Additional Burden of Diseases Associated with Cadmium Exposure: A Case Study of Cadmium Contaminated Rice Fields in Mae Sot District, Tak Province, Thailand
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Cadmium Contaminated Area
2.2. Cadmium Exposure Data of Population in Mae Sot Area

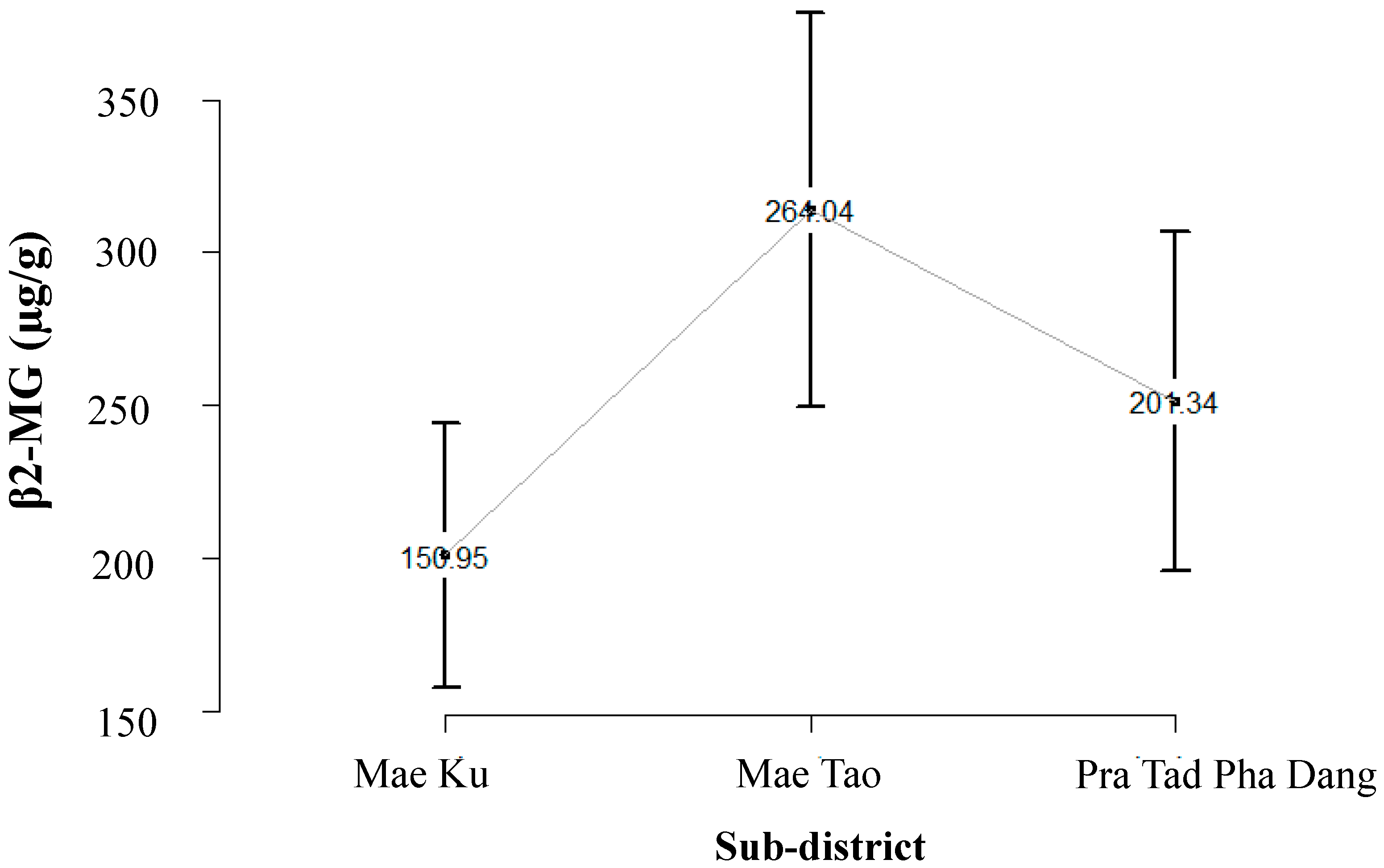
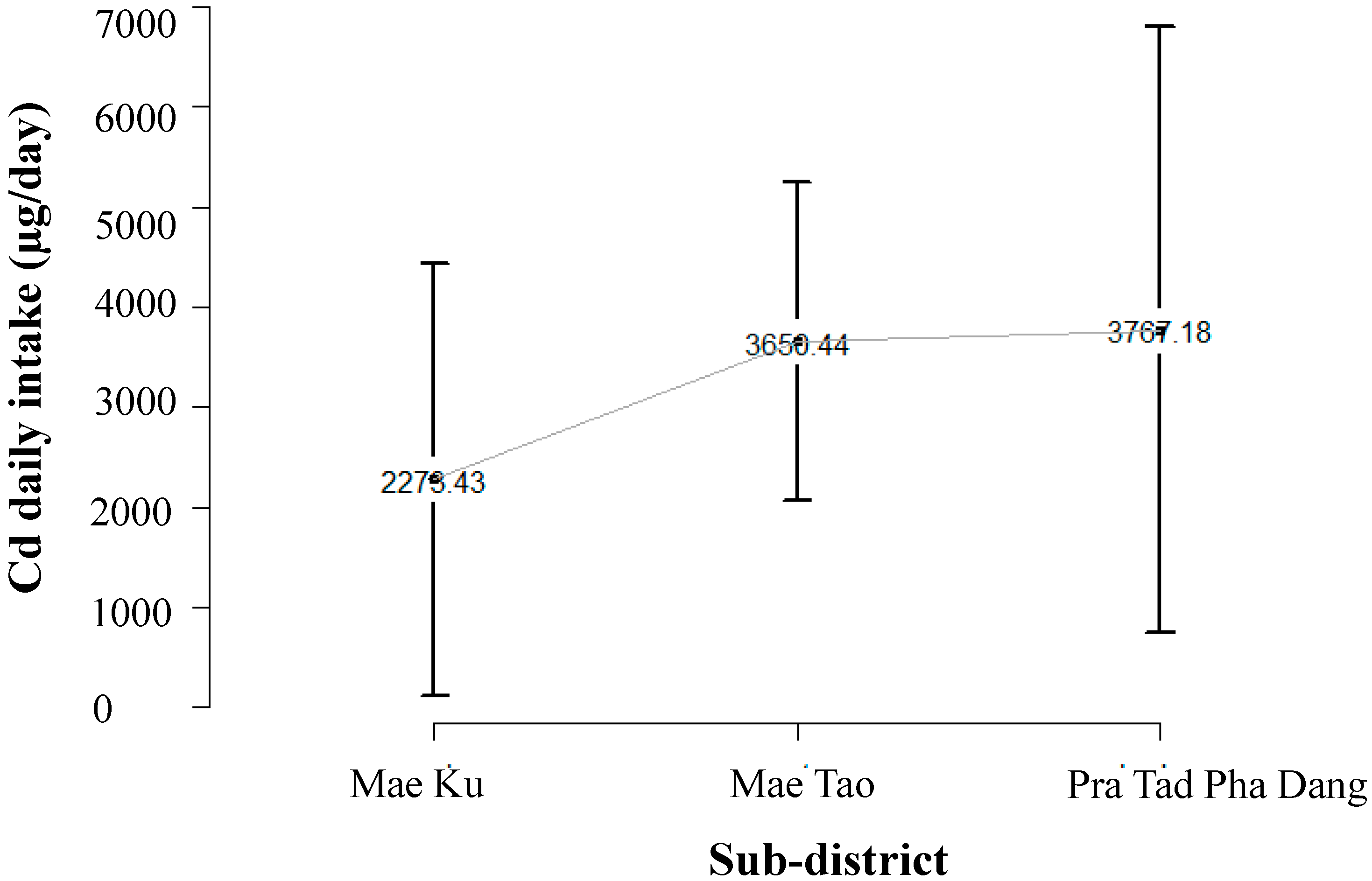
2.3. Literature Review to Identify Risk of Cadmium Related Diseases
2.4. Additional DALYs Attributable to Cadmium Exposure
- PAF total: Total population attributable fraction;
- PFi: Population fraction at exposure level i;
- Riski: Risk of disease (OR, RR or HR) at exposure level i.
| Studies | Age (Years)/Gender | Health Outcome | Mean of Cadmium Exposure (µg/g Cr) (95%CI) | Cadmium Exposure Level (µg/g Cr) | Risk of Disease (95%CI) |
|---|---|---|---|---|---|
| 1.US NHANES; Case control 1988–1994 n = 750 cases/6811 controls [29] | 30–90/All | Osteoporosis | 1.16 | 0–0.99 1.00–1.99 ≥2.00 | Odds ratio 1.00 1.27 (0.88–1.84) 2.59 (1.15–5.83) |
| 2.Swedish mammography cohort (general population) 1997–2009 n = 2688/395 cases [30] | 56–69/female | Osteoporosis | 0.29 (0.14–0.64) | <0.50 0.50–0.75 ≥0.75 | Odds ratio Femoral neck 1.00 |
| 2.09 (1.12–3.93) | |||||
| 3.47 (1.46–8.23) | |||||
| Hip or spine | |||||
| 1.00 | |||||
| 1.27 (0.75–2.14) | |||||
| 4.24 (1.99–9.04) | |||||
| 3.A 22-year follow-up study in Cd-polluted area in Japan (Kakehashi River) 1981–2003 n = 3119/46 cases [31] | ≥50/All | Nephritis/nephrosis | Male 4.6 (4.4–4.7) Female 7.2 (7.0–7.4) | <3.0 3.0–4.9 5.0–9.9 ≥10.0 | Hazard ratio |
| Male | |||||
| 1.0 | |||||
| 2.7 (0.5–13.2) | |||||
| 4.4 (1.0–19.9) | |||||
| 3.4 (0.6–20.5) | |||||
| Female | |||||
| 1.0 | |||||
| 0.7 (0.0–12.4) | |||||
| 1.4 (0.2–13.1) | |||||
| 3.1 (0.4–26.2) | |||||
| 4. A 22-year follow-up study in Cd-polluted area in Japan (Kakehashi River) 1981–2003 n = 3119/156 cases [31] | ≥50/female | Cardiovascular disease | 7.2 (7.0–7.4) | <3.0 3.0–4.9 5.0–9.9 ≥10.0 | Hazard ratio 1.0 |
| 2.1 (0.9–4.7) | |||||
| 2.3 (1.1–4.9) | |||||
| 2.4 (1.1–5.1) | |||||
| 5. A 22-year follow-up study in Cd-polluted area in Japan (Kakehashi River) 1981–2003 n = 3119/115 cases [31] | ≥50/female | Cerebrovascular disease | 7.2 (7.0–7.4) | <3.0 3.0–4.9 5.0–9.9 ≥10.0 | Hazard ratio 1.0 |
| 3.0 (0.9–10.5) | |||||
| 4.3 (1.4–14.0) | |||||
| 3.6 (1.1–11.9) |
| Studies | Age (Years)/Gender | Health Outcome | Cadmium Exposure Level (µg/g Cr) | Risk of Disease (95%CI) |
|---|---|---|---|---|
| 1. A 15 year follow-up study in Cd-polluted area in Japan (Kakehashi River) 1981–1996 n = 3178 [32] | ≥50/All | Nephrosis/Nephritis | <300 300–1000 1000–10,000 ≥10,000 | Hazard ratio |
| Male | ||||
| 1.00 | ||||
| 2.44 (0.53–11.2) | ||||
| 5.67 (1.47–12.8) | ||||
| 18.15 (4.24–77.6) | ||||
| Female | ||||
| 1.00 | ||||
| 5.43 (0.48–61.5) | ||||
| 6.94 (0.59–81.7) | ||||
| 54.98 (5.41–558.5) | ||||
| 2. A 15 year follow-up study in Cd-polluted area in Japan (Kakehashi River) 1981–1996 n = 3178 [32] | ≥50/All | Heart failure | <300 300–1000 1000–10,000 ≥10,000 | Hazard ratio |
| Male | ||||
| 1.00 | ||||
| 0.88 (0.41–1.89) | ||||
| 1.45 (0.74–2.84) | ||||
| 3.69 (1.62–8.39) | ||||
| Female | ||||
| 1.00 | ||||
| 1.94 (1.08–3.48) | ||||
| 3.05 (1.73–5.35) | ||||
| 3.19 (1.19–5.52) | ||||
| 3. A 15 year follow-up study in Cd-polluted area in Japan (Kakehashi River) 1981–1996 n = 3178 [32] | ≥50/All | Cerebral infarction | <300 300–1000 1000–10,000 ≥10,000 | Hazard ratio |
| Male | ||||
| 1.00 | ||||
| 2.4 (1.15–4.98) | ||||
| 4.48 (2.29–8.78) | ||||
| 5.36 (2.04–8.78) | ||||
| Female | ||||
| 1.00 | ||||
| 1.88 (0.82–4.29) | ||||
| 3.58 (1.71–7.51) | ||||
| 3.19 (1.29–7.88) |
| Studies | Age (Years)/Gender | Health Outcome | Mean of Dietary Cadmium (µg/Day) (±SD) | Cadmium Exposure Level (µg/day) | Risk of Disease (95%CI) |
|---|---|---|---|---|---|
| 1. Swedish mammography cohort (general population) 1987–2006 n = 30,210/378 cases [33] | ≥55/female | Post- menopausalendometrial cancer | 15 | <13.7 13.7–16.0 ≥16.0 | Relative risk |
| 1.00 | |||||
| 1.01 (0.77–1.33) | |||||
| 1.39 (1.04–1.86) | |||||
| 2. Swedish mammography cohort (general population) 1987–2008 n = 55,987/2112 cases [34] | ≥55/female | Post-menopausal breast cancer | 15 ± 3.2 | <13 13–16 >16 | Relative risk 1.00 |
| 1.00 (0.90–1.11) | |||||
| 1.05 (0.95–1.17) | |||||
| 3.The cohort of Swedish Men (general population) 1998–2009 n = 41,089/3085 cases [35] | 45–79/male | Prostate cancer | 19 ± 3 | <17 17–20 >20 | Relative risk 1.00 |
| 1.18 (1.00–1.40) | |||||
| 1.29 (1.08–1.53) |

3. Results
3.1. Health Outcomes and Risk Values Associated with Cadmium
3.2. Additional DALYs Attributable to Cadmium in the Mae Sot Area
| Cadmium Exposure Level | Male | Female | Both gender | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Risk | PF | DALY * | Risk | PF | DALY * | Risk | PF | DALY * | |
| 1. Nephrosis/nephritis ** | |||||||||
| <3 | 1.00 | 68 | 0 | 1.00 | 51 | 0 | |||
| 3–4.9 | 2.70 | 19 | 25 | 1.00 | 26 | 0 | |||
| 5–10 | 4.40 | 10 | 26 | 1.40 | 18 | 7 | |||
| ≥10 | 3.40 | 2 | 6 | 3.10 | 6 | 10 | |||
| Total | 43 | 10 | |||||||
| 2. Cardiovascular disease ** | |||||||||
| <3 | 1.00 | 51 | 0 | ||||||
| 3–4.9 | 2.10 | 26 | 22 | ||||||
| 5–10 | 2.30 | 18 | 19 | ||||||
| ≥10 | 2.40 | 6 | 7 | ||||||
| Total | 37 | ||||||||
| 3. Cerebrovascular disease ** | |||||||||
| <3 | 1.00 | 51 | 0 | ||||||
| 3–4.9 | 3.00 | 26 | 34 | ||||||
| 5–10 | 4.30 | 18 | 37 | ||||||
| ≥10 | 3.60 | 6 | 13 | ||||||
| Total | 55 | ||||||||
| 4. Osteoporosis ** | |||||||||
| Femoral neck | |||||||||
| <0.5 | 1.00 | 2 | 0 | ||||||
| 0.5–0.75 | 2.09 | 2 | 2 | ||||||
| ≥0.75 | 3.47 | 96 | 70 | ||||||
| Total | 71 | ||||||||
| Hip or spine | |||||||||
| <0.5 | 1.00 | 2 | 0 | ||||||
| 0.5–0.75 | 1.27 | 2 | 1 | ||||||
| ≥0.75 | 4.24 | 96 | 76 | ||||||
| Total | 76 | ||||||||
| 5. Osteoporosis ** | |||||||||
| 0–0.99 | 1.00 | 22 | 0 | ||||||
| 1.00–1.99 | 1.27 | 28 | 7 | ||||||
| ≥2.00 | 2.59 | 49 | 44 | ||||||
| Total | 46 | ||||||||
| Gender | β2-MG (µg/g Cr) | PF | Nephrosis/Nephritis | Heart Failure | Cerebral Infarction | |||
|---|---|---|---|---|---|---|---|---|
| Risk | DALY * | Risk | DALY * | Risk | DALY * | |||
| Male | <300 | 0.37 | 1.00 | 0 | 1.00 | 0 | 1.00 | 0 |
| 300–1000 | 0.17 | 2.44 | 20 | 0.88 | 0 | 2.40 | 19 | |
| 1000–10,000 | 0.29 | 5.67 | 58 | 1.45 | 12 | 4.48 | 50 | |
| ≥10,000 | 0.17 | 18.15 | 74 | 3.69 | 31 | 5.36 | 43 | |
| 82 | 36 | 67 | ||||||
| Female | <300 | 0.55 | 1.00 | 0 | 1.00 | 0 | 1.00 | 0 |
| 300–1000 | 0.18 | 5.43 | 45 | 1.94 | 15 | 1.88 | 14 | |
| 1000–10,000 | 0.20 | 6.94 | 54 | 3.05 | 29 | 3.58 | 34 | |
| ≥10,000 | 0.07 | 54.98 | 80 | 3.19 | 14 | 3.19 | 14 | |
| 86 | 43 | 46 | ||||||
| Cadmium Exposure Level | Male | Female | ||||
|---|---|---|---|---|---|---|
| Risk | PF | DALY * | Risk | PF | DALY * | |
| 1. Prostate cancer ** | ||||||
| <17 | 1.00 | 0 | 0 | |||
| 17–20 | 1.18 | 0 | 0 | |||
| > 20 | 1.29 | 100 | 12 | |||
| Total | 12 | |||||
| 2. Post-menopausal breast cancer * | ||||||
| <13 | 1.00 | 0 | 0 | |||
| 13–16 | 1.12 | 0 | 0 | |||
| >16 | 1.27 | 100 | 21 | |||
| Total | 21 | |||||
| 3. Post-menopausal endometrial cancer * | ||||||
| <13.7 | 1.00 | 0 | 0 | |||
| 13.7–16 | 1.01 | 0 | 0 | |||
| >16 | 1.39 | 100 | 28 | |||
| Total | 28 | |||||
4. Discussion
Recommendation for Policy Makers
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Satarug, S.; Moore, M.R. Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environ. Health Perspect. 2004, 112, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Garrett, S.H.; Sens, M.A.; Sens, D.A. Cadmium, environmental exposure, and health outcomes. Environ. Health Perspect. 2010, 118, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, T.; Plusquin, M.; Hogervorst, J.; Roels, H.A.; Celis, H.; Thijs, L.; Vangronsveld, J.; Hecke, E.V.; Staessen, J.A. Environmental exposure to cadmium and risk of cancer: A prospective population-based study. Lancet Oncol. 2006, 7, 119–126. [Google Scholar] [CrossRef]
- Bernard, A. Cadmium & its adverse effects on human health. Indian J. Med. Res. 2008, 128, 557–564. [Google Scholar] [PubMed]
- Järup, L.; Åkesson, A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef] [PubMed]
- WHO. International Programme on Chemical Safety: Environmental Health Criteria 134. Cadmium; WHO: Geneva, Switzerland, 1992. [Google Scholar]
- Bernard, A. Renal dysfunction induced by cadmium: Biomarkers of critical effects. Biometals 2004, 17, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Simmons, R.; Pongsakul, P.; Saiyasitpanich, D.; Klinphoklap, S. Elevated levels of cadmium and zinc in paddy soils and elevated levels of cadmium in rice grain downstream of a zinc mineralized area in Thailand: Implications for public health. Environ. Geochem. Health 2005, 27, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Swaddiwudhipong, W.; Mahasakpan, P.; Funkhiew, T.; Limpatanachote, P. Changes in cadmium exposure among persons living in cadmium-contaminated areas in northwestern Thailand: A five-year follow-up. J. Med. Assoc. Thai. 2010, 93, 1217–1222. [Google Scholar] [PubMed]
- Swaddiwudhipong, W.; Limpatanachote, P.; Mahasakpan, P.; Krintratun, S.; Padungtod, C. Cadmium-exposed population in Mae Sot District, Tak Province: 1. Prevalence of high urinary cadmium levels in the adults. J. Med. Assoc. Thai. 2007, 90, 143–148. [Google Scholar] [PubMed]
- Swaddiwudhipong, W.; Limpatanachote, P.; Mahasakpan, P.; Krintratun, S.; Punta, B.; Funkhiew, T. Progress in cadmium-related health effects in persons with high environmental exposure in northwestern Thailand: A five-year follow-up. Environ. Res. 2012, 112, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Honda, R.; Swaddiwudhipong, W.; Nishijo, M.; Mahasakpan, P.; Teeyakasem, W.; Ruangyuttikarn, W.; Satarug, S.; Padungtod, C.; Nakagawa, H. Cadmium induced renal dysfunction among residents of rice farming area downstream from a zinc-mineralized belt in Thailand. Toxicol. Lett. 2010, 198, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Limpatanachote, P.; Swaddiwudhipong, W.; Mahasakpan, P.; Krintratun, S. Cadmium-exposed population in Mae Sot District, Tak Province: 2. Prevalence of renal dysfunction in the adults. J. Med. Assoc. Thai. 2009, 92, 1345–1353. [Google Scholar] [PubMed]
- Limpatanachote, P.; Swaddiwudhipong, W.; Nishijo, M.; Honda, R.; Mahasakpan, P.; Nambunmee, K.; Ruangyuttikarn, W. Cadmium-exposed population in Mae Sot District, Tak Province: 4 bone mineral density in persons with high cadmium exposure. J. Med. Assoc. Thai. 2010, 93, 1451–1457. [Google Scholar] [PubMed]
- Nambunmee, K.; Honda, R.; Nishijo, M.; Swaddiwudhipong, W.; Nakagawa, H.; Ruangyuttikarn, W. Bone resorption acceleration and calcium reabsorption impairment in a Thai population with high cadmium exposure. Toxicol. Mech. Methods 2010, 20, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Swaddiwudhipong, W.; Limpatanachote, P.; Nishijo, M.; Honda, R.; Mahasakpan, P.; Krintratun, S. Cadmium-exposed population in Mae Sot district, Tak province: 3. Associations between urinary cadmium and renal dysfunction, hypertension, diabetes, and urinary stones. J. Med. Assoc. Thai. 2010, 93, 231–238. [Google Scholar] [PubMed]
- Swaddiwudhipong, W.; Mahasakpan, P.; Limpatanachote, P.; Krintratun, S. Correlations of urinary cadmium with hypertension and diabetes in persons living in cadmium-contaminated villages in northwestern Thailand: A population study. Environ. Res. 2010, 110, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Swaddiwudhipong, W.; Mahasakpan, P.; Limpatanachote, P.; Krintratun, S. An association between urinary cadmium and urinary stone disease in persons living in cadmium-contaminated villages in northwestern Thailand: A population study. Environ. Res. 2011, 111, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Teeyakasem, W.; Nishijo, M.; Honda, R.; Satarug, S.; Swaddiwudhipong, W.; Ruangyuttikarn, W. Monitoring of cadmium toxicity in a Thai population with high-level environmental exposure. Toxicol. Lett. 2007, 169, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Padungtod, C.; Swaddiwudhipong, W. Health Risk Management for Cadmium Contamination in Thailand: Are Challenges Overcome? 2003. Available online: http://www.who.int/ifcs/documents/forums/forum5/thai_padungtod.pdf (accessed on 29 July 2015).
- Veerman, J.L.; Barendregt, J.J.; Mackenbach, J.P. Quantitative health impact assessment: Current practice and future directions. J. Epidemiol. Community Health 2005, 59, 361–370. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.; Biddulph, J.P.; Utley, M.; Ferguson, J.; Gallivan, S. A health impact assessment model for environmental changes attributable to development projects. J. Epidemiol. Community Health 2002, 56, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; Amann, M.; Anderson, H.R.; Andrews, K.G.; Aryee, M.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the global burden of disease study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef]
- Murray, C.J.; Ezzati, M.; Lopez, A.D.; Rodgers, A.; Hoorn, S.V. Comparative quantification of health risks conceptual framework and methodological issues. Popul. Health Metr. 2003, 1. [Google Scholar] [CrossRef] [Green Version]
- Faroon, O.; Ashizawa, A.; Wright, S.; Tucker, P.; Jenkins, K.; Ingerman, L.; Rudisill, C. Toxicological Profile for Cadmium; Agency for Toxic Substances and Disease Registry Toxicological Profile: Atlanta, GA, USA, 2012. [Google Scholar]
- Cho, Y.A.; Kim, J.; Woo, H.D.; Kang, M. Dietary cadmium intake and the risk of cancer: A meta-analysis. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Levine, B. What does the population attributable fraction mean? Prev. Chronic Dis. 2007, 4, A14. [Google Scholar] [PubMed]
- Hanley, J.A. A heuristic approach to the formulas for population attributable fraction. J. Epidemiol. Community Health 2001, 55, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Magnus, J.; Hentz, J. Urinary cadmium, osteopenia, and osteoporosis in the US population. Osteoporos. Int. 2010, 21, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Engström, A.; Michaëlsson, K.; Suwazono, Y.; Wolk, A.; Vahter, M.; Åkesson, A. Long-term cadmium exposure and the association with bone mineral density and fractures in a population-based study among women. J. Bone Miner. Res. 2011, 26, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Nishijo, M.; Nakagawa, H.; Morikawa, Y.; Sakurai, M.; Nakamura, K.; Kido, T; Nogawa, K.; Min, D. Relationship between urinary cadmium and mortality in habitants of a cadmium-polluted area: A 22-year follow-up study in Japan. Chin. Med. J (Engl.) 2011, 124, 3504–3509. [Google Scholar] [PubMed]
- Nishijo, M.; Morikawa, Y.; Nakagawa, H.; Tawara, K.; Miura, K.; Kido, T.; Ikawa, A.; Kobayashi, E.; Nogawa, K. Causes of death and renal tubular dysfunction in residents exposed to cadmium in the environment. Occup. Environ. Med. 2006, 63, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Akesson, A.; Julin, B.; Wolk, A. Long-term dietary cadmium intake and postmenopausal endometrial cancer incidence: A population-based prospective cohort study. Cancer Res. 2008, 68, 6435–6441. [Google Scholar] [CrossRef] [PubMed]
- Julin, B.; Wolk, A.; Bergkvist, L.; Bottai, M.; Akesson, A. Dietary cadmium exposure and risk of postmenopausal breast cancer: A population-based prospective cohort study. Cancer Res. 2012, 72, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Julin, B.; Wolk, A.; Johansson, J.E.; Andersson, S.O.; Andren, O.; Akesson, A. Dietary cadmium exposure and prostate cancer incidence: A population-based prospective cohort study. Br. J. Cancer 2012, 107, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Tellez-Plaza, M.; Jones, M.R.; Dominguez-Lucas, A.; Guallar, E.; Navas-Acien, A. Cadmium exposure and clinical cardiovascular disease: A systematic review. Curr. Atherosclero. Rep. 2013, 15. [Google Scholar] [CrossRef] [PubMed]
- Nishijo, M.; Nakagawa, H.; Morikawa, Y.; Kuriwaki, J.; Katsuyuki, M.; Kido, T.; Nogawa, K. Mortality in a cadmium polluted area in Japan. Biometals 2004, 17, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.; Suwazono, Y.; Dochi, M.; Honda, R.; Kido, T. Influence of consumption of cadmium-polluted rice or Jinzu River water on occurrence of renal tubular dysfunction and/or Itai-itai disease. Biol. Trace Elem. Res. 2009, 127, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Salam, S.N.; Eastell, R.; Khwaja, A. Fragility fractures and osteoporosis in CKD: Pathophysiology and diagnostic methods. Am. J. Kidney Dis. 2014, 63, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, K.; Ninomiya, T. Stroke and cerebrovascular diseases in patients with chronic kidney disease. Lancet Neurol. 2014, 13, 823–833. [Google Scholar] [CrossRef]
- Noonan, C.W.; Sarasua, S.M.; Campagna, D.; Kathman, S.J.; Lybarger, J.A.; Mueller, P.W. Effects of exposure to low levels of environmental cadmium on renal biomarkers. Environ. Health Perspect. 2002, 110, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.; Okubo, Y.; Suwazono, Y.; Kido, T.; Nogawa, K. Dose–response relationship between total cadmium intake calculated from the cadmium concentration in rice collected from each household of farmers and renal dysfunction in inhabitants of the Jinzu River basin, Japan. J. Appl. Toxicol. 2002, 22, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Maruzeni, S.; Nishijo, M.; Nakamura, K.; Morikawa, Y.; Sakurai, M.; Nakashima, M.; Kido, T.; Okamoto, R.; Nogawa, K.; Suwazono, Y.; Nakagawa, H. Mortality and causes of deaths of inhabitants with renal dysfunction induced by cadmium exposure of the polluted Jinzu River basin, Toyama, Japan; a 26-year follow-up. Environ. Health 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- Kaji, M. Role of experts and public participation in pollution control: The case of Itai-itai disease in Japan. Ethics Sci. Environ. Polit. 2012, 12, 99–111. [Google Scholar] [CrossRef]
- Ferguson, C.C. Assessing risks from contaminated sites: Policy and practice in 16 European countries. Land Contamin. Reclam. 1999, 7, 87–108. [Google Scholar]
- Besante, J.; Niforatos, J.; Mousavi, A. Cadmium in Rice: Disease and Social Considerations. Environ. Forensic. 2011, 12, 121–123. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Songprasert, N.; Sukaew, T.; Kusreesakul, K.; Swaddiwudhipong, W.; Padungtod, C.; Bundhamcharoen, K. Additional Burden of Diseases Associated with Cadmium Exposure: A Case Study of Cadmium Contaminated Rice Fields in Mae Sot District, Tak Province, Thailand. Int. J. Environ. Res. Public Health 2015, 12, 9199-9217. https://doi.org/10.3390/ijerph120809199
Songprasert N, Sukaew T, Kusreesakul K, Swaddiwudhipong W, Padungtod C, Bundhamcharoen K. Additional Burden of Diseases Associated with Cadmium Exposure: A Case Study of Cadmium Contaminated Rice Fields in Mae Sot District, Tak Province, Thailand. International Journal of Environmental Research and Public Health. 2015; 12(8):9199-9217. https://doi.org/10.3390/ijerph120809199
Chicago/Turabian StyleSongprasert, Nisarat, Thitiporn Sukaew, Khanitta Kusreesakul, Witaya Swaddiwudhipong, Chantana Padungtod, and Kanitta Bundhamcharoen. 2015. "Additional Burden of Diseases Associated with Cadmium Exposure: A Case Study of Cadmium Contaminated Rice Fields in Mae Sot District, Tak Province, Thailand" International Journal of Environmental Research and Public Health 12, no. 8: 9199-9217. https://doi.org/10.3390/ijerph120809199





