Solar-Enhanced Advanced Oxidation Processes for Water Treatment: Simultaneous Removal of Pathogens and Chemical Pollutants
Abstract
:1. Introduction
2. Literature Search and Selection Criteria
3. Simultaneous Pathogen Inactivation and Pollutant Degradation
| Substrates, Initial Concentration | Experimental Conditions * (Light Source, Reactor Type and Volume) | Results Obtained (Degree of Degradation/Inactivation **, Irradiation Time) | Reference |
|---|---|---|---|
| 17α-ethynylestradiol (0.1 mg/L) + E. coli (1 × 103 CFU/mL) (in synthetic wastewater) | Solar simulator system,5.8 × 10−7 Einstein/L·s Batch-type photoreactor, 300 mL | 17α-EE: ca. 80%, 90 min E. coli: > 95%, 90 min The degree of degradation/inactivation was less than in deionized water and when the substrates were treated separately. | [27] |
| Either Resorcinol/ Hydroquinone (10 mg/L) + Either E. coli/ S.typhimurium (106 CFU/mL) | Solar simulator lamp, 1000 W/m2 Reactor: Pyrex bottle, 80 mL | Resorcinol: ca. 50%, 90 min (in the presence of either E. coli / S.typhimurium) Hydroquinone: ca. 30%, 120 min (in the presence of either E. coli / S.typhimurium) E. coli: ca. 3 logs, 120 min (in the presence of either Resorcinol/Hydroquinone) S.typhimurium: ca. 1–2 logs, 120 min (in the presence of either Resorcinol/Hydroquinone) The simultaneous presence of dixydroxybenzenes and bacteria negatively affected both the degradation and inactivation processes. | [26] |
| Either Resorcinol/ Hydroquinone/ Catechol (2 mmol/L) + E. coli (107 CFU/mL) | Solar simulator lamp,1000 W/m2 Reactor: Pyrex bottle, 50 mL | Resorcinol/ Hydroquinone/ Catechol: ca. 25%, 2 h/ca. 12%, 2 h/ca. 18%, 2 h E. coli: 100%, 40 min (in the presence of either Resorcinol/Hydroquinone/Catechol) | [23] |
| Substrates, Initial Concentration | Experimental Conditions (Fenton’s Reagent Concentration, Initial pH, Light Source, Reactor Type And Volume) | Results Obtained (Degree of Degradation/Inactivation *, Irradiation Time) | Reference |
|---|---|---|---|
| Resorcinol (10 mg/L) + E.faecalis (106 CFU/mL) | [Fe2+] =20 mg/L; [H2O2] = 50 mg/L pH = 6–7 Natural sunlight, 30 ± 2 W/m2 Glass reactor, 250 mL | Resorcinol: 100%, <5 min E.faecalis: 100%, 10 min | [29] |
| Either Resorcinol/ Hydroquinone (10 mg/L) + Either E. coli/ S.typhimurium, S.sonnei, (106 CFU/ml) | [Fe3+] = 1mg/L; [H2O2] = 60 mg/L pH = 5.0 (initial)Solar simulator lamp, 1000 W/m2 Reactor: Pyrex bottle, 80 mL | Resorcinol: ca. 60%–80%, 40 min (in the presence of either E. coli / S.typhimurium / S.sonnei) Hydroquinone: ca. 55%–90%, 40 min(in the presence of either E. coli / S.typhimurium / S.sonnei) E. coli / S.typhimurium / S.sonnei: ca. 2.5–4 logs, 40 min (in the presence of Resorcinol) / ca. 4.5–5.5 logs, 40 min (in the presence of Hydroquinone) The simultaneous presence of dixydroxybenzenes and bacteria negatively affected both the degradation and inactivation processes. | [26] |
| Either Ofloxacin/ Trimethoprim (100 µg/L) + Enterococci (2.53 × 103 CFU/mL) (in secondary treated WW, 6.29–8.6 mg DOC **/L) | [Fe2+] = 5 mg/L; [H2O2] = 75 mg/L Natural sunlight Reactor: CPC ***, 250 L total volume, 85.4 L irradiated volume, circulation speed 600 L/h | Ofloxacin / Trimethoprim: 100% removal (for both) Enterococci at the start of the experiment: 5.00 × 102 CFU/mL (in the presence of Ofloxacin); 2.67 × 102 CFU/mL (in the presence of Trimethoprim) Enterococci at the end of the experiment: 0 CFU/mL, 180 min (in the presence of either Ofloxacin/Trimethoprim) | [28] |
3.1. Effect of the Simultaneous Presence of Chemical Pollutants and Pathogens on Their Degradation/Inactivation
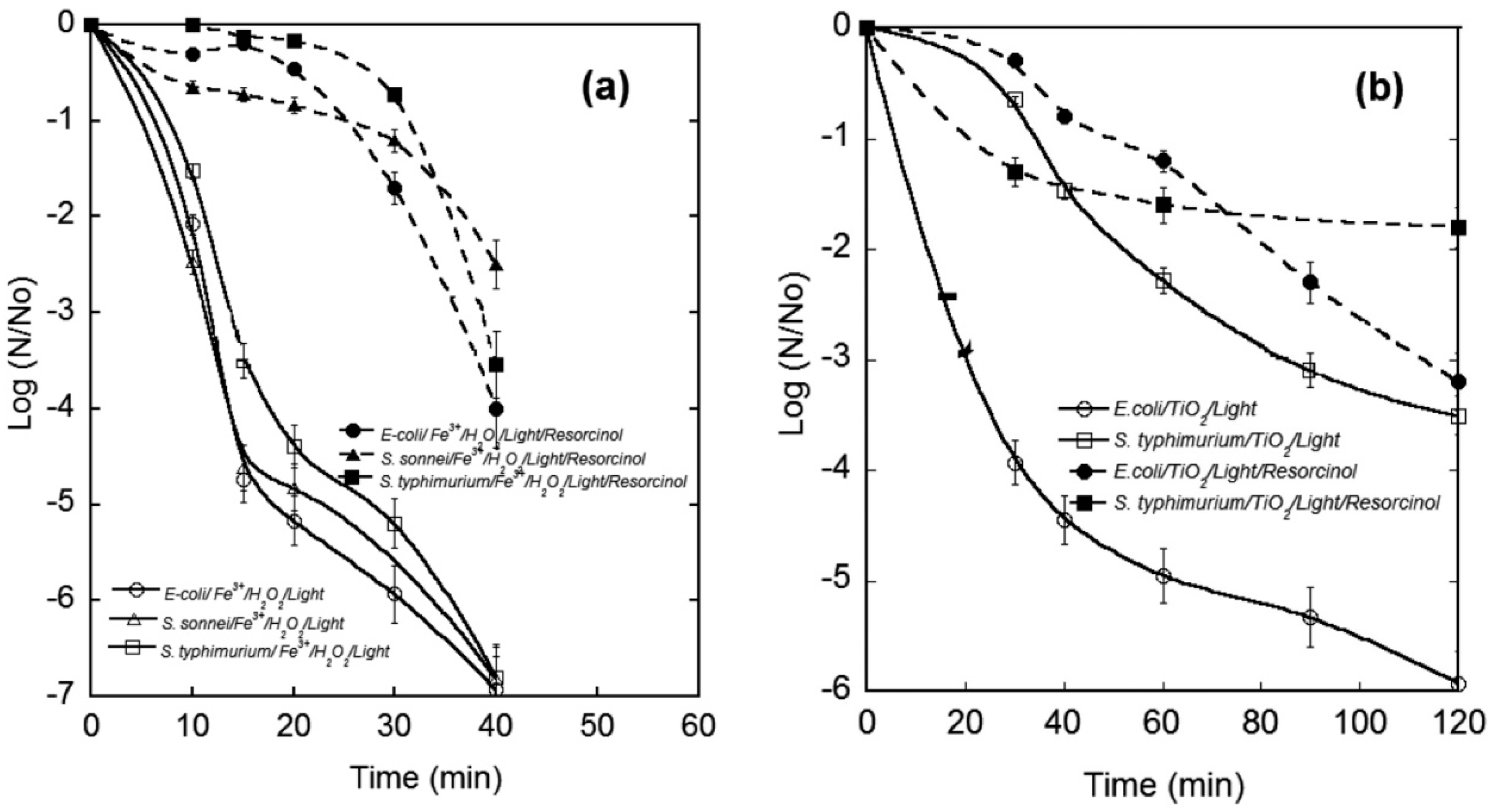
3.2. Effect of Water Matrix on Pathogen Inactivation and Chemical Pollutant Degradation
4. Overcoming the Detrimental Effects of the Simultaneous Presence of Chemical Pollutants and Pathogens
4.1. Catalyst Concentration
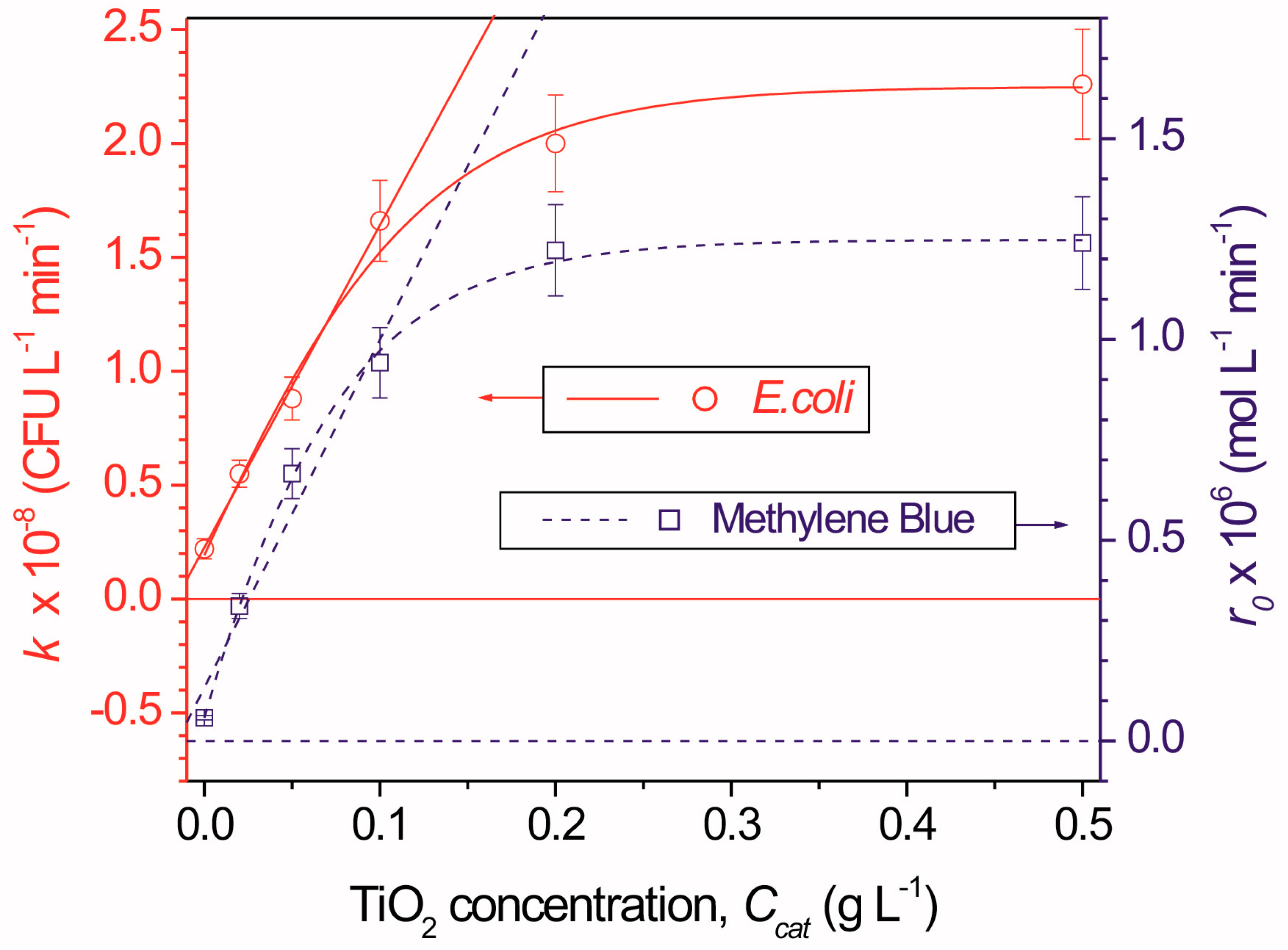
4.2. Irradiance

4.3. Oxidant Concentration
4.4. pH
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Progress on Drinking Water and Sanitation: 2014 Update. Available online: http://www.unicef.org/publications/files/JMP_report_2014_webEng.pdf (accessed on 22 June 2015).
- Comninellis, C.; Kapalka, A.; Malato, S.; Parsons, S.A.; Poulios, I.; Mantzavinos, D. Advanced oxidation processes for water treatment: Advances and trends for R&D. J. Chem. Technol. Biotechnol. 2008, 83, 769–776. [Google Scholar]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment I: Oxidation technologies at ambient conditions. Adv. Environ. Res. 2004, 8, 501–551. [Google Scholar] [CrossRef]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment II: Hybrid methods. Adv. Environ. Res. 2004, 8, 553–597. [Google Scholar] [CrossRef]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Wols, B.; Hofman-Caris, C. Review of photochemical reaction constants of organic micropollutants required for UV advanced oxidation processes in water. Water Res. 2012, 46, 2815–2827. [Google Scholar] [CrossRef] [PubMed]
- Robertson, P.K.; Robertson, J.M.; Bahnemann, D.W. Removal of microorganisms and their chemical metabolites from water using semiconductor photocatalysis. J. hazard. Mater. 2012, 211, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.; Malato, S.; Fernández-Ibañez, P.; Alarcón, D.; Gernjak, W.; Maldonado, M. Review of feasible solar energy applications to water processes. Renew. Sust. Energ. Rev. 2009, 13, 1437–1445. [Google Scholar] [CrossRef]
- Byrne, J.A.; Fernandez-Ibanez, P.A.; Dunlop, P.S.; Alrousan, D.; Hamilton, J.W. Photocatalytic enhancement for solar disinfection of water: A review. Int. J. Photoenergy 2011, 2011. [Google Scholar] [CrossRef]
- Byrne, J.A.; Dunlop, P.S.; Hamilton, J.W.; Fernandez-Ibanez, P.; Polo-Lopez, I.; Sharma, P.K.; Vennard, A.S. A review of heterogeneous photocatalysis for water and surface disinfection. Molecules 2015, 20, 5574–5615. [Google Scholar] [CrossRef] [PubMed]
- Fatta-Kassinos, D.; Meric, S.; Nikolaou, A. Pharmaceutical residues in environmental waters and wastewater: Current state of knowledge and future research. Anal Bioanal Chem 2011, 399, 251–275. [Google Scholar] [CrossRef] [PubMed]
- Oller, I.; Malato, S.; Sánchez-Pérez, J. Combination of advanced oxidation processes and biological treatments for wastewater decontamination—A review. Sci. Total Environ. 2011, 409, 4141–4166. [Google Scholar] [CrossRef] [PubMed]
- Munoz, I.; Peral, J.; Ayllon, J.A.; Malato, S.; Passarinho, P.; Domenech, X. Life cycle assessment of a coupled solar photocatalytic-biological process for wastewater treatment. Water Res. 2006, 40, 3533–3540. [Google Scholar] [CrossRef] [PubMed]
- Sarathy, S.; Mohseni, M. An overview of UV-based advanced oxidation processes for drinking water treatment. IUVA News 2006, 8, 16–27. [Google Scholar]
- Maldonado, M.; Passarinho, P.; Oller, I.; Gernjak, W.; Fernández, P.; Blanco, J.; Malato, S. Photocatalytic degradation of EU priority substances: A comparison between TiO2 and Fenton plus photo-Fenton in a solar pilot plant. J. Photoch. Photobio. A 2007, 185, 354–363. [Google Scholar] [CrossRef]
- Klamerth, N.; Rizzo, L.; Malato, S.; Maldonado, M.I.; Aguera, A.; Fernandez-Alba, A.R. Degradation of fifteen emerging contaminants at microg l(-1) initial concentrations by mild solar photo-Fenton in MWTP effluents. Water Res. 2010, 44, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Klamerth, N.; Malato, S.; Aguera, A.; Fernandez-Alba, A.; Mailhot, G. Treatment of municipal wastewater treatment plant effluents with modified photo-Fenton as a tertiary treatment for the degradation of micro pollutants and disinfection. Environ. Sci. Technol. 2012, 46, 2885–2892. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.R.; Silva, A.M.T. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Foster, H.A.; Ditta, I.B.; Varghese, S.; Steele, A. Photocatalytic disinfection using titanium dioxide: Spectrum and mechanism of antimicrobial activity. Appl. Microbial. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef] [PubMed]
- Agulló-Barceló, M.; Polo-López, M.; Lucena, F.; Jofre, J.; Fernández-Ibáñez, P. Solar advanced oxidation processes as disinfection tertiary treatments for real wastewater: Implications for water reclamation. Appl. Catal. B Environ. 2013, 136, 341–350. [Google Scholar] [CrossRef]
- Ndounla, J.; Pulgarin, C. Evaluation of the efficiency of the photo fenton disinfection of natural drinking water source during the rainy season in the Sahelian region. Sci. Total Environ. 2014, 493, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Chueca, J.; Ormad, M.; Mosteo, R.; Sarasa, J.; Ovelleiro, J. Conventional and advanced oxidation processes used in disinfection of treated urban wastewater. Water Environ. Res. 2015, 87, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Rincon, A.-G.; Pulgarin, C. Effect of pH, inorganic ions, organic matter and H2O2 on E. Coli k12 photocatalytic inactivation by TiO2: Implications in solar water disinfection. Appl. Catal. B Environ. 2004, 51, 283–302. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.; Polo-López, M.; Mosteo, R.; Ormad, M.; Fernández-Ibáñez, P. Disinfection of real and simulated urban wastewater effluents using a mild solar photo-Fenton. Appl. Catal. B Environ. 2014, 150, 619–629. [Google Scholar] [CrossRef]
- Moncayo-Lasso, A.; Sanabria, J.; Pulgarin, C.; Benitez, N. Simultaneous E. coli inactivation and NOM degradation in river water via photo-Fenton process at natural pH in solar CPC reactor. A new way for enhancing solar disinfection of natural water. Chemosphere 2009, 77, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Moncayo-Lasso, A.; Mora-Arismendi, L.E.; Rengifo-Herrera, J.A.; Sanabria, J.; Benitez, N.; Pulgarin, C. The detrimental influence of bacteria (E. coli, Shigella and Salmonella) on the degradation of organic compounds (and vice versa) in TiO2 photocatalysis and near-neutral photo-Fenton processes under simulated solar light. Photoch. Photobio. Sci. 2012, 11, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Fanourgiakis, S.; Frontistis, Z.; Chatzisymeon, T.; Venieri, D.; Mantzavinos, D. Simultaneous removal of estrogens and pathogens from secondary treated wastewater by solar photocatalytic treatment. Global Nest J. 2014, 16, 543–552. [Google Scholar]
- Michael, I.; Hapeshi, E.; Michael, C.; Varela, A.R.; Kyriakou, S.; Manaia, C.M.; Fatta-Kassinos, D. Solar photo-Fenton process on the abatement of antibiotics at a pilot scale: Degradation kinetics, ecotoxicity and phytotoxicity assessment and removal of antibiotic resistant Enterococci. Water Res. 2012, 46, 5621–5634. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Gómez, E.; Martín, M.B.; García, B.E.; Pérez, J.S.; Ibáñez, P.F. Solar photo-Fenton for water disinfection: An investigation of the competitive role of model organic matter for oxidative species. Appl. Catal. B Environ. 2014, 148, 484–489. [Google Scholar] [CrossRef]
- Pablos, C.; van Grieken, R.; Marugan, J.; Muñoz, A. Simultaneous photocatalytic oxidation of pharmaceuticals and inactivation of Escherichia coli in wastewater treatment plant effluents with suspended and immobilised TiO2. Water Sci. Technol. 2012, 65, 2016–2023. [Google Scholar] [CrossRef] [PubMed]
- Pavelescu, G.; Uyguner-Demirel, C.; Bekbolet, M.; Ghervase, L.; Ioja, C. Comparison of photocatalytic treatment effectiveness on sewage and industrial wastewaters. Environ. Eng. Manag. J. 2014, 13, 2015–2021. [Google Scholar]
- Polo-López, M.I.; García-Fernández, I.; Velegraki, T.; Katsoni, A.; Oller, I.; Mantzavinos, D.; Fernández-Ibáñez, P. Mild solar photo-Fenton: An effective tool for the removal of Fusarium from simulated municipal effluents. Appl. Catal. B Environ. 2012, 111, 545–554. [Google Scholar] [CrossRef]
- Spuhler, D.; Rengifo-Herrera, J.A.; Pulgarin, C. The effect of Fe2+, Fe3+, H2O2 and the photo–Fenton reagent at near neutral pH on the solar disinfection (SODIS) at low temperatures of water containing Escherichia Coli k12. Appl. Catal. B Environ. 2010, 96, 126–141. [Google Scholar] [CrossRef]
- Moncayo-Lasso, A.; Pulgarin, C.; Benitez, N. Degradation of DBPS’ precursors in river water before and after slow sand filtration by photo-Fenton process at pH 5 in a solar CPC reactor. Water Res. 2008, 42, 4125–4132. [Google Scholar] [CrossRef] [PubMed]
- Rosado-Lausell, S.L.; Wang, H.; Gutiérrez, L.; Romero-Maraccini, O.C.; Niu, X.-Z.; Gin, K.Y.; Croué, J.-P.; Nguyen, T.H. Roles of singlet oxygen and triplet excited state of dissolved organic matter formed by different organic matters in bacteriophage MS2 inactivation. Water Res. 2013, 47, 4869–4879. [Google Scholar] [CrossRef] [PubMed]
- Rubio, D.; Nebot, E.; Casanueva, J.F.; Pulgarin, C. Comparative effect of simulated solar light, UV, UV/H2O2 and photo-Fenton treatment (UV-VIS/H2O2/Fe2+,3+) in the Escherichia coli inactivation in artificial seawater. Water Res. 2013, 47, 6367–6379. [Google Scholar] [CrossRef] [PubMed]
- Rincón, A.-G.; Pulgarin, C. Solar photolytic and photocatalytic disinfection of water at laboratory and field scale. Effect of the chemical composition of water and study of the postirradiation events. J. Solar Energy Eng. 2007, 129, 100–110. [Google Scholar] [CrossRef]
- Radjenović, J.; Sirtori, C.; Petrović, M.; Barcelo, D.; Malato, S. Solar photocatalytic degradation of persistent pharmaceuticals at pilot-scale: Kinetics and characterization of major intermediate products. Appl. Catal. B Environ. 2009, 89, 255–264. [Google Scholar] [CrossRef]
- Marugán, J.; van Grieken, R.; Pablos, C.; Sordo, C. Analogies and differences between photocatalytic oxidation of chemicals and photocatalytic inactivation of microorganisms. Water Res. 2010, 44, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.A.; He, X.; Khan, H.M.; Shah, N.S.; Dionysiou, D.D. Oxidative degradation of atrazine in aqueous solution by UV/H2O2/Fe2+, UV/S2O82−/Fe2+ and UV/HSO5−/Fe2+ processes: A comparative study. Chem. Eng. J. 2013, 218, 376–383. [Google Scholar] [CrossRef]
- Pablos, C.; Marugán, J.; van Grieken, R.; Serrano, E. Emerging micropollutant oxidation during disinfection processes using UV-C, UV-C/H2O2, UV-A/TiO2 and UV-A/TiO2/H2O2. Water Res. 2013, 47, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Neamţu, M.; Grandjean, D.; Sienkiewicz, A.; Le Faucheur, S.; Slaveykova, V.; Colmenares, J.J.V.; Pulgarín, C.; de Alencastro, L.F. Degradation of eight relevant micropollutants in different water matrices by neutral photo-Fenton process under UV 254 and simulated solar light irradiation—A comparative study. Appl. Catal. B Environ. 2014, 158, 30–37. [Google Scholar] [CrossRef]
- Gogniat, G.; Thyssen, M.; Denis, M.; Pulgarin, C.; Dukan, S. The bactericidal effect of TiO2 photocatalysis involves adsorption onto catalyst and the loss of membrane integrity. FEMS microbial. Let. 2006, 258, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Giannakis, S.; Darakas, E.; Escalas-Canellas, A.; Pulgarin, C. Environmental considerations on solar disinfection of wastewater and the subsequent bacterial (re)growth. Photochem. Photobiol. Sci. 2015, 14, 618–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rincón, A.-G.; Pulgarin, C. Field solar E.Coli inactivation in the absence and presence of TiO2: Is UV solar dose an appropriate parameter for standardization of water solar disinfection? Sol. Energy 2004, 77, 635–648. [Google Scholar] [CrossRef]
- Chen, F.; Yang, X.; Xu, F.; Wu, Q.; Zhang, Y. Correlation of photocatalytic bactericidal effect and organic matter degradation of TiO2 part I: Observation of phenomena. Environ. Sci. Technol. 2009, 43, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Hu, A.; Hatat-Fraile, M.; Zhou, N. Development of TiO2 nanowires for membrane filtration applications. In Nanotechnology for Water Treatment and Purification; Hu, A., Apblett, A., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 47–77. [Google Scholar]
- Zong, X.; Lu, G.M.; Wang, L. Nonmetal doping in TiO2 toward visible-light-induced photocatalysis. In Environmental Photochemistry Part III; Bahnemann, D., Robertson, P., Eds.; Springer-Verlag: Berlin Heidelberg, Germany, 2015; pp. 87–113. [Google Scholar]
- Hu, A.; Liang, R.; Zhang, X.; Kurdi, S.; Luong, D.; Huang, H.; Peng, P.; Marzbanrad, E.; Oakes, K.; Zhou, Y.; et al. Enhanced photocatalytic degradation of dyes by TiO2 nanobelts with hierarchical structures. J. Photoch. Photobio. A 2013, 256, 7–15. [Google Scholar] [CrossRef]
- Kositzi, M.; Poulios, I.; Malato, S.; Caceres, J.; Campos, A. Solar photocatalytic treatment of synthetic municipal wastewater. Water Res. 2004, 38, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Gernjak, W.; Fuerhacker, M.; Fernández-Ibañez, P.; Blanco, J.; Malato, S. Solar photo-Fenton treatment - process parameters and process control. Appl. Catal. B Environ. 2006, 64, 121–130. [Google Scholar] [CrossRef]
- Frontistis, Z.; Xekoukoulotakis, N.P.; Hapeshi, E.; Venieri, D.; Fatta-Kassinos, D.; Mantzavinos, D. Fast degradation of estrogen hormones in environmental matrices by photo-Fenton oxidation under simulated solar radiation. Chem. Eng. J. 2011, 178, 175–182. [Google Scholar] [CrossRef]
- Carra, I.; Malato, S.; Jiménez, M.; Maldonado, M.; Pérez, J.S. Microcontaminant removal by solar photo-Fenton at natural pH run with sequential and continuous iron additions. Chem. Eng. J. 2014, 235, 132–140. [Google Scholar] [CrossRef]
- Carra, I.; Garcia Sanchez, J.L.; Casas Lopez, J.L.; Malato, S.; Sanchez Perez, J.A. Phenomenological study and application of the combined influence of iron concentration and irradiance on the photo-Fenton process to remove micropollutants. Sci. Total Environ. 2014, 478, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Sichel, C.; Tello, J.; De Cara, M.; Fernández-Ibáñez, P. Effect of UV solar intensity and dose on the photocatalytic disinfection of bacteria and fungi. Catal. Today 2007, 129, 152–160. [Google Scholar] [CrossRef]
- Bahena, C.L.; Martínez, S.S. Photodegradation of chlorbromuron, atrazine, and alachlor in aqueous systems under solar irradiation. Int. J. Photoenergy 2006, 2006. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Brienza, M.; Goetz, V.; Chiron, S. Solar photo-Fenton using peroxymonosulfate for organic micropollutants removal from domestic wastewater: Comparison with heterogeneous TiO2 photocatalysis. Chemosphere 2014, 117, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, D.H.; Álvarez, P.M.; Rey, A.; Contreras, S.; Beltrán, F.J. Application of solar photocatalytic ozonation for the degradation of emerging contaminants in water in a pilot plant. Chem. Eng. J. 2015, 260, 399–410. [Google Scholar] [CrossRef]
- Nadtochenko, V.A.; Rincon, A.G.; Stanca, S.E.; Kiwi, J. Dynamics of E. coli membrane cell peroxidation during TiO2 photocatalysis studied by ATR-FTIR spectroscopy and AFM microscopy. J. Photoch. Photobio. A 2005, 169, 131–137. [Google Scholar] [CrossRef]
- Wilson, W.W.; Wade, M.M.; Holman, S.C.; Champlin, F.R. Status of methods for assessing bacterial cell surface charge properties based on zeta potential measurements. J. microbial. Meth. 2001, 43, 153–164. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Env. Sci. Tec. 2006, 36, 1–84. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsydenova, O.; Batoev, V.; Batoeva, A. Solar-Enhanced Advanced Oxidation Processes for Water Treatment: Simultaneous Removal of Pathogens and Chemical Pollutants. Int. J. Environ. Res. Public Health 2015, 12, 9542-9561. https://doi.org/10.3390/ijerph120809542
Tsydenova O, Batoev V, Batoeva A. Solar-Enhanced Advanced Oxidation Processes for Water Treatment: Simultaneous Removal of Pathogens and Chemical Pollutants. International Journal of Environmental Research and Public Health. 2015; 12(8):9542-9561. https://doi.org/10.3390/ijerph120809542
Chicago/Turabian StyleTsydenova, Oyuna, Valeriy Batoev, and Agniya Batoeva. 2015. "Solar-Enhanced Advanced Oxidation Processes for Water Treatment: Simultaneous Removal of Pathogens and Chemical Pollutants" International Journal of Environmental Research and Public Health 12, no. 8: 9542-9561. https://doi.org/10.3390/ijerph120809542





