Cross-Sectional Study of Hepatitis A Virus Infection in the Pantanal Population before Vaccine Implementation in Brazil: Usage of Non-Invasive Specimen Collection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Aspects
2.2. Sample Collection and Processing
2.3. Sample Screening
2.4. Studied Population
2.5. Statistical Analysis
3. Results
3.1. Study Design and Patients
3.2. Sociodemographic Characteristics of the Studied Population
| Variable | Studied Population (n) | Studied Population (%) |
|---|---|---|
| Age group (years) | ||
| 0–10 | 46 | 20.53% |
| 11–20 | 50 | 22.32% |
| 21–30 | 47 | 20.98% |
| 31–40 | 26 | 11.61% |
| 41–50 | 29 | 12.95% |
| >50 | 26 | 11.61% |
| Gender | ||
| Female | 100 | 43.90% |
| Male | 124 | 56.10% |
| Race/Ethnicity | ||
| Caucasian | 67 | 29.91% |
| Brown | 55 | 29.00% |
| Black | 41 | 18.30% |
| Amerindian | 37 | 16.52% |
| Asian | 8 | 3.57% |
| Not reported | 6 | 2.70% |
| Educational level | ||
| None | 19 | 8.48% |
| First grade | 156 | 69.60% |
| Second grade | 32 | 14.29% |
| Graduated | 14 | 6.25% |
| Not reported | 3 | 1.38% |
| Family income | ||
| 1 minimun salary | 107 | 47.80% |
| 2 minimun salary | 95 | 42.40% |
| 3 minimun salary | 19 | 8.50% |
| Not reported | 3 | 1.30% |
| Community | ||
| Serra do Amolar/São Lourenço | 45 | 20.09% |
| Paraguai-Mirim | 60 | 26.78% |
| Porto da Manga | 23 | 10.27% |
| Passo do Lontra | 96 | 42.86% |
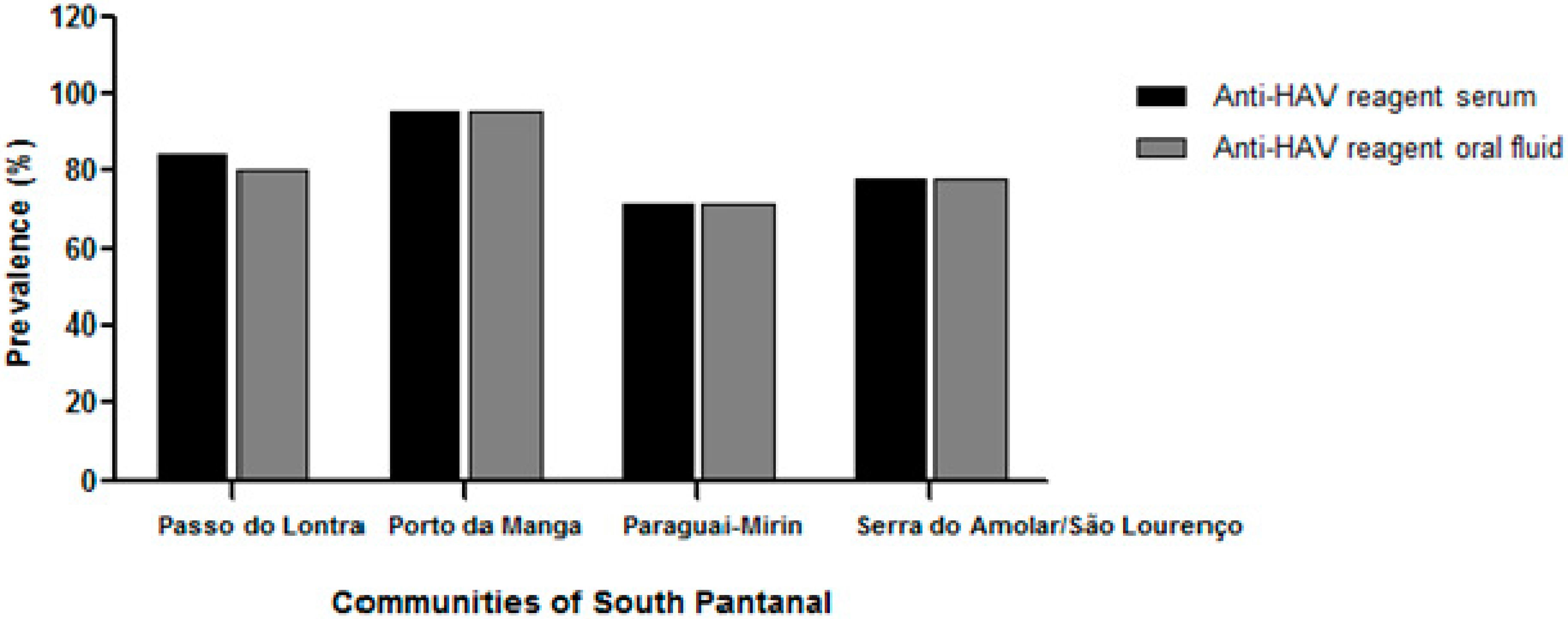
3.3. Anti-HAV Antibodies Detection in Serum and Oral Fluid Samples
3.4. Factors Associated with Total Anti-HAV Positivity
| Variable | Serum | Oral Fluid (ChemBio®) | ||||
|---|---|---|---|---|---|---|
| Positive (n = 181) | Negative (n = 43) | p | Positive (n = 177) | Negative (n = 47) | p | |
| Age (years), median (interval) | 27 (5.0–86.0) | 10 (3.0–72.0) | <0.0001 | 26 (5.0–86.0) | 11 (3.0–77.0) | <0.0001 |
| Gender, n (%) | 0.9175 | 0.9953 | ||||
| Male | 101 (55.8) | 23 (53.4) | 98 (53.3) | 26 (55.3) | ||
| Female | 80 (44.2) | 20 (46.6) | 79 (46.7) | 21 (44.7) | ||
| Race/Ethnicity, n (%) | 0.0312 | 0.2797 | ||||
| Caucasian | 33 (18.2) | 14 (32.6) | 51 (28.8) | 16 (34.0) | ||
| Brown | 58 (32.0) | 7 (16.3) | 57 (32.2) | 8 (17.0) | ||
| Black | 53 (29.3) | 8 (18.6) | 32 (18.1) | 9 (19.3) | ||
| Amerindian | 26 (14.4) | 11 (25.6) | 26 (14.7) | 11 (23.4) | ||
| Asian | 6 (3.3) | 2 (4.6) | 6 (6.4) | 2 (4.2) | ||
| NR a | 5 (2.8) | 1 (2.3) | 5 (2.8) | 1 (2.1) | ||
| Domestic residents, n (%) | 0.1589 | 0.7919 | ||||
| 0–1 | 17 (9.4) | 5 (11.6) | 16 (9.0) | 6 (12.8) | ||
| 2–3 | 56 (30.9) | 4 (9.3) | 53 (29.9) | 7 (14.9) | ||
| 4–5 | 41 (22.7) | 14 (32.6) | 40 (22.6) | 15 (31.9) | ||
| 6–7 | 33 (18.2) | 9 (20.9) | 32 (18.1) | 10 (21.3) | ||
| >7 | 33 (18.2) | 10 (23.3) | 35 (19.8) | 8 (17.0) | ||
| NR | 1 (0.6) | 1 (2.3) | 1 (0.6) | 1 (2.1) | ||
| Educational level, n (%) | 0.8396 | 0.7248 | ||||
| None | 14 (7.7) | 5 (11.6) | 14 (7.9) | 5 (10.7) | ||
| First grade | 127 (70.2) | 29 (67.5) | 126 (71.2) | 30 (63.8) | ||
| Second grade | 28 (15.5) | 4 (9.3) | 25 (14.1) | 7 (14.9) | ||
| Graduated | 10 (5.5) | 4 (9.3) | 10 (5.6) | 4 (8.5) | ||
| NR | 2 (1.1) | 1 (2.3) | 2 (1.2) | 1 (2.1) | ||
| Familiar income (minimum salary), n (%) | 0.7331 | 0.8599 | ||||
| ≤1 | 82 (45.3) | 25 (58.1) | 81 (45.7) | 26 (55.3) | ||
| 2 | 85 (47.0) | 10 (23.3) | 82 (46.3) | 13 (27.7) | ||
| 3 | 12 (6.6) | 7 (16.3) | 12 (6.8) | 7 (14.9) | ||
| NR | 2 (1.1) | 1 (2.3) | 2 (1.2) | 1 (2.1) | ||
| Drinking water (source), n (%) | 0.2302 | 0.4174 | ||||
| Untreated (river) | 56 (30.9) | 11 (25.6) | 56 (31.6) | 11 (23.4) | ||
| Treated (river) b | 107 (59.2) | 30 (69.8) | 104 (58.6) | 33 (70.2) | ||
| Bottled (mineral water) | 16 (8.8) | 1 (2.3) | 5 (2.8) | 2 (4.2) | ||
| NR | 2 (1.1) | 1 (2.3) | 2 (1.2) | 2 (4.2) | ||
| History of hepatitis A, n (%) | 0.0903 | 0.2506 | ||||
| No | 161 (89.0) | 42 (97.7) | 159 (89.8) | 44 (93.7) | ||
| Yes | 16 (8.8) | 0 (0.0) | 15 (8.5) | 1 (2.1) | ||
| NR | 4 (2.2) | 1 (2.3) | 3 (1.7) | 2 (4.2) | ||
| Community, n (%) | 0.0562 | 0.1157 | ||||
| Passo do Lontra | 81 (44.8) | 15 (34.2) | 77 (43.5) | 19 (40.4) | ||
| Porto da Manga | 22 (12.2) | 1 (2.3) | 22 (12.4) | 1 (2.1) | ||
| Paraguai-Mirim | 43 (23.7) | 17 (39.5) | 43 (24.3) | 17 (36.2) | ||
| Serra do Amolar/São Lourenço | 35 (19.3) | 10 (23.3) | 35 (19.7) | 10 (21.3) | ||
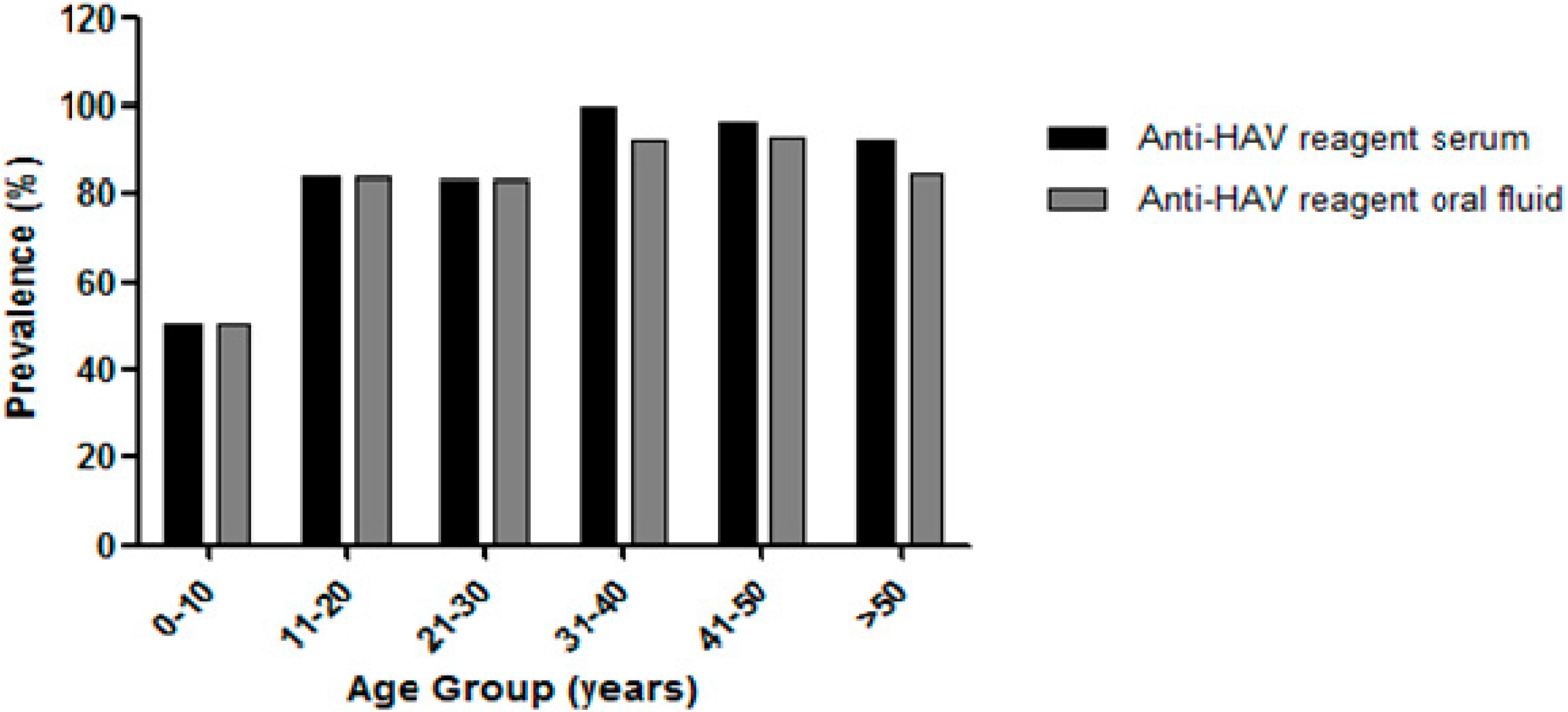
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tourinho, R.S.; de Almeida, A.J.; Amado, L.A.; Villar, L.M.; Castro, A.R.; de Paula, V.S. Could oral fluid be used to evaluate anti-hepatitis A virus status in individuals living in difficult-to-access areas? Vaccine 2012, 30, 6421–6426. [Google Scholar] [CrossRef] [PubMed]
- Sartori, A.M.; de Soárez, P.C.; Novaes, H.M.; Amaku, M.; de Azevedo, R.S.; Moreira, R.C.; Pereira, L.M.; Ximenes, R.A.; Martelli, C.M. Cost-effectiveness analysis of universal childhood hepatitis A vaccination in Brazil: Regional analyses according to the endemic context. Vaccine 2012, 30, 7489–7497. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Lee, H.S. Hepatitis A: Clinical manifestations and management. Intervirology 2010, 53, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Matheny, S.C.; Kingery, J.E. Hepatitis A. Am. Fam. Phys. 2012, 86, 1027–1034. [Google Scholar]
- Shapiro, C.N.; Margolis, H.S. Worldwide epidemiology of hepatitis A virus infection. J. Hepatol. 1993, 18, S11–S14. [Google Scholar] [CrossRef]
- Mackinney-Novelo, I.; Barahona-Garrido, J.; Castillo-Albarran, F.; Santiago-Hernández, J.J.; Méndez-Sánchez, N.; Uribe, M.; Chávez-Tapia, N. Clinical course and management of acute hepatitis A infection in adults. Ann. Hepatol. 2012, 11, 652–657. [Google Scholar] [PubMed]
- Jayakumar, S.; Chowdhury, R.; Ye, C.; Karvellas, C.J. Fulminant viral hepatitis. Crit. Care Clin. 2013, 29, 677–697. [Google Scholar] [CrossRef] [PubMed]
- De Alencar Ximenes, R.A.; Martelli, C.M.; Merchán-Hamann, E.; Montarroyos, U.R.; Braga, M.C.; de Lima, M.L.; Cardoso, M.R.; Turchi, M.D.; Costa, M.A.; de Alencar, L.C.; et al. Hepatitis Study Group. Multilevel analysis of hepatitis A infection in children and adolescents: A household survey in the Northeast and Central-west regions of Brazil. Int. J. Epidemiol. 2008, 37, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, R.A.; Pereira, L.M.; Martelli, C.M.; Merchán-Hamann, E.; Stein, A.T.; Figueiredo, G.M.; Braga, M.C.; Montarroyos, U.R.; Brasil, L.M.; Turchi, M.D.; et al. Methodology of a nationwide cross-sectional survey of prevalence and epidemiological patterns of hepatitis A, B and C infection in Brazil. Cad. Saude Pública 2010, 26, 1693–1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parry, J.V. Detection of viral antibodies in saliva specimens as an alternative to serum. Diagnosis of hepatitis A and B by testing saliva. J. Clin. Chem. Clin. Biochem. 1989, 27, 245–246. [Google Scholar] [PubMed]
- Morris-Cunnington, M.C.; Edmunds, W.J.; Miller, E.; Brown, D.W.G. A population-based seroprevalence study of hepatitis A virus using oral fluid in England and Wales. Am. J. Epidemiol. 2004, 159, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.H. Prevenção da hepatite B e delta. Braz. J. Infect. Dis. 2006, 10, S72–S78. (In Portuguese) [Google Scholar]
- Alho, C.J.R.; Lacher, T.E., Jr.; Gonçalves, H.C. Environmental degradation in the Pantanal ecosystem. BioScience 1988, 38, 164–171. [Google Scholar]
- Moraes, A.S.; Resende, E.K.; Rodrigues, C.A.G.; Mauro, R.A.; Galdino, S.; Oliveira, M.D.; Crispim, S.M.A.; Vieira, L.M.; Soriano, B.M.A.; Abreu, U.G.P.; et al. Embrapa Pantanal: 25 anos de pesquisas em prol da conservação do Pantanal, os desafios do novo milênio. In III Simpósio Sobre Recursos Naturais e Sócio-Econômicos do Pantanal: O Desafio do novo Milênio; Embrapa Pantanal: Corumbá, Brazil, 2001; p. 55. (In Portuguese) [Google Scholar]
- Adámoli, J. Fisiografia do Pantanal. In Recursos Forrageiros Nativos do Pantanal Mato-Grossense; Allem, A.C., Valls, J.F.M., Eds.; Documento 8, Embrapa Cenargen: Brasília, Brazil, 1987; pp. 15–22. [Google Scholar]
- Silva, J.V.; Abdon, M.M. Delimitação do Pantanal brasileiro e suas sub-regiões. Pesqui. Agropecu. Bras. 1998, 33, 1703–1711. (In Portuguese) [Google Scholar]
- Magalhães, N.W. O Pantanal: Aspectos gerais. In Conheça o Pantanal, 1st ed.; Cap 1, Terragraph: São Paulo, Brazil, 1992; pp. 7–12. [Google Scholar]
- Altman, D.G. Practical Statistics for Medical Research; Chapman and Hall: London, UK, 1991. [Google Scholar]
- Fiore, A.E.; Wasley, A.; Bell, B.P. Prevention of hepatitis A through active or passive immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Rep. Recomm. Rep. 2006, 55, 1–23. [Google Scholar]
- FitzSimons, D.; Hendrickx, G.; Vorsters, A.; van Damme, P. Hepatitis A and E: Update on prevention and epidemiology. Vaccine 2010, 28, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Parry, J.V. Simple and reliable salivary test for HIV and hepatitis A and B virus diagnosis and surveillance. Ann. N.Y. Acad. Sci. 1993, 694, 216–233. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, P.G.; Laszlo, J.; Appleyard, K.; Ogden, G.R. Modified enzyme immunoassay to detect hepatitis C virus antibodies in oral fluid. Eur. J. Microbiol. Infect. Dis. 1996, 15, 882–884. [Google Scholar] [CrossRef]
- Ochnio, J.J.; Scheifele, D.H.; Ho, M.; Mitchell, L.A. New, ultrasensitive enzyme immunoassay for detecting vaccineand disease-induced hepatitis A virus-specific immunoglobulin G in saliva. J. Clin. Microbiol. 1997, 35, 98–101. [Google Scholar] [PubMed]
- Oba, I.T.; Spina, A.M.M.; Saraceni, C.P.; Lemos, M.F.; Senhoras, R.C.F.A.; Moreira, R.C.; Granato, C.F.H. Detection of hepatitis A antibodies by ELISA using saliva as clinical samples. Rev. Med. Trop. São Paulo 2000, 42, 197–200. [Google Scholar] [CrossRef]
- Chohan, B.H.; Lavreys, L.; Mandaliya, K.N.; Kreiss, J.K.; Bwayo, J.J.; Ndinya-Achola, J.O.; Martin, H.L., Jr. Validation of a modified commercial enzyme-linked immunoassay for detection of human immunodeficiency virus type 1 immunoglobulin G antibodies in saliva. Clin. Diagn. Lab. Immunol. 2001, 8, 346–348. [Google Scholar] [CrossRef] [PubMed]
- Amado, L.A.; Villar, L.M.; de Paula, V.S.; de Almeida, A.J.; Gaspar, A.M.C. Detection of hepatitis A, B and C virus-specific antibodies using oral fluid for epidemiological studies. Mem. Inst. Oswaldo Cruz 2006, 101, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.K.; Cohen, M.E.; Bienek, D.R. Efficiency of oral fluid collection devices in extracting antibodies. Oral Microbiol. Immunol. 2009, 24, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.E.; Cutts, F.T.; Samuel, R.; Diaz-Ortega, J.L. Control of rubella and congenital rubella syndrome (CRS) in developing countries: A global review, Part II, Vaccination against Rubella. Bull. World Health Organ. 1997, 75, 69–80. [Google Scholar] [PubMed]
- Clemens, S.A.; Da Fonseca, J.C.; Azevedo, T.; Cavalcanti, A.; Silveira, T.R.; Castilho, M.C.; Clemens, R. Hepatitis A and hepatitis B seroprevalence in 4 centers in Brazil. Rev. Soc. Bras. Med. Trop. 2000, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Barzaga, N.G. Hepatitis A shifting epidemiology in South-East Asia and China. Vaccine 2000, 18, S61–S64. [Google Scholar] [CrossRef]
- Tufenkeji, H. Hepatitis A shifting epidemiology in the Middle East and Africa. Vaccine 2000, 18, S65–S67. [Google Scholar] [CrossRef]
- Arankalle, V.A.; Chadha, M.S. Who should receive hepatitis A vaccine? J. Viral Hepat. 2003, 10, 157–158. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.; Tavares-Neto, J.; Queiroz-Andrade, M.; Dias, C.; Ribeiro, T.; Silva, F.; Silva-Araújo, J.; Tatsch, F.; Paraná, R. Sociodemographical aspects of seroprevalence of hepatitis A virus in the settlement of Cavunge, a semi-arid region of Bahia State. Rev. Soc. Bras. Med. Trop. 2006, 39, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Braga, W.S.; Borges, F.G.; Barros Junior, G.M.; Martinho, A.C.; Rodrigues, I.S.; Azevedo, E.P.; Davis, G.H.; Queiroz, M.B.; Santos, S.H.; Barbosa, T.V.; et al. Prevalence of hepatitis A virus infection: The paradoxical example of isolated communities in the western Brazilian Amazon region. Rev. Soc. Bras. Med. Trop. 2009, 42, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Gröschl, M.; Köhler, H.; Topf, H.G.; Rupprecht, T.; Rauh, M. Evaluation of saliva collection devices for the analysis of steroids, peptides and therapeutic drugs. J. Pharm. Biomed. Anal. 2008, 47, 478–486. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tourinho, R.S.; De Almeida, A.J.; Villar, L.M.; Murat, P.G.; Capelin, G.J.M.; Castro, A.R.C.M.; De Paula, V.S. Cross-Sectional Study of Hepatitis A Virus Infection in the Pantanal Population before Vaccine Implementation in Brazil: Usage of Non-Invasive Specimen Collection. Int. J. Environ. Res. Public Health 2015, 12, 7357-7369. https://doi.org/10.3390/ijerph120707357
Tourinho RS, De Almeida AJ, Villar LM, Murat PG, Capelin GJM, Castro ARCM, De Paula VS. Cross-Sectional Study of Hepatitis A Virus Infection in the Pantanal Population before Vaccine Implementation in Brazil: Usage of Non-Invasive Specimen Collection. International Journal of Environmental Research and Public Health. 2015; 12(7):7357-7369. https://doi.org/10.3390/ijerph120707357
Chicago/Turabian StyleTourinho, Renata Santos, Adilson José De Almeida, Livia Melo Villar, Paula Guerra Murat, Gina Jonasson Mousquer Capelin, Ana Rita Coimbra Motta Castro, and Vanessa Salete De Paula. 2015. "Cross-Sectional Study of Hepatitis A Virus Infection in the Pantanal Population before Vaccine Implementation in Brazil: Usage of Non-Invasive Specimen Collection" International Journal of Environmental Research and Public Health 12, no. 7: 7357-7369. https://doi.org/10.3390/ijerph120707357





