Prevalence of Genes of OXA-23 Carbapenemase and AdeABC Efflux Pump Associated with Multidrug Resistance of Acinetobacter baumannii Isolates in the ICU of a Comprehensive Hospital of Northwestern China
Abstract
:1. Introduction
2. Experimental Section
2.1. Bacterial Isolation
2.2. Test of Antimicrobial Susceptibility
2.3. Identification of the Drug Resistance Genes
| Target genes | Primer sets | Primer sequence (5′→3′) | Amplicon Size (bp) |
|---|---|---|---|
| Carbapenemase | blaNDM-1 F | GCATTGGCGGCGAAAGTCA | 921 |
| blaNDM-1 R | CTCGCACCGAATGTCTGGC | ||
| blaSIM F | TACAAGGGATTCGGCATCG | 741 | |
| blaSIM R | TAATGGCCTGTTCCCATGTG | ||
| blaVIM F | TTATGGAGCAACCGATGT | 920 | |
| blaVIM R | CAAAAGTCCCGCTCCAACGA | ||
| blaIMP-1 F | ATCCAAGCAGCAAGCGCGTTA | 474 | |
| blaIMP-1 R | AGGCGTGCTGCTGCAACGACTTGT | ||
| blaIMP-4 F | CTACCGCAGCAGAGTCTTTG | 879 | |
| blaIMP-4 R | AACCAGTTTTGCCTTACCAT | ||
| blaOXA-23 F | GATGTGTCATAGTATTCGTCG | 774 | |
| blaOXA-23 R | TCACAACAACTAAAAGCACTG | ||
| blaOXA-24 F | TTCCCCTAACATGAATTTGT | 828 | |
| blaOXA-24 R | GTACTAATCAAAGTTGTGAA | ||
| blaOXA-58 F | AAGTATTGGGGCTTGTGCTG | 800 | |
| blaOXA-58 R | CCCCTCTGCGCTCTACATAC | ||
| blaOXA-51 F | TAATGCTTTGATCGGCCTTG | 760 | |
| blaOXA-51 R | TGGATTGCACTTCATCTTGG | ||
| Multidrug resistance efflux pumps | AdeA F | GGCGTATTGGGCAATCTTTTGT | 1157 |
| AdeA R | GTCACCGACTTTCAAGCCTTTG | ||
| AdeB F | TGGCGGAATGAAGTATGT | 1323 | |
| AdeB R | GCAGTGCGGCAGGTTAG | ||
| AdeC F | GACAATCGTATCTCGTGGACTC | 1331 | |
| AdeC R | AGCAATTTTCTGGTCAGTTTCC | ||
| AdeR F | TCACATGGCTATCTACGGTTGG | 538 | |
| AdeR R | TGAAGGCATGAGTGTTATTCGG | ||
| AdeS F | GTGGACGTTAGGTCAAGTTCTG | 949 | |
| AdeS R | TGTTATCTTTTGCGGCTGTATT |
2.4. Genetic Relationship among A. Baumannii Isolates Determined by Pulse Field Gel Electrophoresis (PFGE)
2.5. Statistical Analysis
3. Results
3.1. Epidemiological Data of Drug-Resistant A. Baumannii Infection in ICU
| Antibiotics | Drug-resistant isolates | MIC ranges (mg/mL) | Drug-susceptible isolates |
|---|---|---|---|
| Trimethoprim/sulfamethoazole | 102/102, (100%) | 4–32/64 | 0/102, (0.0%) |
| Piperacillin | 99/102, (97.1%) | 128–512 | 3/102, (2.9%) |
| Ceftriaxome | 99/102, (97.1%) | 16–256 | 3/102, (2.9%) |
| Ampicillin-sulbactam | 98/102, (96.1%) | 8–256/8 | 4/102, (3.9%) |
| Ticarcillin-clavulanicac | 98/102, (96.1%) | 32–1224/1 | 4/102, (3.9%) |
| Cefepime | 94/102, (92.2%) | 8–512 | 8/102, (7.8%) |
| Ceftazidime | 94/102, (92.2%) | 4–128 | 8/102, (7.8%) |
| Levofloxacin | 92/102, (90.2%) | 2–64 | 10/102, (9.8%) |
| Tobramycin | 90/102, (88.2%) | 4–256 | 12/102, (9.8%) |
| Imipenem | 83/102, (81.4%) | 2–64 | 19/102, (18.6%) |
| Polymyxin B | 13/102, (12.7%) | 1–16 | 89/102, (87.3%) |
3.2. Genes of Antimicrobial Resistance Identified by Multiplex PCR Assays
| Genes | AdeA | AdeB | AdeC | AdeS | AdeR |
|---|---|---|---|---|---|
| blaOXA-23 | 93/78 | 93/73 | 93/83 | 93/78 | 93/76 |
| blaOXA-24 | 0/78 | 0/73 | 0/83 | 0/78 | 0/76 |
| blaOXA-51 | 93/78 | 93/73 | 93/83 | 93/78 | 93/76 |
| blaOXA-58 | 0/78 | 0/73 | 0/83 | 0/78 | 0/76 |
| BlaNDM-1 | 0/78 | 0/73 | 0/83 | 0/78 | 0/76 |
| BlaSIM | 0/78 | 0/73 | 0/83 | 0/78 | 0/76 |
| BlaVIM | 0/78 | 0/73 | 0/83 | 0/78 | 0/76 |
| BlaIMP-1 | 0/78 | 0/73 | 0/83 | 0/78 | 0/76 |
| BlaIMP-4 | 0/78 | 0/73 | 0/83 | 0/78 | 0/76 |
3.3. Impact of Efflux Pumps on the Susceptibility of Clinical MDR A. Baumannii Isolates to Antibiotics
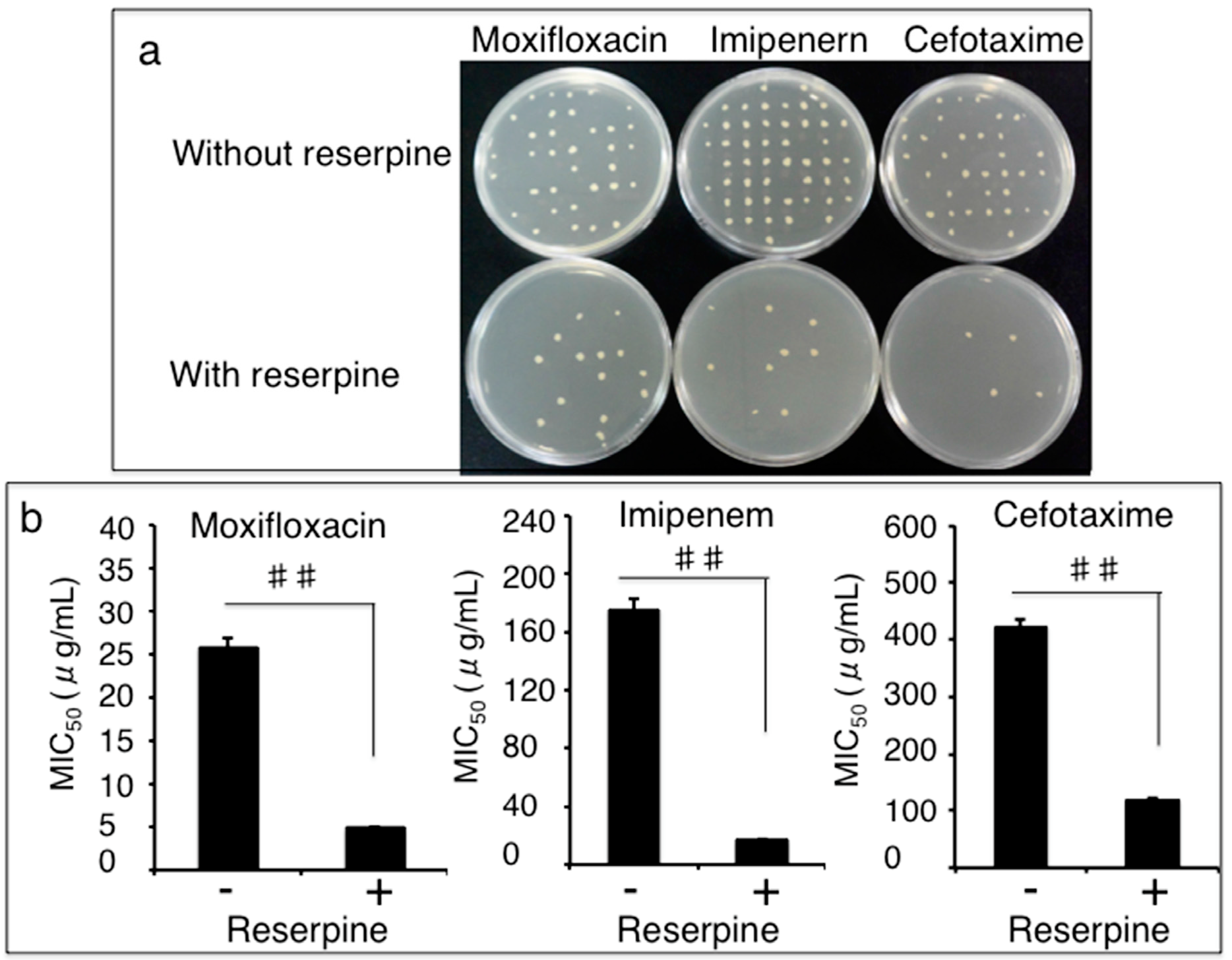
3.4. Clonal Relationship of Drug-Resistant A. Baumannii Isolates Determined by a Pulsed-Field Gel Electrophoresis (PFGE) Method
| Efflux pump AdeABC genes | PFGE groups | Constituent ratio | |||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | Other | ||
| AdeABC, AdeRS | 11 | 12 | 18 | 5 | 3 | 19 | 68/102 (66.7%) |
| AdeABC | 0 | 1 | 0 | 0 | 0 | 3 | 4/102 (3.9%) |
| AdeABC, AdeR | 0 | 0 | 0 | 0 | 0 | 2 | 2/102 (1.9%) |
| AdeABC, AdeS | 2 | 0 | 2 | 0 | 0 | 0 | 4/102 (3.9%) |
| Other genotypes | 3 | 0 | 1 | 3 | 0 | 5 | 12/102 (11.8%) |
| Negative | 0 | 0 | 4 | 7 | 0 | 1 | 12/102 (11.7%) |
| Sum | 16 | 13 | 25 | 15 | 3 | 30 | 102/102 (100%) |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
List of abbreviations
| A. baumannii | Acinetobacter baumannii |
| Ade | Acinetobacter drug efflux |
| ICU | Intensive care unit |
| IMP | metallo-β-lactamase resistant to imipenem |
| MDR | multidrug-resistant |
| NDM | New Delhi metallo-β-lactamase |
| MIC | Minimal inhibitory concentration |
| OXA | oxacillinase |
| PCR | polymerase chain reaction |
| PFGE | pulsed-field gel electrophoresis |
| SIM | seoul imipeneamase |
| VIM | verona integron encoded metallo-β-lactamase |
References
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.T.; Sun, F.J.; Chen, Z.H.; Luo, G.M.; Feng, W.; Xiong, W.; Xia, P.Y. The epidemiology and resistance mechanisms of Acinetobacter baumannii isolates from the respiratory department ICU of a hospital in China. Microb. Drug Resist. 2014, 20, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.B.; Shin, K.S.; Ha, J.; Han, K. Co-existence of blaOXA-23 and armA in multidrug-resistant Acinetobacter baumannii isolated from a hospital in South Korea. J. Med. Microbiol. 2013, 62, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, Y.R.; Kim, J.; Park, Y.J.; Song, W.; Shin, J.H.; Uh, Y.; Lee, K.; Lee, S.H.; Cho, J.H.; et al. Increasing prevalence of blaOXA-23-carrying Acinetobacter baumannii and the emergence of blaOXA-182-carrying Acinetobacter nosocomialis in Korea. Diagn. Microbiol. Infect. Dis. 2013, 77, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.Y.; Koo, S.H.; Cho, H.H.; Kwon, K.C. Nosocomial infection by sequence type 357 multidrug-resistant Acinetobacter baumannii isolates in a neonatal intensive care unit in Daejeon, Korea. Ann Lab Med. 2013, 33, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Lean, S.S.; Suhaili, Z.; Ismail, S.; Rahman, N.I.; Othman, N.; Abdullah, F.H.; Jusoh, Z.; Yeo, C.C.; Thong, K.L. Prevalence and genetic characterization of carbapenem- and polymyxin-resistant Acinetobacter baumannii isolated from a tertiary hospital in Terengganu, Malaysia. ISRN Microbiol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Hasan, B.; Perveen, K.; Olsen, B.; Zahra, R. Emergence of carbapenem-resistant Acinetobacter baumannii in hospitals in Pakistan. J. Med. Microbiol. 2014, 63, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Peymani, A.; Higgins, P.G.; Nahaei, M.R.; Farajnia, S.; Seifert, H. Characterisation and clonal dissemination of OXA-23-producing Acinetobacter baumannii in Tabriz, northwest Iran. Int. J. Antimicrob. Agents 2012, 39, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Aly, M.; Tayeb, H.T.; Al Johani, S.M.; Alyamani, E.J.; Aldughaishem, F.; Alabdulkarim, I.; Balkhy, H.H. Genetic diversity of OXA-51-like genes among multidrug-resistant Acinetobacter baumannii in Riyadh, Saudi Arabia. Euro. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Chen, T.L.; Lee, Y.T.; Huang, L.; Kuo, S.C.; Yu, K.W.; Hsueh, P.R.; Dou, H.Y.; Su, I.J.; Fung, C.P. Dissemination of multidrug-resistant Acinetobacter baumannii carrying blaOXA-23 from hospitals in central Taiwan. J. Microbiol. Immunol. Infect. 2013, 46, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Apisarnthanarak, A.; Pinitchai, U.; Warachan, B.; Warren, D.K.; Khawcharoenporn, T.; Hayden, M.K. Effectiveness of infection prevention measures featuring advanced source control and environmental cleaning to limit transmission of extremely-drug resistant Acinetobacter baumannii in a Thai intensive care unit: An analysis before and after extensive flooding. Am. J. Infect. Control 2014, 42, 116–121. [Google Scholar] [PubMed]
- Xiao, Y.; Wei, Z.; Shen, P.; Ji, J.; Sun, Z.; Yu, H.; Zhang, T.; Ji, P.; Ni, Y.; Hu, Z.; et al. Bacterial-resistance among outpatients of county hospitals in China: Significant geographic distinctions and minor differences between central cities. Microbes Infect. 2015. [Google Scholar] [CrossRef] [PubMed]
- Perez, F.; Hujer, A.M.; Hujer, K.M.; Decker, B.K.; Rather, P.N.; Bonomo, R.A. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2007, 51, 3471–3484. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Z.; Nikaido, H. Efflux-mediated drug resistance in bacteria: An update. Drugs 2009, 69, 1555–1623. [Google Scholar] [CrossRef] [PubMed]
- Rumbo, C.; Gato, E.; Lopez, M.; Ruiz de Alegria, C.; Fernandez-Cuenca, F.; Martinez-Martinez, L.; Vila, J.; Pachon, J.; Cisneros, J.M.; Rodriguez-Bano, J.; et al. Contribution of efflux pumps, porins, and beta-lactamases to multidrug resistance in clinical isolates of Acinetobacter baumannii. Antimicrob. Agents Chemother. 2013, 57, 5247–5257. [Google Scholar] [CrossRef] [PubMed]
- Siroy, A.; Cosette, P.; Seyer, D.; Lemaitre-Guillier, C.; Vallenet, D.; Van Dorsselaer, A.; Boyer-Mariotte, S.; Jouenne, T.; De, E. Global comparison of the membrane subproteomes between a multidrug-resistant Acinetobacter baumannii strain and a reference strain. J. Proteome Res. 2006, 5, 3385–3398. [Google Scholar] [CrossRef] [PubMed]
- Toledo, P.V.M.; Arend, L.N.; Pilonetto, M.; Costa Oliveira, J.C.; Luhm, K.R. Surveillance programme for multidrug-resistant bacteria in healthcare-associated infections: An urban perspective in South Brazil. J. Hosp. Infect. 2012, 80, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Dolejska, M.; Villa, L.; Poirel, L.; Nordmann, P.; Carattoli, A. Complete sequencing of an IncHI1 plasmid encoding the carbapenemase NDM-1, the ArmA 16s RNA methylase and a resistance-nodulation-cell division/multidrug efflux pump. J. Antimicrob. Chemother. 2013, 68, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.S.; Yao, S.M.; Fung, C.P.; Hsieh, Y.P.; Liu, C.P.; Lin, J.F. An OXA-66/OXA-51-like carbapenemase and possibly an efflux pump are associated with resistance to imipenem in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2007, 51, 3844–3852. [Google Scholar] [CrossRef] [PubMed]
- Koh, T.H.; Sng, L.H.; Wang, G.C.; Hsu, L.Y.; Zhao, Y. Carbapenemase and efflux pump genes in Acinetobacter calcoaceticus—Acinetobacter baumannii complex strains from Singapore. J. Antimicrob. Chemother. 2007, 60, 1173–1174. [Google Scholar] [CrossRef] [PubMed]
- Seecoomar, G.D.; Marmol, B.C.; Kwon, D.H. Promoter deletions of Klebsiella pneumoniae carbapenemase (KPC)-encoding genes (blaKPC-2) and efflux pump (AcrAB) on β-lactam susceptibility in KPC-producing Enterobacteriaceae. FEMS Microbiol. Lett. 2013, 348, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard-9th Edition; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2014. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.F.; Jiang, J.P.; Xu, N.; Huang, Z.M.; Wang, Y.Y. Inhibitory effects of reserpine and carbonyl cyanide m-chloro-phenylhydrazone on fluoroquinolone resistance of Acinetobacter baumannii. Chin. Med. J. 2005, 118, 340–343. [Google Scholar] [PubMed]
- Jia, W.; Li, G.; Wang, W. Prevalence and antimicrobial resistance of Enterococcus species: A hospital-based study in China. Int. J. Environ. Res. Public Health 2014, 11, 3424–3442. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Manos, J. Pulsed-field gel electrophoresis of Pseudomonas aeruginosa. Methods Mol. Biol. 2015, 1301, 157–170. [Google Scholar] [PubMed]
- Seifert, H.; Dolzani, L.; Bressan, R.; van der Reijden, T.; van Strijen, B.; Stefanik, D.; Heersma, H.; Dijkshoorn, L. Standardization and interlaboratory reproducibility assessment of pulsed-field gel electrophoresis-generated fingerprints of Acinetobacter baumannii. J. Clin. Microbiol. 2005, 43, 4328–4335. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.R. Emerging carbapenemases: A global perspective. Int. J. Antimicrob. Agents 2010, 36, S8–S14. [Google Scholar] [CrossRef]
- Liu, L.L.; Ji, S.J.; Ruan, Z.; Fu, Y.; Fu, Y.Q.; Wang, Y.F.; Yu, Y.S. Dissemination of blaOXA-23 in Acinetobacter spp. in China: Main roles of conjugative plasmid pAZJ221 and transposon TN2009. Antimicrob. Agents Chemother. 2015, 59, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.E.; Bell, J.M.; Turnidge, J.D.; Castanheira, M.; Jones, R.N. Emergence and widespread dissemination of OXA-23, -24/40 and -58 carbapenemases among Acinetobacter spp. in Asia-Pacific nations: Report from the SENTRY Surveillance Program. J. Antimicrob. Chemother. 2009, 63, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.L.; Lee, Y.T.; Kuo, S.C.; Hsueh, P.R.; Chang, F.Y.; Siu, L.K.; Ko, W.C.; Fung, C.P. Emergence and distribution of plasmids bearing the blaOXA-51-like gene with an upstream ISAba1 in carbapenem-resistant Acinetobacter baumannii isolates in Taiwan. Antimicrob. Agents Chemother. 2010, 54, 4575–4581. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ruan, Z.; Feng, Y.; Fu, Y.; Jiang, Y.; Wang, H.; Yu, Y. Species distribution of clinical Acinetobacter isolates revealed by different identification techniques. PloS ONE 2014, 9, e104882. [Google Scholar] [CrossRef] [PubMed]
- Abbott, I.; Cerqueira, G.M.; Bhuiyan, S.; Peleg, A.Y. Carbapenem resistance in Acinetobacter baumannii: Laboratory challenges, mechanistic insights and therapeutic strategies. Expert Rev. Anti Infect. Ther. 2013, 11, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Novovic, K.; Mihajlovic, S.; Vasiljevic, Z.; Filipic, B.; Begovic, J.; Jovcic, B. Carbapenem-resistant Acinetobacter baumannii from Serbia: Revision of CarO classification. PloS ONE 2015, 10, e0122793. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.Y.; Jiang, D.Y.; Xu, P.C.; Zhang, Y.K.; Shi, H.F.; Cao, H.L.; Wu, Q. An investigation of drug-resistant Acinetobacter baumannii infections in a comprehensive hospital of East China. Ann. Clin. Microbiol. Antimicrob. 2015. [Google Scholar] [CrossRef] [PubMed]
- Higgins, P.G.; Poirel, L.; Lehmann, M.; Nordmann, P.; Seifert, H. OXA-143, a novel carbapenem-hydrolyzing class D β-lactamase in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2009, 53, 5035–5038. [Google Scholar] [CrossRef] [PubMed]
- Coelho, J.M.; Turton, J.F.; Kaufmann, M.E.; Glover, J.; Woodford, N.; Warner, M.; Palepou, M.F.; Pike, R.; Pitt, T.L.; Patel, B.C.; et al. Occurrence of carbapenem-resistant Acinetobacter baumannii clones at multiple hospitals in London and Southeast England. J. Clin. Microbiol. 2006, 44, 3623–3627. [Google Scholar] [CrossRef] [PubMed]
- Yoon, E.J.; Courvalin, P.; Grillot-Courvalin, C. RND-type efflux pumps in multidrug-resistant clinical isolates of Acinetobacter baumannii: Major role for adeabc overexpression and adeRS mutations. Antimicrob. Agents Chemother. 2013, 57, 2989–2995. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.F.; Chen, X.Y.; Yan, G.F.; Wang, Y.P.; Ying, C.M. Study of the correlation of imipenem resistance with efflux pumps AdeABC, AdeIJK, AdeDE and AbeM in clinical isolates of Acinetobacter baumannii. Chemotherapy 2012, 58, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Yum, J.H.; Kim, C.K.; Yong, D.; Jeon, E.H.; Jeong, S.H.; Ahn, J.Y.; Lee, K. Role of OXA-23 and AdeABC efflux pump for acquiring carbapenem resistance in an Acinetobacter baumannii strain carrying the blaOXA-66 gene. Ann. Clin. Lab. Sci. 2010, 40, 43–48. [Google Scholar] [PubMed]
- Ruzin, A.; Keeney, D.; Bradford, P.A. AdeABC multidrug efflux pump is associated with decreased susceptibility to tigecycline in Acinetobacter calcoaceticus—Acinetobacter baumannii complex. J. Antimicrob. Chemother. 2007, 59, 1001–1004. [Google Scholar] [CrossRef] [PubMed]
- Coyne, S.; Courvalin, P.; Perichon, B. Efflux-mediated antibiotic resistance in Acinetobacter spp. Antimicrob. Agents Chemother. 2011, 55, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Yoon, E.J.; Nait Chabane, Y.; Goussard, S.; Snesrud, E.; Courvalin, P.; De, E.; Grillot-Courvalin, C. Contribution of resistance-nodulation-cell division efflux systems to antibiotic resistance and biofilm formation in Acinetobacter baumannii. mBio 2015. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, W.; Li, C.; Zhang, H.; Li, G.; Liu, X.; Wei, J. Prevalence of Genes of OXA-23 Carbapenemase and AdeABC Efflux Pump Associated with Multidrug Resistance of Acinetobacter baumannii Isolates in the ICU of a Comprehensive Hospital of Northwestern China. Int. J. Environ. Res. Public Health 2015, 12, 10079-10092. https://doi.org/10.3390/ijerph120810079
Jia W, Li C, Zhang H, Li G, Liu X, Wei J. Prevalence of Genes of OXA-23 Carbapenemase and AdeABC Efflux Pump Associated with Multidrug Resistance of Acinetobacter baumannii Isolates in the ICU of a Comprehensive Hospital of Northwestern China. International Journal of Environmental Research and Public Health. 2015; 12(8):10079-10092. https://doi.org/10.3390/ijerph120810079
Chicago/Turabian StyleJia, Wei, Caiyun Li, Haiyun Zhang, Gang Li, Xiaoming Liu, and Jun Wei. 2015. "Prevalence of Genes of OXA-23 Carbapenemase and AdeABC Efflux Pump Associated with Multidrug Resistance of Acinetobacter baumannii Isolates in the ICU of a Comprehensive Hospital of Northwestern China" International Journal of Environmental Research and Public Health 12, no. 8: 10079-10092. https://doi.org/10.3390/ijerph120810079





