The Significance of Experiences of Nature for People with Parkinson’s Disease, with Special Focus on Freezing of Gait—The Necessity for a Biophilic Environment. A Multi-Method Single Subject Study
Abstract
:1. Introduction
- Can FOG be triggered by walking through narrow passages in Nature?
- Can FOG be triggered to the same extent in passages in natural contexts when adding a built element?
- Can FOG be triggered when the peripheral visual field is limited?
- Can our findings be explained by or generate any theories or hypotheses?
2. Experimental Section
2.1. Subjects
2.2. Venue and Experimental Conditions
2.3. Quantitative Data
2.3.1. Questionnaire
2.3.2. Accelerometer
2.4. Qualitative Data
2.4.1. Interviews
2.4.2. Observations
2.5. Pre-Test Procedure
2.6. Test Procedure
- (i)
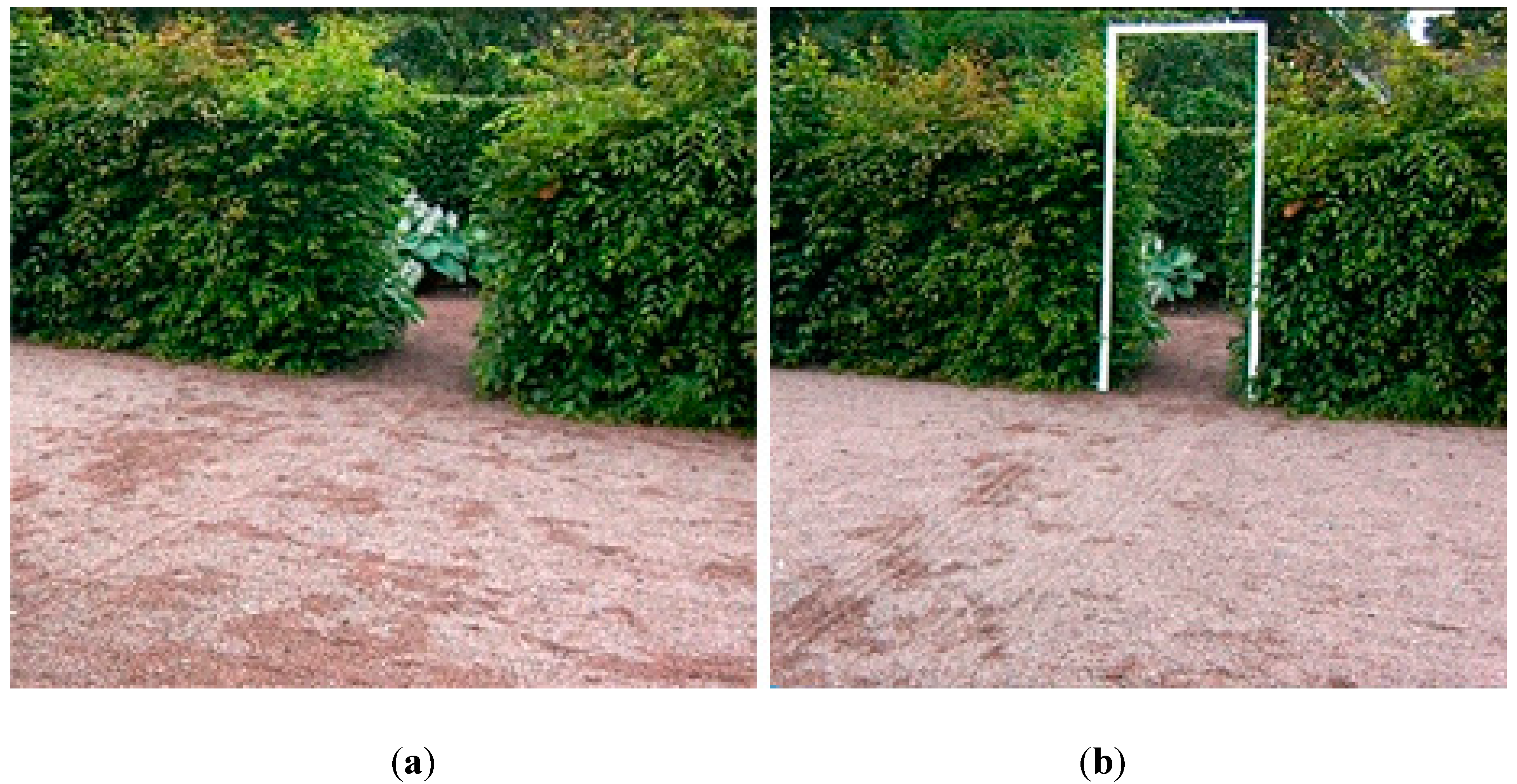
- Walking test through a hedge opening 80 cm wide. This was repeated three times.
- (ii)
- A white doorframe (Figure 1(b)) was placed in the hedge opening. Its inner measurements were 80 cm wide by 210 cm high. The second walking test included the frame, highly visible in the opening; all other aspects were the same. This was repeated three times.
- (iii)
- Walking test through the hedge opening without the frame. This was repeated three times.
- (i)
- Hedge opening with a white doorframe which was placed in the hedge opening. It measured 80 cm wide by 210 cm high. The walking test included the frame, highly visible in the opening; all other aspects were the same. This was repeated three times.
- (ii)
- Hedge opening with a white doorframe. This was repeated three times.
- (iii)
- Hedge opening with a white doorframe. This was repeated three times.
- (i)
- Walking test through a hedge opening 67.5 cm wide. This was repeated three times.
- (ii)
- A white doorframe was placed in the hedge opening. It measured 67.5 cm wide by 210 cm high. The second walking test included the frame, highly visible in the opening; all other aspects were the same. This was repeated three times.
- (iii)
- Walking test through the hedge opening without the frame. This was repeated three times.
- (i)
- Walking test through a hedge opening 67.5 cm wide. This was repeated three times.
- (ii)
- Walking test through a hedge opening 67.5 cm wide while limiting the subject’s field of peripheral vision using a paper cylinder to blinker him. This was repeated three times.
- (iii)
- Walking test through the hedge opening without a paper cylinder to blinker the subject. This was repeated three times.
- (i)
- Walking test through a hedge opening 67.5 cm wide. This was repeated three times.
- (ii)
- A white doorframe was placed in the hedge opening. It measured 67.5 cm wide by 210 cm high. The second walking test included the frame, and limiting the subject’s field of peripheral vision using a paper cylinder to blinker him. This was repeated three times.
- (iii)
- Walking test through the hedge opening without the frame or the paper cylinder. This was repeated three times.
3. Results
3.1. Interviews before the Test
| Subject | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Cognitive experience in connection with FOG | Feeling of insecurity and discomfort | Having trouble thinking and expressing himself | Unfocused, having difficulties thinking and expressing himself, having difficulties remembering | Problems concentrating when tired, having trouble keeping his eyes open | Difficulties with speech; slurs his words. Better when in a good mood. |
| Physical experience in connection with FOG | Experiencing strong locking | Stiff and locked, finding it difficult to control his body, getting uncontrolled body movements | Stiff, difficulties lifting legs | Noticing a distinct stiffness, having difficulties with balance. Falling sometimes | Distinct stiffness, and aches |
| Strategy | Deliberately uses unusual behavior to walk, “unnatural”, for example, walks backwards, skips or jump with both feet together. | Walks with small, skipping steps, which were more common in the past, now used more rarely | Skips, concentrates on lifting legs | Dances. Eyes closed upon passage | Skips, and turns in his elbow upon passage |
| Differences in walking, outdoors/indoors | Moves more easily outdoors | Doesn’t know | Moves more easily outdoors | Moves more easily outdoors | Missing data |
3.2. Tests
| Subject | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Weather conditions during the tests | 20 °C nice weather, cloudy with “rain in the air” | 20 °C Sunny, variable with rainfall. High humidity. Light wind. | 14 °C. Light rain before and after the tests. Light wind | 8 °C Cloudy, no wind | 8°C Cloudy, no wind |
| 360 degree turn | Very strong FOG-reaction, jump with rollator | Very strong FOG-reaction, very stiff | Strong FOG-reaction, did not use his stick | Observable FOG-reaction, skipping steps | Observable FOG-reaction, turning on his heel |
| Walking aids | Walker | No aids | Stick | No aids | No aids |
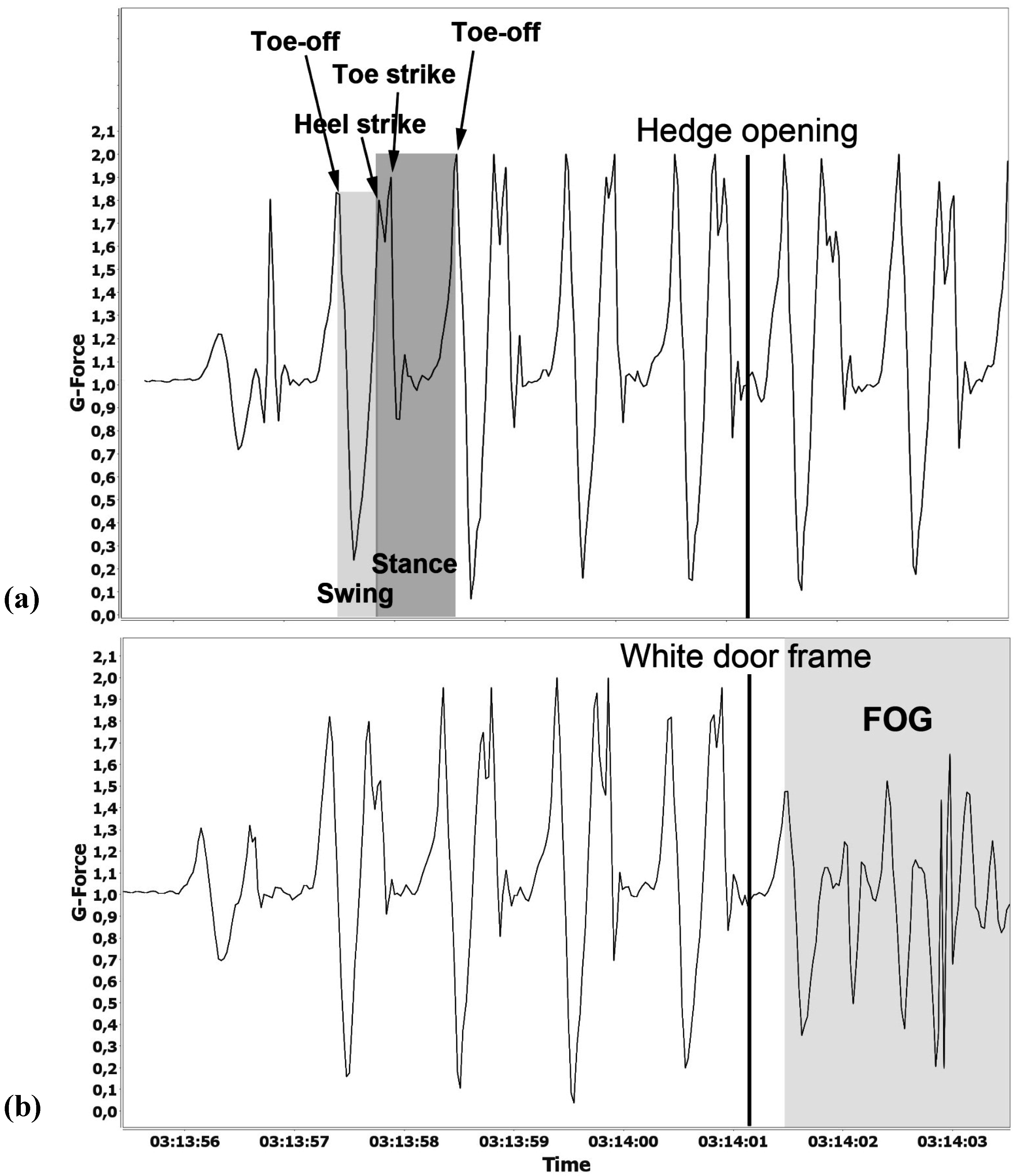
| Observed FOG, without doorframe | Not observable | Not observable | Not observable | Not observable | Not observable |
| Accelerometer, without doorframe | No deviations | No deviations | No deviations | No deviations | No deviations |
| Self-estimated FOG, Without doorframe (mean values) | 0.3 | 0 | 0 | 5.0 | 0 |
| Observed FOG, doorframe | Very strong | Not observable | Not observable | Strong | Not observable |
| Accelerometer, doorframe | Strong deviations | No deviations | No deviations | Strong deviations | Deviations |
| Self-estimated FOG at doorframe (mean values) | 7.3 | 0 | 0 | 6.0 | 6.0 |
| Self-estimated FOG Overall, during the test situation, (mean values) | 5.0 | 5.4 | 4.7 | 7.7 | 6.0 |
| Observed changes in walking patterns. Overall, during the test situation | More constrained walking pattern; e.g., pulling up shoulders, change regarding the placement of one of his feet | Increased stiffness, less co-movement | Increased stiffness, stronger extra movements. Impaired coordination, does not use the walking cane anymore—Carries it instead | Increased stiffness, increased absence of co-movements | Increased rigidity, narrower step width, more bent knees and hip joints, increased posture leaning forward |
| Observed changes in walking patterns when passing through the hedge opening with the doorframe | Very strong FOG-reaction, his right foot turned inward, and there was a distinct slowness and stiffness (tardiness) in movements, and strong lockings. | No FOG reaction could be observed | No observable effect on walking behavior through the actual passages | A distinct FOG reaction and a clear observable decrease in stride length and walking speed before and during passing through the doorframe. Rigid movements, and observable that it becomes harder and harder to move as the FOG increases | No observable change in stride length, walking speed or posture when passing through the openings |
| Differences between upper and lower body regarding FOG reactions. Overall, during the test | None | FOG reactions only in the upper body | Apparent FOG reaction in the upper body, not regarding walking pattern, but the subject felt himself that his legs were affected | None | None, but he felt that his legs were affected |
| Observed strategies to avoid FOG | Jumping with the aid of the walker. | No change in the gait pattern can be observed. Marches. No tendency to FOG | Gait pattern changes due to increased stiffness, but no tendency to FOG | He closes his eyes | Increases his walking pace, half running |
| Subject | 1 | 2 |
|---|---|---|
| Self-estimated overall FOG when walking through the hedge opening (mean value) | 6.00 | 1.60 |
| FOG reaction when walking through the hedge opening | No | No |
| Self-estimated overall FOG when walking through the doorframe (mean value) | 6.05 | 6.60 |
| FOG reaction when walking through the doorframe | Yes | No |
| Subject number one | Overall FOG | FOG when Passing through the Doorframe | FOG Reaction |
|---|---|---|---|
| Self-estimated FOG hedge opening (mean value) | 8 | 0,5 | No |
| Self-estimated FOG doorframe (mean value) | 7 | 7 | Yes |
| Self-estimated FOG hedge opening and paper cylinder (mean value) | 7 | 4.9 | Yes |
| Self-estimated FOG doorframe and paper cylinder (mean value) | 8 | 7,5 | Yes |
3.3. Observations by a Physical Therapist
4.Discussion
4.1. Main Findings
4.2. Discussion, Related to Mechanisms of Information and Action
5. Conclusions: A Need for a Biophilic Environment?
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yao, S.C.; Hart, A.D.; Terzella, M.J. An evidence-based osteopathic approach to Parkinson disease. Osteopath. Fam. Phys. 2013, 5, 96–101. [Google Scholar] [CrossRef]
- De Lau, L.M.L.; Breteler, M.M.B. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006, 5, 525–535. [Google Scholar] [CrossRef]
- 1177 Vårdguiden. Parkinsons sjukdom. Available online: http://www.1177.se/Fakta-och-rad/Sjukdomar/Parkinsons-sjukdom/ (accessed on 28 August 2014).
- Knobl, P.; Kiestra, L.; Almeida, Q. The relationship between motor planning and freezing of gait in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2012, 83, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.J.G.; Barker, R.A. A pathophysiological model of freezing of gait in Parkinson’s disease. Parkinsonism Relat. Disord. 2009, 15, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Giladi, N.; Hausdorff, J. The role of mental function in the pathogenesis of freezing of gait in Parkinson’s disease. J. Neurol. Sci. 2006, 248, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Bartels, A.L.; Balash, T.; Gurevich, T.; Schaafsma, J.D.; Hausdorff, J.M.; Giladi, N. Relationship between freezing of gait (FOG) and other features of Parkinson’s: FOG is not correlated with bradykinesia. J. Clin. Neurosci. 2003, 10, 584–588. [Google Scholar] [CrossRef]
- Okuma, Y. Freezing of gait in Parkinson’s disease. J. Neurol. 2006, 253, VII27–VII32. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, J.D.; Balash, Y.; Gurevich, T.; Bartels, A.L.; Hausdorff, J.M.; Giladi, N. Characterization of freezing of gait subtypes and the response of each to levodopa in Parkinson’s disease. Eur. J. Neurol. 2003, 10, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Amboni, M.; Cozzolino, A.; Longo, K.; Picillo, M.; Barone, P. Freezing of gait and executive functions in patients with Parkinson’s disease. Mov. Disord. 2008, 23, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.Q.; Lebold, A.C. Freezing of gait in Parkinson’s disease: A perceptual cause for a motor impairment? J. Neurol. Neurosurg. Psychiatry 2010, 81, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Giladi, N. Freezing of gait: Risk factors and clinical characteristics. Parkinsonism Relat. Disord. 2006, 12. [Google Scholar] [CrossRef]
- Bodis-Wollner, I. Neuropsychological and perceptual defects in Parkinson’s disease. Parkinsonism Relat. Disord. 2003, 9, 83–89. [Google Scholar] [CrossRef]
- Giladi, N.; Huber-Mahlin, V.; Herman, T.; Hausdorff, J.M. Freezing of gait in older adults with high level gait disorders: Association with impaired executive function. J. Neural. Transm. 2007, 114, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- Lundström, J.; Ullberg, J. Upplevelsen av Kroppen hos Personer med Parkinsons Sjukdom; Luleå University of Technology: Luleå, Sweden, 2013. (In Swedish) [Google Scholar]
- Liebermann, A. Are freezing of gait (FOG) and panic related? J. Neurol. Sci. 2006, 248, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Sunvisson, H.; Lökk, J.; Ericson, K.; Winblad, B.; Ekman, S-L. Changes in motor performance in persons with Parkinson’s disease after exercise in a mountain area. J. Neurosci. Nurs. 1997, 29, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Sunvisson, H.; Ekman, S. Environmental influences on the experiences of people with Parkinson’s disease. Nurs. Inq. 2001, 8, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Annerstedt, M.; Währborg, P. Nature assisted therapy: Systematic review of controlled and observational studies. Scand. J. Public Health 2011, 39, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.O.; Gang, M.H.; Jung, K.S. Effects of horticultural therapy program on state-anxiety, fatigue and quality of life among women cancer survivors. Asian Oncol. Nurs. 2012, 12, 125–131. [Google Scholar] [CrossRef]
- Verra, M.L.; Angst, F.; Beck, T.; Lehmann, S.; Brioschi, R.; Schneider, R.; Aeschlimann, A. Horticultural therapy for patients with chronic musculoskeletal pain. Altern. Ther. Health Med. 2012, 18, 44–50. [Google Scholar] [PubMed]
- Wang, H-M.; Shih, H.Y.; Hsu, P.Y.; Li, M.H.; Kao, M.Y.; Lai, Y.L. Rainbow in life—Horticultural therapy for terminal cancer patients. J. Exp. Clin. Med. 2013, 5, 85. [Google Scholar] [CrossRef]
- Kotozaki, Y. The psychological effect of horticultural therapy intervention on earthquake-related stress in women of earthquake-related areas. J. Transl. Med. Epidemiol. 2014. Available online: http://www.jscimedcentral.com/TranslationalMedicine/Articles/translationalmedicine-2-1008.pdf (accessed on 29 June 2015). [Google Scholar]
- Jarrot, S.E.; Gigliotti, C.M. Comparing responses to horticultural-based and traditional activities in dementia care programs. Amer. J. Alzheimer’s Dis. 2010, 25, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-Y.; Park, S.-A.; Song, J.-E.; Son, K.-C. Horticultural therapy program for the improvement of attention and sociality in children with intellectual disabilities. Horttechnology. 2012, 22, 320–324. [Google Scholar]
- Yun, S.-Y.; Choi, B.-J. Effect of horticultural therapy on the stress and serum cortisol of demented elders. Korean J. Hortic. Sci. 2010, 28, 891–894. [Google Scholar]
- Bay-Richter, C.; Träskman-Bendz, L.; Grahn, P.; Brundin, L. Garden rehabilitation stabilises IFN-gamma and IL-2 levels but does not relieve depressive-symptoms. Neurol. Psychiatry Brit. 2012. [Google Scholar] [CrossRef]
- Währborg, P.; Petersson, I.F.; Grahn, P. Nature-assisted rehabilitation for reactions to severe stress and/or depression in a rehabilitation garden: Long-term follow-up including comparisons with a matched population-based reference cohort. J. Rehabil. Med. 2014, 46, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Kim, G.S.; Mattson, N.S.; Kim, W.S. Effects of horticultural occupational therapy on the physical and psychological rehabilitation of patients with hemiplegia after stroke. Korean J. Hortic. Sci. 2010, 28, 884–890. [Google Scholar]
- Bundy, A.C.; Lane, S.J.; Murray, E.A. Sensory Integration—Theory and Practice; F.A. Davis Company: Philadelphia, PA, USA, 2002. [Google Scholar]
- Ulrich, R.S. Biophilia, biophobia and natural landscapes. In The Biophilia Hypothesis; Kellert, S.R., Wilson, E.O., Eds.; Island Press Shearwater: Washington, DC, USA, 1993; pp. 73–137. [Google Scholar]
- Ulrich, R.S.; Simons, R.F.; Losito, B.D.; Fiorito, E.; Miles, M.A.; Zelson, M. Stress recovery during exposure to natural and urban environments. J. Environ. Psychol. 1991, 11, 201–230. [Google Scholar] [CrossRef]
- Hartig, T. Three steps to understanding restorative environments as health resources. In Open Space: People Space; Ward Thompson, C., Travlou, P., Eds.; Taylor & Francis: London, UK, 2007; pp. 163–179. [Google Scholar]
- Bell, S. Landscape: Pattern, Perception and Process.; E & FN Spon: New York, NY, USA, 1999. [Google Scholar]
- Grahn, P.; Tenngart Ivarsson, C.; Stigsdotter, U.K.; Bengtsson, I.-L. Using affordances as a health-promoting tool in a therapeutic garden. In Innovative Approaches to Researching Landscape and Health; Ward Thompson, C., Bell, S., Aspinall, P., Eds.; Taylor & Francis: London, UK, 2010; pp. 116–154. [Google Scholar]
- Ulrich, R.S. Effects of gardens on health outcomes: Theory and research. In Healing Gardens: Therapeutic Benefits and Design Recommendations; Cooper-Marcus, C., Barnes, M., Eds.; John Wiley: New York, NY, USA, 1999; pp. 27–86. [Google Scholar]
- Kaplan, R.; Kaplan, S. The Experience of Nature. A Psychological Perspective; Cambridge University Press: New York, NY, USA, 1989. [Google Scholar]
- Singer, W.; Gray, C.M. Visual feature integration and the temporal correlation hypothesis. Annu. Rev. Neurosci. 1995, 18, 555–586. [Google Scholar] [CrossRef] [PubMed]
- Ward Thompson, C. Linking landscape and health: The recurring theme. Landsc. Urban. Plan. 2011, 99, 187–195. [Google Scholar] [CrossRef]
- Hausdorff, J.M.; Schaafsma, J.D.; Balash, Y.; Bartels, A.L.; Gurevich, T.; Giladi, N. Impaired regulation of stride variability in Parkinson’s disease subjects with freezing of gait. Exp. Brain Res. 2003, 149, 187–194. [Google Scholar] [PubMed]
- Redmond, L.; Suddick, K. The lived experience of freezing in people with Parkinson’s. Int. J. Ther. Rehabil. 2013, 19, 169–177. [Google Scholar] [CrossRef]
- Cooper, J.O.; Heron, T.E.; Heward, W.L. Applied Behavior Analysis, 2nd ed.; Prentice Hall: New York, NY, USA, 2007. [Google Scholar]
- Tripodi, T. A Primer on Single-Subject Design for Clinical Social Workers; National Association of Social Workers (NASW) Press: Washington, DC, USA, 1998. [Google Scholar]
- Kazdin, A. Single-Case Research Designs; Oxford University Press: New York, NY, USA, 1982. [Google Scholar]
- Scollon, S.N.; Kim-Prieto, C.; Diener, E. Experience sampling: Promises and pitfalls, strengths and weaknesses. J. Happiness Stud. 2003, 4, 5–34. [Google Scholar] [CrossRef]
- Heavey, C.L. Confronting the challenges of observing inner experience: The descriptive experience sampling method. In Self-Observation in the Social Sciences; Clegg, J.W., Ed.; Transaction Publishers: New Brunswick, NJ, USA, 2013; pp. 103–120. [Google Scholar]
- Clegg, J.W. Developing an adequate theory of self-observation. In Self-Observation in the Social Sciences; Clegg, J.W., Ed.; Transaction Publishers: New Brunswick, NJ, USA, 2013; pp. 3–21. [Google Scholar]
- Shiffman, S.; Stone, A.A.; Hufford, M.R. Ecological momentary assessment. Annu. Rev. Clin. Psychol. 2008, 4, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Gillies, G.E.; Pienaar, I.S.; Vohra, S.; Qamhawi, Z. Sex differences in Parkinson’s disease. Front. Neuroendocrinol. 2014, 35, 370–384. [Google Scholar] [CrossRef] [PubMed]
- Haaxma, C.A.; Bloem, B.R.; Borm, G.F.; Oyen, W.J.G.; Leenders, K.L.; Eshuis, S.; Booij, J.; Dluzen, D.E.; Horstink, M.W.I.M. Gender differences in Parkinson’s disease. J. Neurol. Neurosurg. Psyciatry. 2007, 78, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Colombo, D.; Abbruzzese, G.; Antonini, A.; Barone, P.; Bellia, G.; Franconi, F.; Simoni, L.; Attar, M.; Zagni, E.; Haggiag, S.; et al. The “Gender Factor” in wearing-off among patients with Parkinson’s disease. Sci. World J. 2015, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yin, R. Case Study Research; Sage Publications: Thousand Oaks, CA, USA, 2003. [Google Scholar]
- Giorgi, A. The Descriptive Phenomenological Method in Psychology; Duquesne University Press: Pittsburgh, PA, USA, 2009. [Google Scholar]
- Aspers, P. Empirical phenomenology: A qualitative research approach. Indo-Pac. J. Phenomenol. 2009, 9, 1–12. [Google Scholar] [CrossRef]
- Stigsdotter, U.; Grahn, P. Experiencing a garden: A healing garden for people suffering from burnout diseases. J. Ther. Hortic. 2003, 14, 38–48. [Google Scholar]
- Kvale, S. Den. kvalitativa forskningsintervjun; Studentlitteratur: Lund, Sweden, 1996. (In Swedish) [Google Scholar]
- Graneheim, U.H.; Lundman, B. Qualitative content analysis in nursing research: Concepts, procedures and measures to achieve trustworthiness. Nurse Educ. Today 2004, 24, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Spildooren, J.; Vercruysse, S.; Desloovere, K.; Vandenberghe, W.; Kerckhofs, E.; Nieuwboer, A. Freezing of gait in Parkinson’s disease: The impact of dual-tasking and turning. Mov. Disord. 2010, 25, 2563–2570. [Google Scholar] [CrossRef] [PubMed]
- Ottosson, J.; Grahn, P. The role of natural settings in crisis rehabilitation. How does the level of crisis influence the response to experiences of nature with regard to measures of rehabilitation? Landsc. Res. 2008, 33, 51–70. [Google Scholar] [CrossRef]
- Ottosson, J.; Lavesson, L.; Pinzke, S. Frysningar vanligast inomhus. Parkinson Journalen. 2012, 3, 24–25. (In Swedish) [Google Scholar]
- Chaudhuri, K.R.; Martinez-Martin, P.; Schapira, A.H.V.; Stocchi, F.; Sethi, K.; Odin, P.; Brown, R.G.; Koller, W.; Barone, P.; MacPhee, G.; et al. International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson’s disease: The NMSQuest study. Mov. Disord. 2006, 21, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Hüllert, M. Informativa Strukturer och Egenskaper som stöd för Automatiska Processer i Människans Informationshantering; Cognition Program, University of Skövde: Skövde, Sweden, 1998. (In Swedish) [Google Scholar]
- Ulrich, R.S.; Lundén, O.; Eltinge, J.L. Effects of exposure to nature and abstract pictures on patients recovering from heart surgery. Psychophysiology 1993, 30. [Google Scholar] [CrossRef]
- Appleton, J. The Experience of Landscape; John Wiley and Sons: New York, NY, USA, 1975. [Google Scholar]
- Nesse, R.M. Evolutionary explanations of Emotions. Hum. Nat.-Int. Biosoc. 1990, 1, 261–289. [Google Scholar] [CrossRef] [PubMed]
- Marks, I.; Nesse, R.M. An evolutionary perspective on panic disorder and agoraphobia. Ethol. Sociobiol. 1994, 15, 247–261. [Google Scholar] [CrossRef]
- Kaplan, S. Meditation, restoration, and the management of mental fatigue. Environ. Behav. 2001, 33, 480–506. [Google Scholar] [CrossRef]
- Kaplan, S.; Berman, M.G. Directed attention as a common resource for executive functioning and self-regulation. Perspect. Psychol. Sci. 2010, 5, 43–57. [Google Scholar] [CrossRef]
- Kahneman, D. Thinking, Fast and Slow; Farrar, Straus and Giroux: New York, NY, USA, 2012. [Google Scholar]
- Buschman, T.J.; Miller, E.K. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science 2007, 315, 1860–1862. [Google Scholar] [CrossRef] [PubMed]
- Dominey, P.; Decety, J.; Broussolle, E. Motor imagery of a lateralized sequential task is asymmetrically slowed in hemi-Parkinson’s patients. Neuropsychologia. 1995, 33, 727–741. [Google Scholar] [CrossRef]
- Gibson, E.J.; Walk, R.D. The “visual cliff”. Sci. Am. 1960, 202, 67–71. [Google Scholar] [CrossRef]
- Damasio, A. Self Comes to Mind: Constructing the Conscious. Brain; Pantheon Books: New York, NY, USA, 2010. [Google Scholar]
- Öhman, A. Fear and anxiety. Overlaps and dissociations. In Handbook of Emotions; Lewis, M., Haviland-Jones, J.M., Feldman Barrett, L., Eds.; The Guilford Press: New York, USA, 2008; pp. 709–729. [Google Scholar]
- Heerwagen, J.; Hase, B. Building biophilia: Connecting people to nature in building design. Environ. Des. Construct. 2001, 30–36. [Google Scholar]
- Markevych, I.; Thiering, E.; Fuertes, E.; Sugiri, D.; Berdel, D.; Koletzko, S.; von Berg, A.; Bauer, C-P.; Heinrich, J. A cross-sectional analysis of the effects of residential greenness on blood pressure in 10-year old children: Results from the GINIplus and LISAplus studies. BMC Public Health 2014. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.O. Biophilia.: The Human Bond. with Other Species; Harvard University Press: Cambridge, MA, USA, 1984. [Google Scholar]
- Kellert, S.A.; Wilson, E.O. (Eds.) The Biophilia. Hypothesis; Island Press Shearwater: Washington, DC, USA, 1993.
- Ottosson, J. The Importance of Nature in Coping; Swedish University of Agricultural Sciences: Alnarp, Sweden, 2007. [Google Scholar]
- Bar, M. The proactive brain: Using analogies and associations to generate predictions. Trends Cogn. Sci. 2007, 11, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Seligman, M.E.P. Phobias and preparedness. Behav. Ther. 1971, 2, 307–320. [Google Scholar] [CrossRef]
- Öhman, A.; Mineka, S. Fear, phobias and preparedness: Toward an evolved module of fear and fear learning. Psychol. Rev. 2001, 108, 483–522. [Google Scholar] [CrossRef] [PubMed]
- LeDoux, J. The emotional brain, fear, and the amygdala. Cell. Mol. Neurobiol. 2003, 23, 727–738. [Google Scholar] [CrossRef] [PubMed]
- LeDoux, J. Information flow from sensation to emotion. In Learning and Computational Neuroscience; Gabriel, M., Moore, J., Eds.; Bradford Books, MIT Press: New York, NY, USA, 1990; pp. 3–51. [Google Scholar]
- Ottosson, J.; Grahn, P. Measures of restoration in geriatric care residences. J. Hous. Elder. 2006, 19, 229–258. [Google Scholar] [CrossRef]
- Luria, A.R. Higher Cortical Functions in Man; Basic Books: New York, NY, USA, 1980. [Google Scholar]
- Ambrose, A.F.; Paul, G.; Hausdorff, J.M. Risk factors for falls among older adults: A review of the literature. Maturitas. 2013, 75, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Lord, S.R.; Dayhew, J. Visual risk factors for falls in older people. J. Amer. Geriatr. Soc. 2001, 49, 508–515. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ottosson, J.; Lavesson, L.; Pinzke, S.; Grahn, P. The Significance of Experiences of Nature for People with Parkinson’s Disease, with Special Focus on Freezing of Gait—The Necessity for a Biophilic Environment. A Multi-Method Single Subject Study. Int. J. Environ. Res. Public Health 2015, 12, 7274-7299. https://doi.org/10.3390/ijerph120707274
Ottosson J, Lavesson L, Pinzke S, Grahn P. The Significance of Experiences of Nature for People with Parkinson’s Disease, with Special Focus on Freezing of Gait—The Necessity for a Biophilic Environment. A Multi-Method Single Subject Study. International Journal of Environmental Research and Public Health. 2015; 12(7):7274-7299. https://doi.org/10.3390/ijerph120707274
Chicago/Turabian StyleOttosson, Johan, Lillian Lavesson, Stefan Pinzke, and Patrik Grahn. 2015. "The Significance of Experiences of Nature for People with Parkinson’s Disease, with Special Focus on Freezing of Gait—The Necessity for a Biophilic Environment. A Multi-Method Single Subject Study" International Journal of Environmental Research and Public Health 12, no. 7: 7274-7299. https://doi.org/10.3390/ijerph120707274





