standing water, as noted in the Methods section). The surface of the media bed in columns A1, A2 and G1 were intentionally scoured to remove the schmutzdecke when the filtration rate decreased to approximately 10% of the initial maximum. This cleaning procedure produced a rapid increase in filtration rate from less than 0.1 m/h to 0.6–0.9 m/h. As noted in the Methods section, current BSF design guidance calls for filtration rates no greater than 0.4 m/h [
24]; these experiments were conducted using the BSF design standards at the time [
26].
Figure 4.
Initial daily filtration rate during Column Test No. 1. Accusand-loaded filter columns A1-A3 (a); and Granite-loaded filter columns G1-G3 (b). Spikes in filtration rate are due to either intentional removal (cleaning) of the schmutzdecke (A1 day 39, A2 day 29, G1 day 47) or unintentional disturbance of the schmutzdecke during filter loading (A3 day 17, G3 day 41).
Figure 4.
Initial daily filtration rate during Column Test No. 1. Accusand-loaded filter columns A1-A3 (a); and Granite-loaded filter columns G1-G3 (b). Spikes in filtration rate are due to either intentional removal (cleaning) of the schmutzdecke (A1 day 39, A2 day 29, G1 day 47) or unintentional disturbance of the schmutzdecke during filter loading (A3 day 17, G3 day 41).
Figure 5.
Initial daily filtration rate during Column Test No. 2. Accusand-loaded filter columns A1-A3 (a); and Granite-loaded filter columns G1-G3 (b). Spikes in filtration rate are due to intentional removal (cleaning) of the schmutzdecke (A1 day 49; A2 day 37; A3 day 37; G1 days 37 and 56; G2 days 29 and 56; G3 days 23 and 56).
Figure 5.
Initial daily filtration rate during Column Test No. 2. Accusand-loaded filter columns A1-A3 (a); and Granite-loaded filter columns G1-G3 (b). Spikes in filtration rate are due to intentional removal (cleaning) of the schmutzdecke (A1 day 49; A2 day 37; A3 day 37; G1 days 37 and 56; G2 days 29 and 56; G3 days 23 and 56).
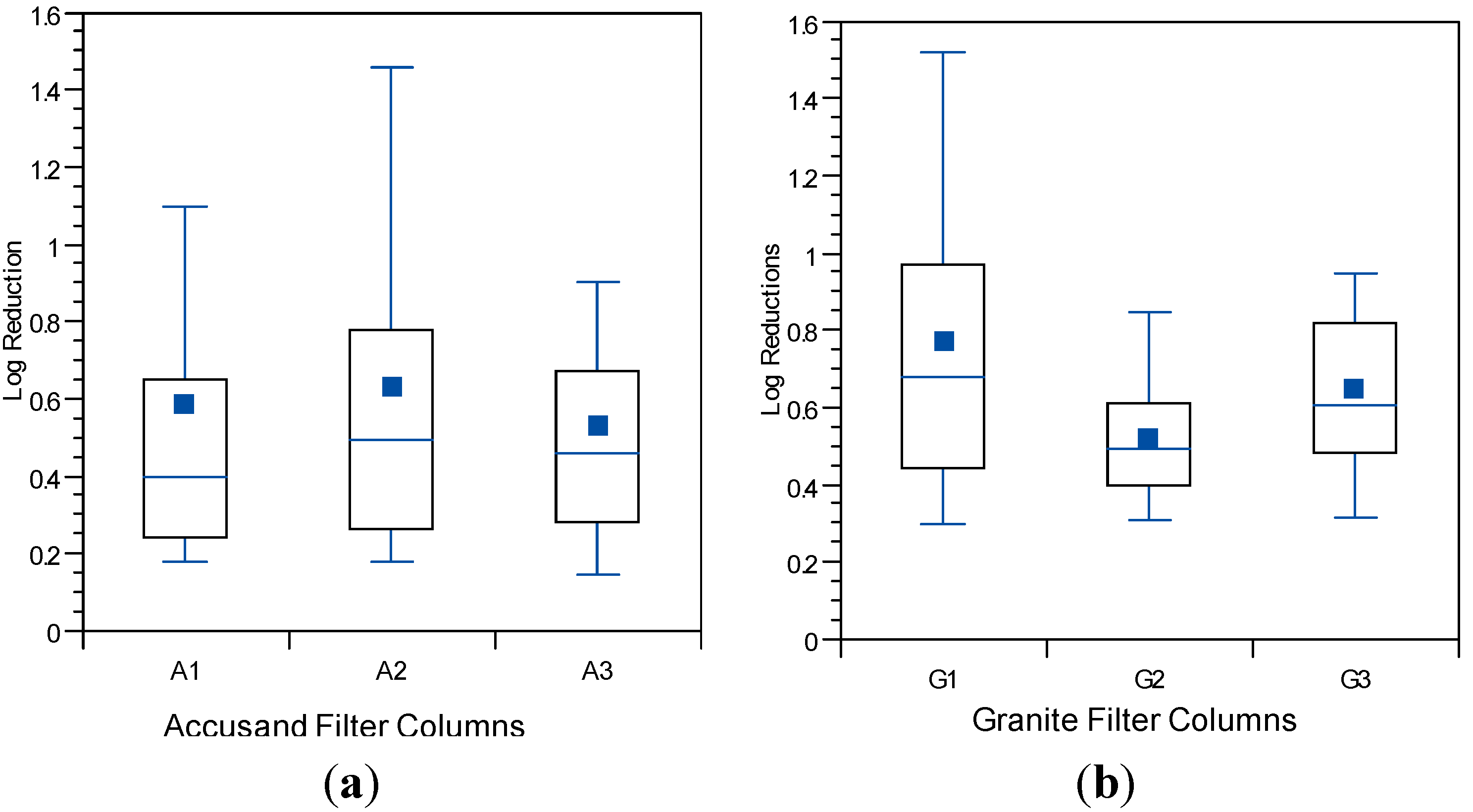
The decline in filtration rate for each filter column during Column Test No. 2 is presented in
Figure 5a (Accusand media), 5b (granite media). The reproducibility of filtration rate decline improved among replicate columns containing the granite media over that observed in Column Test No. 1 (
Figure 4b). The improvement could be due to backwashing which may have made the media size more consistent. Filtration rates also seem more reproducible among replicate columns containing Accusand than seen in Column Test No. 1 (
Figure 4b) although this may not be explained by backwashing as the grain size for Accusand was relatively uniform (
Figure 3).
Despite some variability still evident in Column Test No. 2, the filtration rate decline for columns filled with the granite media appears faster than with the decline for Accusand columns. Head loss development may be more rapid in the granite than Accusand media due to smaller grains sizes of granite near the surface of the column where particles are efficiently entrapped. Particle entrapment may not influence head loss development in the Accusand columns due to larger and more uniform grain size. Instead, head loss development may be caused mainly by microbial growth in the schmutzdecke.
The filtration rate decline, particularly for columns containing Accusand (
Figure 4a and
Figure 5a), appears slower following the scouring of the schmutzdecke. The implication is retarded regrowth of the schmutzdecke compared to startup of the column test. Microorganisms that contribute to schmutzdecke growth are derived from the feed water. Therefore, one possible explanation for retarded schmutzdecke growth could be microbial inactivation that may have occurred during storage of the feed water for many weeks at 4 °C prior to the scouring event. The granite columns in Test No. 2 recovered head loss after scouring at a similar rate to startup (
Figure 5b); it is possible that the much finer grains in the top layer of the backwashed granite (
Figure 3) were better able to retain, during scouring, the organisms responsible for schmutzdecke growth.
3.4. Reductions in Challenge E. coli
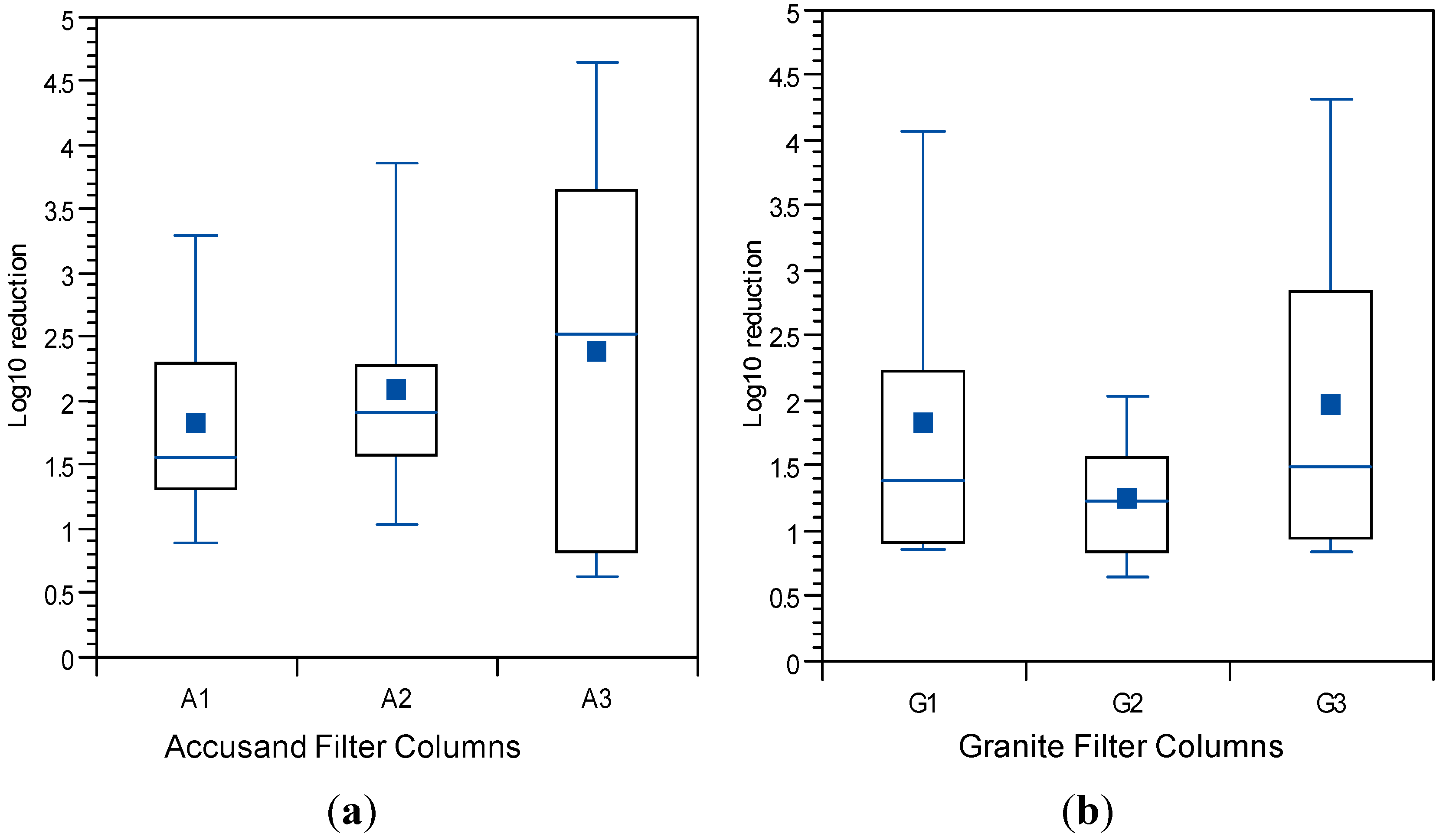
The
E. coli reductions from each column over the eight weeks of operation in Column Tests No. 1 and Test No. 2 are summarized in the box and whisker plots shown in
Figure 6a (Accusand media) and
Figure 6b (granite media) in Column Test No. 1 and in
Figure 7a (Accusand media) and
Figure 7b (granite media) in Column Test No. 2. Reductions ranged from less than 1-log to greater than 5-log and the variability is both within each column and among replicate columns. Maturation is primarily responsible for variability within each column. Variability among columns containing the same media could be due to differences in head loss development, as indicated by differences in filtration rate decline (
Figure 4 and
Figure 5); increases in filtration rate indicate either intentional removal or unintentional disturbance of the schmutzdecke as noted in the figures.
The effect of filtration media on
E. coli reduction can be qualitatively compared through box and whisker plots in
Figure 6a,b (Column Test No. 1) and in
Figure 7a,b (Column Test No. 2). No significant differences in
E. coli reductions were found between the two media types in either Column Test No. 1 (
p = 0.11) or Test No. 2 (
p = 0.965). The box and whisker plots show that the range in reductions over each column experiment were similar for the Accusand and granite media despite the fact that the (1) the granite was much more angular and (2) metals content was at least two orders of magnitude higher for granite than the Accusand media (
Table 4). Increased angularity can lead to increased straining and wedging of colloids in saturated media [
42]. Metals in the form of iron and aluminum oxide as well as hydroxide coatings have been shown to enhance bacterial reductions [
43,
44]. However, the chemical form of the iron and aluminum on the surface of the granite media is unknown. Additionally, dissolved organic matter commonly found in surface waters has been shown to block the metal oxide and hydroxide sites that can sorb
E. coli [
45]. Therefore, while enhanced bacterial reduction due to metal oxides and hydroxides is possible, it likely to be short-lived and sensitive to feed water quality.
Figure 6.
E. coli reductions for (a) Accusand-filled columns; and (b) granite-filled columns in Column Test No. 1 for samples collected throughout the eight-week experiment. N = 10 for G3; 11 for A1, A2 and G1; 12 for A3; 13 for G2.
Figure 6.
E. coli reductions for (a) Accusand-filled columns; and (b) granite-filled columns in Column Test No. 1 for samples collected throughout the eight-week experiment. N = 10 for G3; 11 for A1, A2 and G1; 12 for A3; 13 for G2.
Figure 7.
E. coli reductions for (a) Accusand-filled columns; and (b) granite-filled columns in Column Test No. 2 for samples collected throughout the eight-week experiment. N = 17 for G2; 18 for A1; 19 for A2; 20 for G1, G3 and A3.
Figure 7.
E. coli reductions for (a) Accusand-filled columns; and (b) granite-filled columns in Column Test No. 2 for samples collected throughout the eight-week experiment. N = 17 for G2; 18 for A1; 19 for A2; 20 for G1, G3 and A3.
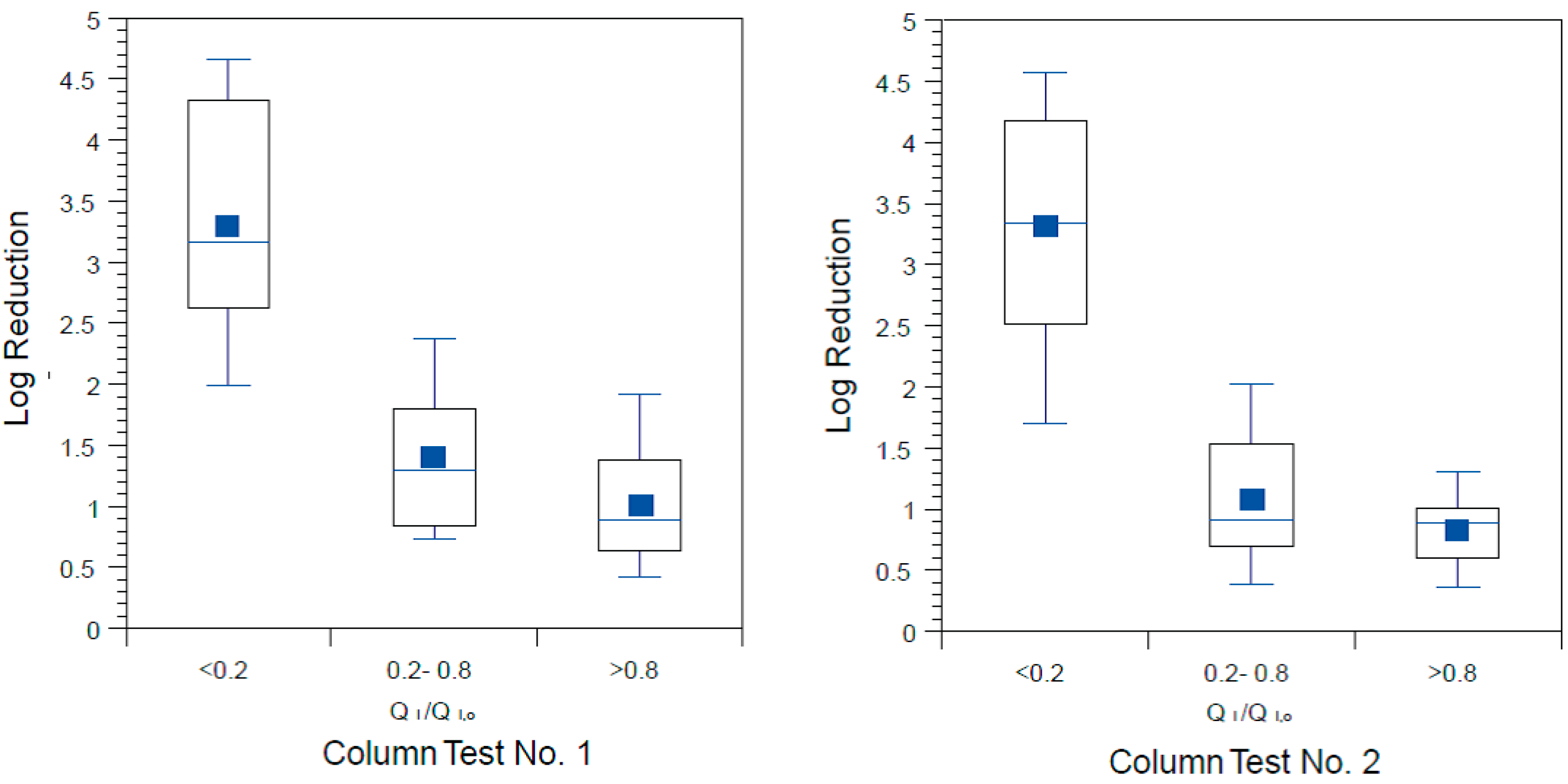
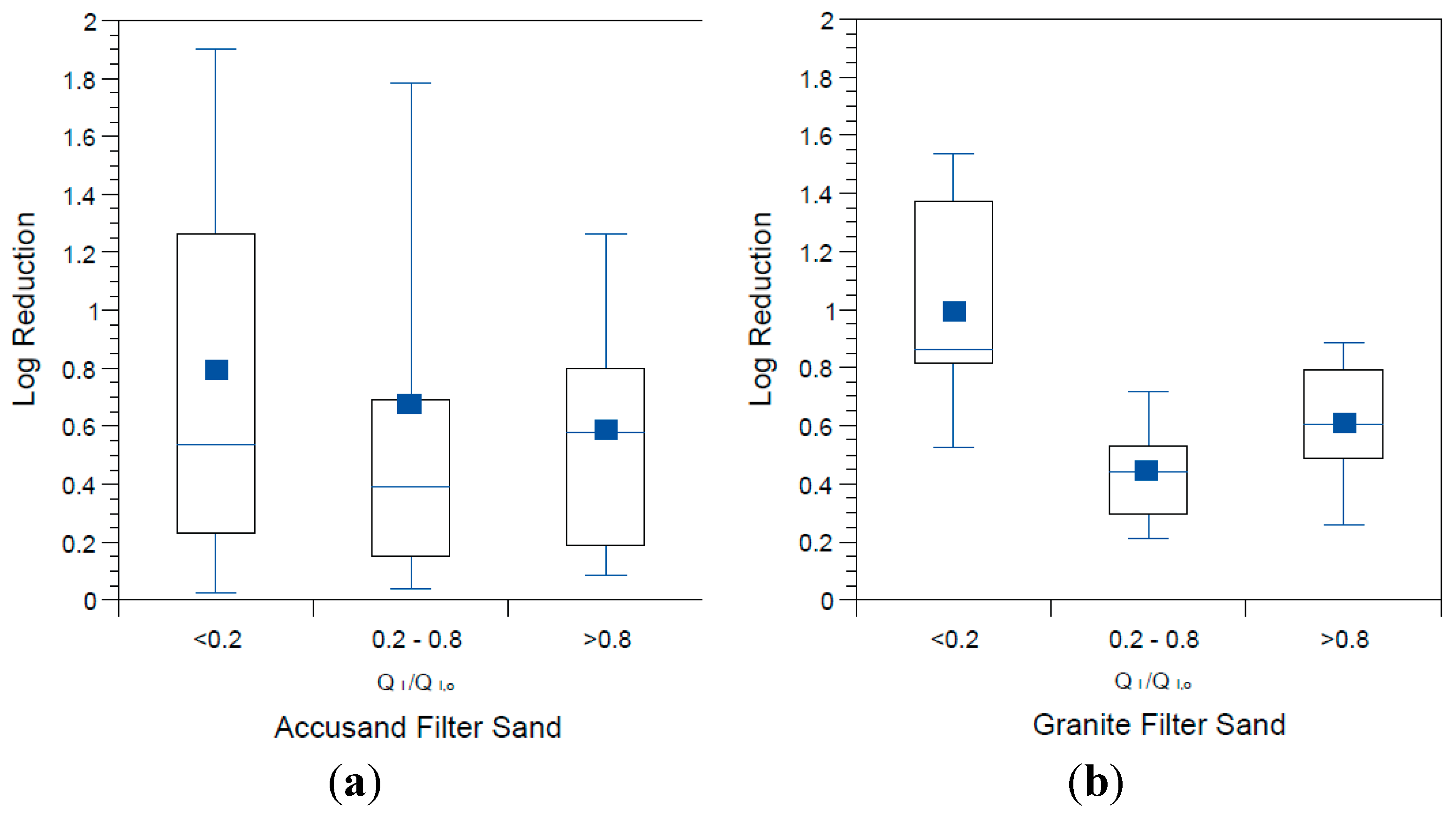
The influence of schmutzdecke development on
E. coli reductions in Column Tests No. 1 and No. 2 is shown in
Figure 8a,b, respectively. In these box and whisker plots, the extent of schmutzdecke development is expressed by normalized filtration rate, Q
I/Q
I,o where Q
I is the initial daily filtration rate (
i.e., upon introduction of the daily charge) for each day and Q
I,o is the initial daily filtration rate on the first day of operation. Normalized filtration rates during the eight weeks of operation were classified into three bins as shown in the figures. The largest Q
I/Q
I,o bin (values greater than 0.8) represents samples taken during the stages of column operation without a substantial schmutzdecke (the earliest samples and those shortly after schmutzdecke scouring) while the smallest Q
I/Q
I,o bin (values less than 0.2) represents stage of column operation when the schmutzdecke is fully formed. Log reductions of
E. coli increased substantially as Q
I/Q
I,o declined, as illustrated in
Figure 8; comparing samples collected on days with Q
I/Q
I,o > 0.2 to those < 0.2 yielded a highly significant difference for both Accusand and granite columns (
p < 0.0001). These results demonstrate the importance of schmutzdecke growth for increasing microbial reductions through (1) physical straining and/or (2) decreasing flow rate (caused by increased head loss) leading to more efficient depth filtration. Physical straining is likely to be important given that the top few centimeters of media surface where the schmutzdecke develops has been shown to be responsible for enhanced bacterial reductions [
46,
47]. Furthermore, it has been reported that slowing the flow rate by using an outlet valve also improved
E. coli reductions [
14]. However, the relative contributions of these two mechanisms of bacterial reductions require further investigation.
Figure 8.
E. coli reductions in Column Test No. 1 and Column Test No. 2 organized by bins of normalized filtration rate where QI is the initial daily filtration rate on each day of charging the column and QI,o is the comparable initial filtration rate on the first day. N for Column Test No. 1: 14 for <0.2, 13 for 0.2–0.8, 23 for >0.8. N for Column Test No. 2: 15 for <0.2, 34 for 0.2–0.8, 30 for >0.8.
Figure 8.
E. coli reductions in Column Test No. 1 and Column Test No. 2 organized by bins of normalized filtration rate where QI is the initial daily filtration rate on each day of charging the column and QI,o is the comparable initial filtration rate on the first day. N for Column Test No. 1: 14 for <0.2, 13 for 0.2–0.8, 23 for >0.8. N for Column Test No. 2: 15 for <0.2, 34 for 0.2–0.8, 30 for >0.8.
3.5. Reductions in Challenge Viruses
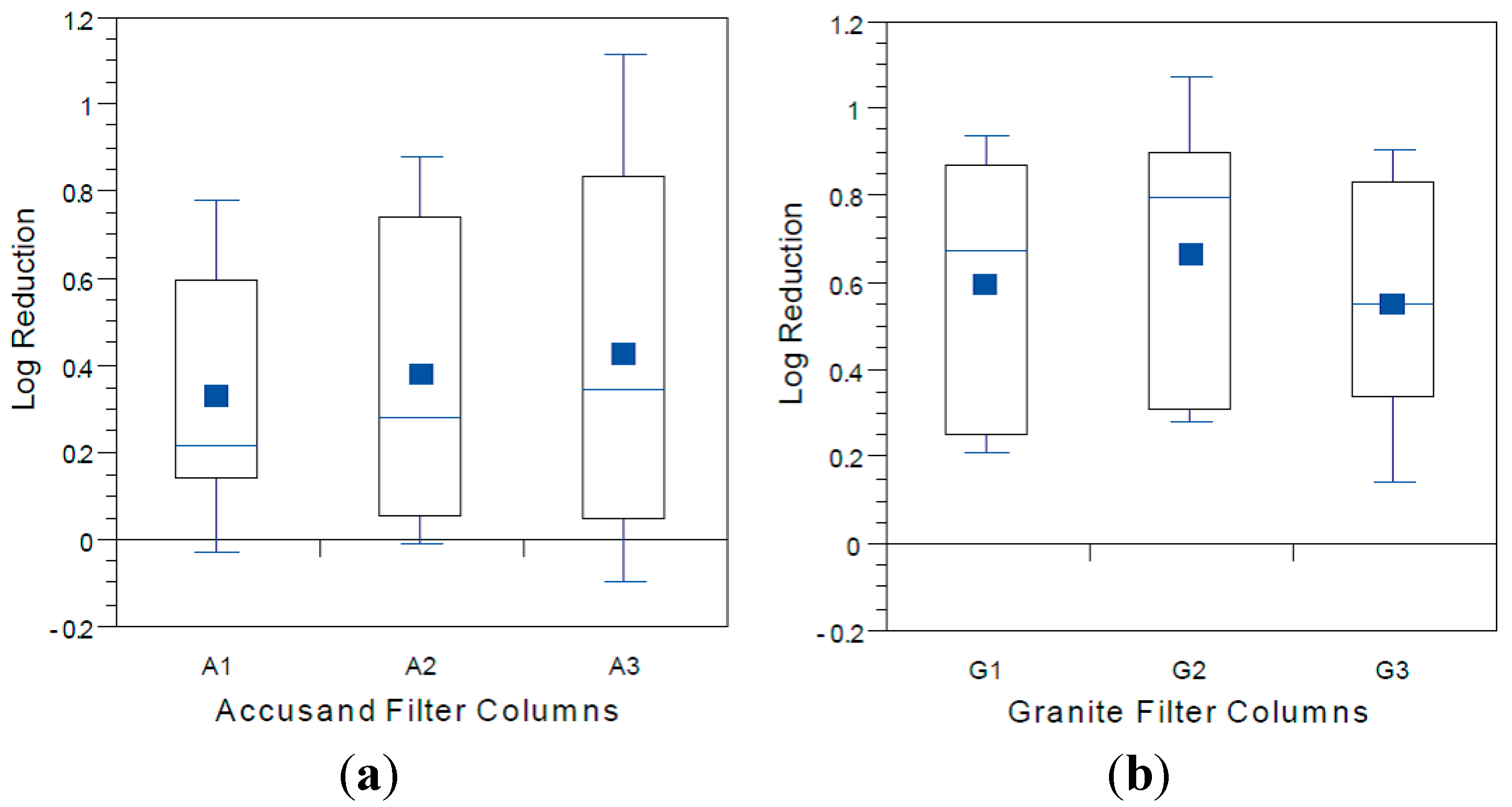
Box and whisker plots of log reduction of MS2 coliphage are given in
Figure 9a (Accusand media) and
Figure 9b (granite media) for Column Test No. 1 and in
Figure 10a (Accusand media) and
Figure 10b (granite media) for Column Test No. 2. Reductions were generally less than about 1-log (90%) although varied throughout the eight weeks of operation. The extents of virus reductions in these bench-scale tests were similar to those observed previously in full-scale tests [
10].
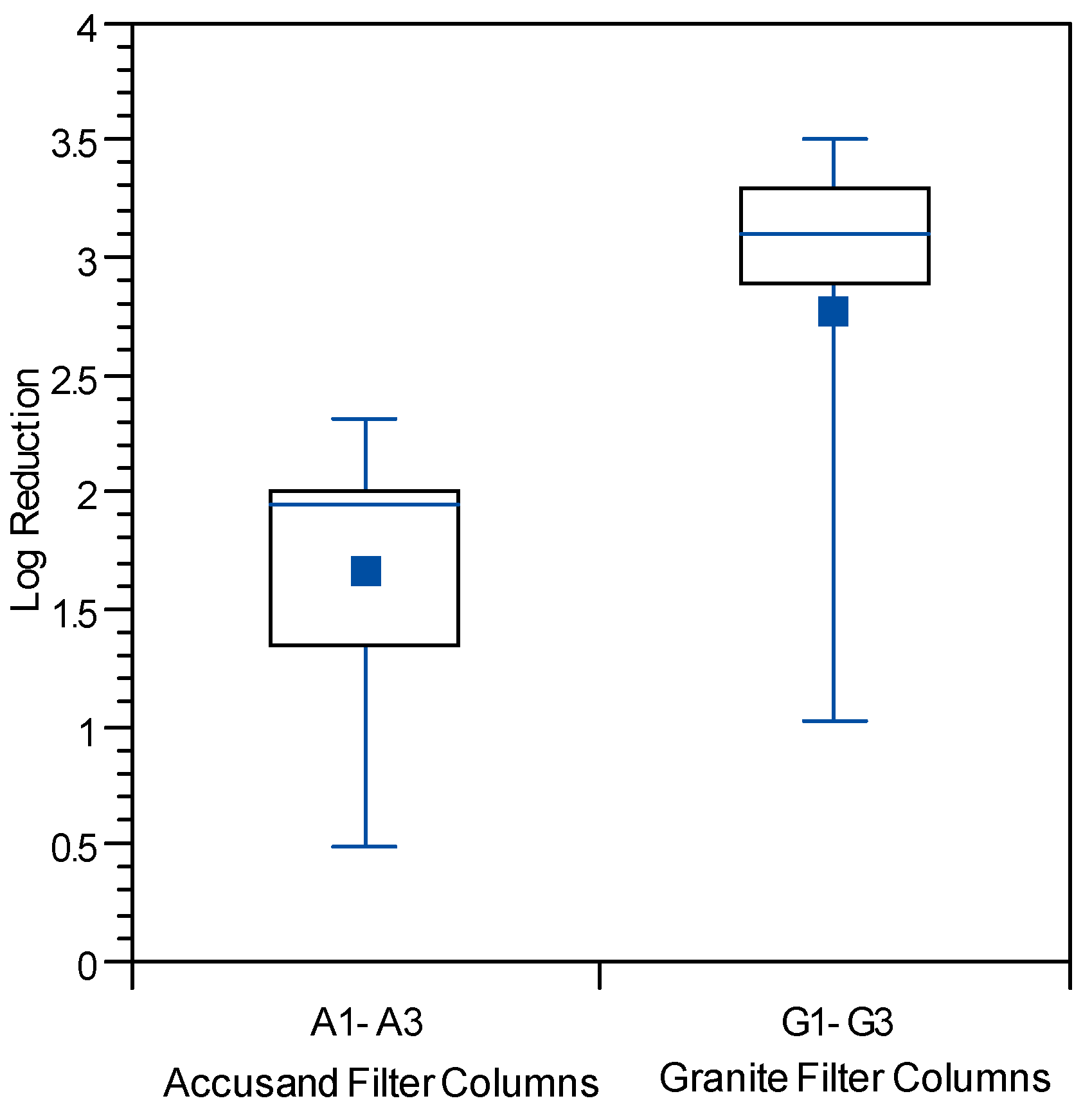
Because less data were available on reductions of PRD-1 phage and echovirus 12, combined log reduction results for all three columns having the same media are presented in box and whisker plots. Log reductions for echovirus 12 are shown in
Figure 10 as box and whisker plots for Column Test No. 1 only as this was not used during Column Test No. 2. Likewise log reductions for PRD-1 are shown in
Figure 11 as box and whisker plots for Column Test No. 2 only as it was not used in Column Test No. 1. Mean PRD-1 reductions of about 0.64 log (range 0-to-2.5 log) were not significantly different from those for MS2 at 0.57 log (range 0-to-2.7 log), whereas mean reduction for echovirus 12 was much greater at 2.21 (range 0.35-to-3.69) (
p < 0.0001 for comparison with MS2). Direct comparisons between PRD-1 and echovirus 12 reductions were not possible in this experiment.
Figure 9.
MS2 reductions for (a) Accusand media columns; and (b) granite media columns in Column Test No. 1 for samples collected throughout the eight-week experiment. N = 7 for G3; 8 for A1; 9 for G1, G2, A2 and A3.
Figure 9.
MS2 reductions for (a) Accusand media columns; and (b) granite media columns in Column Test No. 1 for samples collected throughout the eight-week experiment. N = 7 for G3; 8 for A1; 9 for G1, G2, A2 and A3.
Figure 10.
Echovirus 12 reductions for Accusand media columns and granite media columns in Column Test No. 1 for samples collected throughout the eight-week experiment. N = 11 for Accusand and 11 for granite media.
Figure 10.
Echovirus 12 reductions for Accusand media columns and granite media columns in Column Test No. 1 for samples collected throughout the eight-week experiment. N = 11 for Accusand and 11 for granite media.
However, echovirus 12 reductions have been reported to be of greater magnitude than those of MS2 and PRD-1 previously [
10].
Although the effect of media type on reductions of all three challenge viruses was investigated, the results should be interpreted with caution due to difference in the experimental conditions of Column Tests No. 1 and 2. Mean reductions of both challenge viruses were significantly greater in the granite than in Accusand media in Column Test No. 1; MS2 mean reductions were 0.62-log in granite
vs. 0.37-log in Accusand (
p < 0.01) and echovirus 12 reductions were likewise greater in the granite than Accusand (2.8-log
vs. 1.6-log) (
p < 0.01). However, log reductions of viruses did not always differ between granite and Accusand media, with no significant differences found in Column Test No. 2 reductions of MS2 (
Figure 11a,b) or PRD-1 (
Figure 12a,b). Thus, the effect of column media type on virus reductions was not consistent among the different viruses tested or across the two column tests.
Figure 11.
MS2 reductions for (a) Accusand media columns; and (b) granite media columns in Column Test No. 2 for samples collected throughout the eight-week experiment. N = 13 for G2; 14 for A1–A3; 15 for G1 and G2.
Figure 11.
MS2 reductions for (a) Accusand media columns; and (b) granite media columns in Column Test No. 2 for samples collected throughout the eight-week experiment. N = 13 for G2; 14 for A1–A3; 15 for G1 and G2.
Figure 12.
PRD-1 reductions for (a) Accusand media columns; and (b) granite media columns in Column Test No. 2 for samples collected throughout the eight-week experiment. N = 13 for G2; 14 for A1–A3; 15 for G1 and G2.
Figure 12.
PRD-1 reductions for (a) Accusand media columns; and (b) granite media columns in Column Test No. 2 for samples collected throughout the eight-week experiment. N = 13 for G2; 14 for A1–A3; 15 for G1 and G2.
The initial hypothesis, virus reductions would be greater in columns containing the granite than Accusand media because of increased virus attachment to more positively charged sites on the granite surface [
48,
49], are consistent with the results for MS2 and Echo 12 in Column Test No. 1. However, this hypothesis was not supported by virus reductions in Column Test No. 2 wherein there was no significant difference between columns filled with granite and Accusand. The likely explanation for the observed differences in results is that the large surface area of fine silty material present in the granite media during Column Test No. 1 was lost during the backwashing procedure prior to Column Test No. 2. Increased surface area could enhance virus reduction by adsorption mechanisms or possibly through biological mechanisms. Possible mechanisms of virus reduction related to depth filtration and maturation are discussed in more detail by Elliott
et al. [
10] and Wang
et al. [
21].
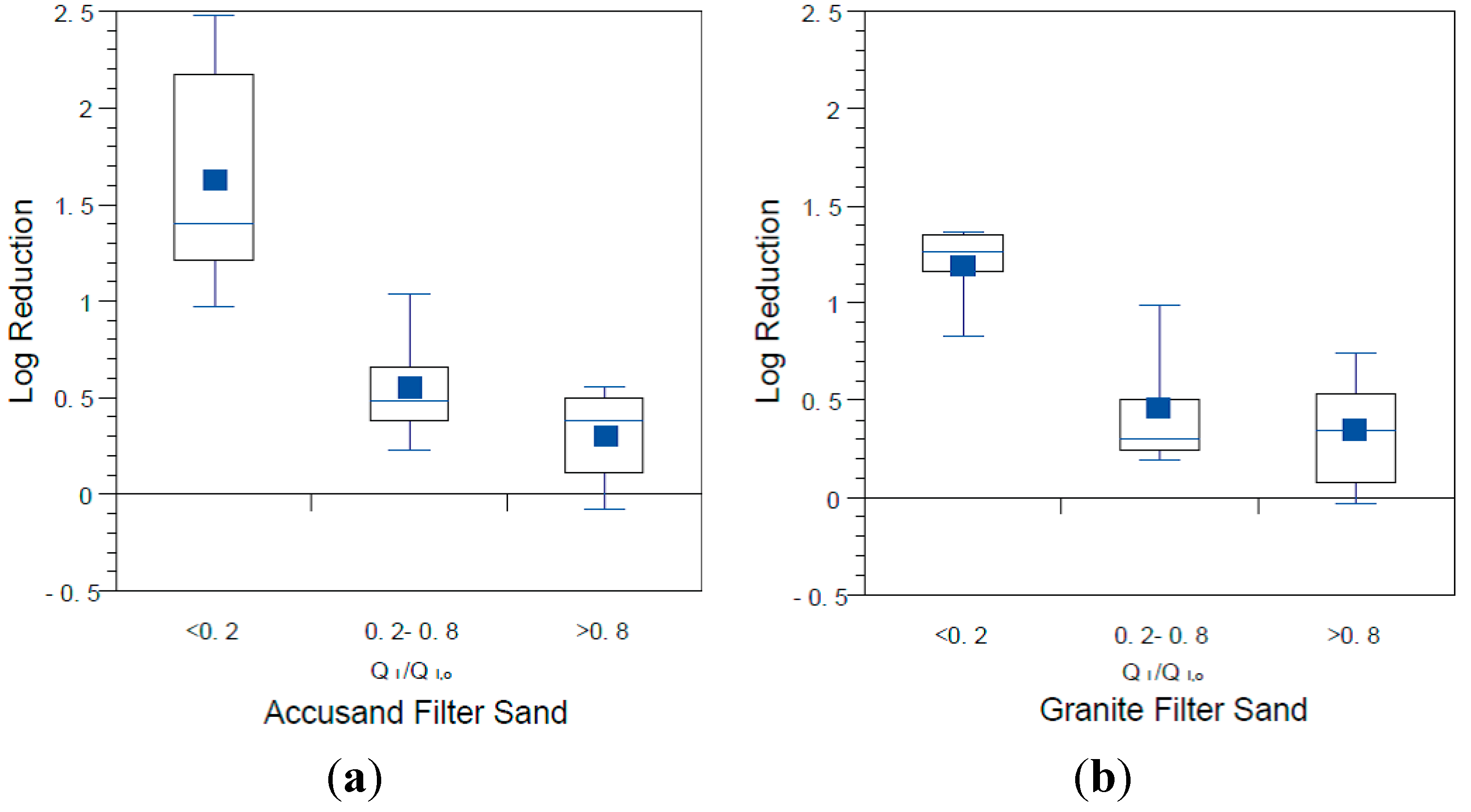
The influence of filter maturation on MS2 and PRD-1 log reductions was investigated by grouping the data by normalized, initial daily filtration rate (Q
I/Q
I,o). There were insufficient data for echovirus 12 reductions to do the same analysis. The results for MS2 and PRD-1, which are shown in
Figure 13 and
Figure 14, respectively, indicate that greater reductions are associated with lower values of Q
I/Q
I,o, (
p < 0.0001 for MS2 and PRD-1) as was also noted for
E. coli reductions (
Figure 8). These results indicate that filter maturation, directly or indirectly, contributed to enhanced virus reductions.
Figure 13.
MS2 reductions for (a) Accusand media columns; and (b) granite media columns in Column Tests No. 1 and No. 2 organized by bins of normalized filtration rate, where QI is the initial daily filtration rate on each day of charging the column and QI,o is the initial filtration rate on the first day. N for Accusand: 16 for <0.2, 17 for 0.2–0.8, 17 for >0.8. N for granite: 12 for <0.2, 25 for 0.2–0.8, 11 for >0.8.
Figure 13.
MS2 reductions for (a) Accusand media columns; and (b) granite media columns in Column Tests No. 1 and No. 2 organized by bins of normalized filtration rate, where QI is the initial daily filtration rate on each day of charging the column and QI,o is the initial filtration rate on the first day. N for Accusand: 16 for <0.2, 17 for 0.2–0.8, 17 for >0.8. N for granite: 12 for <0.2, 25 for 0.2–0.8, 11 for >0.8.
The log virus reductions observed in the granular media BSF column experiments of this study and presented in
Figure 9,
Figure 10,
Figure 11,
Figure 12,
Figure 13 and
Figure 14 raise questions concerning the responsible mechanism(s). Physical straining of viruses by the schmutzdecke is unlikely to be an important mechanism of virus reduction because of their small particle size (see
Table 3). However, the schmutzdecke could increase viral reductions by decreasing flow rate, leading to more efficient depth filtration by physical-chemical mechanisms. Wang and colleagues found that rates of MS2 reduction per depth in the top 5 cm of the filter bed were nearly 10× those in the rest of the bed, indicating that the schmutzdecke zone contributes greatly to virus reductions [
21]. However, several reports of virus reduction in conventional SSF suggest no effect or only a small effect by removing the schmutzdecke while not allowing the flow rate to increase [
46,
50,
51]. Schmutzdecke growth also occurs in parallel with other biological maturation processes that have been shown to affect depth filtration of viruses in SSF through “media aging” [
23,
51,
52]. Therefore, while the active microbial community in the top layers of the BSF almost certainly plays a major role in virus reductions, the presence of a physically intact schmutzdecke may contribute to virus reductions primarily by slowing flow and increasing residence time throughout the media bed.
Figure 14.
PRD-1 reductions for (a) Accusand media columns and (b) granite media columns in Column Tests No. 2 organized by bins of normalized filtration rate where QI is the initial daily filtration rate on each day of charging the column and QI,o is the initial filtration rate on the first day. N for Accusand: 6 for <0.2, 10 for 0.2–0.8, 9 for >0.8. N for granite: 6 for <0.2, 15 for 0.2–0.8, 6 for >0.8.
Figure 14.
PRD-1 reductions for (a) Accusand media columns and (b) granite media columns in Column Tests No. 2 organized by bins of normalized filtration rate where QI is the initial daily filtration rate on each day of charging the column and QI,o is the initial filtration rate on the first day. N for Accusand: 6 for <0.2, 10 for 0.2–0.8, 9 for >0.8. N for granite: 6 for <0.2, 15 for 0.2–0.8, 6 for >0.8.
3.6. Microbial Reductions in Context
The absolute microbial reductions reported here may not be representative of those in BSFs that meet current design guidelines [
24]. As discussed in
Section 2, column design and media preparation were based on the guidelines at the time of these experiments [
25,
26]. Additionally, log reductions reported here include data from the first few weeks of operation, whereas other researchers may report microbial reductions only after a period of ripening. However, these results do provide useful comparisons between the design parameters and microorganisms studied here.
Log reductions of
E. coli were typical of those reported in the literature [
8,
9,
10,
12]; 1–2 log
10 reductions were observed following a period of ripening and increased to 4-log
10 or greater when the schmutzdecke became clogged and flow rates declined to a small fraction (<1/10th) of those seen with newly loaded sand.
MS2 reductions were modest (0.3–1.0 log
10), similar to those reported by most other research groups [
9,
10,
11,
12,
23]. In contrast, one group has reported 3-to-6 log reductions of MS2 in BSFs with sand media [
20,
21]. While the reason for these differences are not clear from the literature, Bradley and colleagues reported modest MS2 reductions averaging around 1-log10 for the first 75 days of their experiment, with MS2 reductions increasing to 3-to-5 log10 after 150–300 days of operation. This length of operation is not typical of the other studies referenced. Possible explanations include: (1) that BSF maturation can continue to improve treatment of viruses beyond the typical duration of laboratory challenge experiments; and/or (2) variable experimental conditions or characteristics (e.g., feed water, media, microbial community) led to the increased MS2 reductions reported by Bradley
et al., and Wang
et al. [
20,
21]. These questions warrant further research.
PRD-1 and echovirus 12 have not been tested by other research groups. As reported above, PRD-1 reductions were similar to those of MS2 whereas echovirus 12 reductions were significantly greater. MS2 and PRD-1 have a lower isoelectric point than echovirus 12 (
Table 3); thus, all things being equal, greater physical-chemical removal of echovirus would be expected. Further investigation of physical-chemical virus reduction mechanisms in BSF is warranted.
Although it is often stated that BSFs are more effective at treating bacteria than viruses, this is not always true. Comparison of E. coli reductions with virus reductions (for experiments and days on which both were tested) did show that E. coli reductions were significantly greater than those of MS2 and PRD-1 (p < 0.0001 for both comparisons). However, E.coli and echovirus 12 reductions were not significantly different (p = 0.22). Additionally, BSF challenge experiments have only been conducted using members of the coliform group (E. coli, thermotolerant coliforms, total coliforms). Further investigation of BSF performance using different bacteria is warranted.