Introducing Simple Detection of Bioavailable Arsenic at Rafaela (Santa Fe Province, Argentina) Using the ARSOlux Biosensor
Abstract
:1. Introduction
1.1. The Problem of Arsenic in the World and in Argentina
1.2. Commonly Used Detection Methods and Their Limitations
1.3. Arsenic Biosensor Measurement
2. Materials and Methods
2.1. Site Description and Water Sampling

2.2. Water Testing with the ARSOlux Biosensor
2.3. Specific Considerations/Preferences for the Correct Use of the Biosensor
2.4. Water Testing with the Arsenic Quick™ Kit
2.5. Chemical Analysis
2.6. Correlation Analysis
3. Results
3.1. Correlations
| Parameter | Pearson Correlation Coefficient | Significance | Spearman Correlation Coefficient | Significance |
|---|---|---|---|---|
| pH | 0.601 | 0.018 | 0.835 | 0.00011 |
| EC | −0.107 | 0.705 | −0.557 | 0.031 |
| DO | −0.443 | 0.098 | −0.361 | 0.187 |
| IC | 0.344 | 0.209 | 0.246 | 0.376 |
| TOC | −0.439 | 0.102 | −0.486 | 0.0664 |
| Al | −0.147 | 0.602 | −0.258 | 0.354 |
| B | 0.570 | 0.026 | 0.259 | 0.351 |
| Ca | −0.288 | 0.298 | −0.836 | 0.0001 |
| Cr | 0.058 | 0.838 | −0.091 | 0.746 |
| Fe | −0.307 | 0.265 | −0.721 | 0.0024 |
| Mn | −0.201 | 0.472 | −0.611 | 0.0156 |
| Si | −0.559 | 0.0302 | −0.753 | 0.0012 |
| V | 0.960 | <0.0001 | 0.909 | <0.0001 |
| F− | 0.687 | 0.0066 | 0.663 | 0.0011 |
| NO3− | −0.214 | 0.462 | −0.499 | 0.0694 |
| Cl− | −0.132 | 0.652 | −0.574 | 0.032 |
| HCO3− | 0.331 | 0.228 | 0.229 | 0.412 |
| SO42− | −0.104 | 0.712 | −0.55 | 0.0337 |
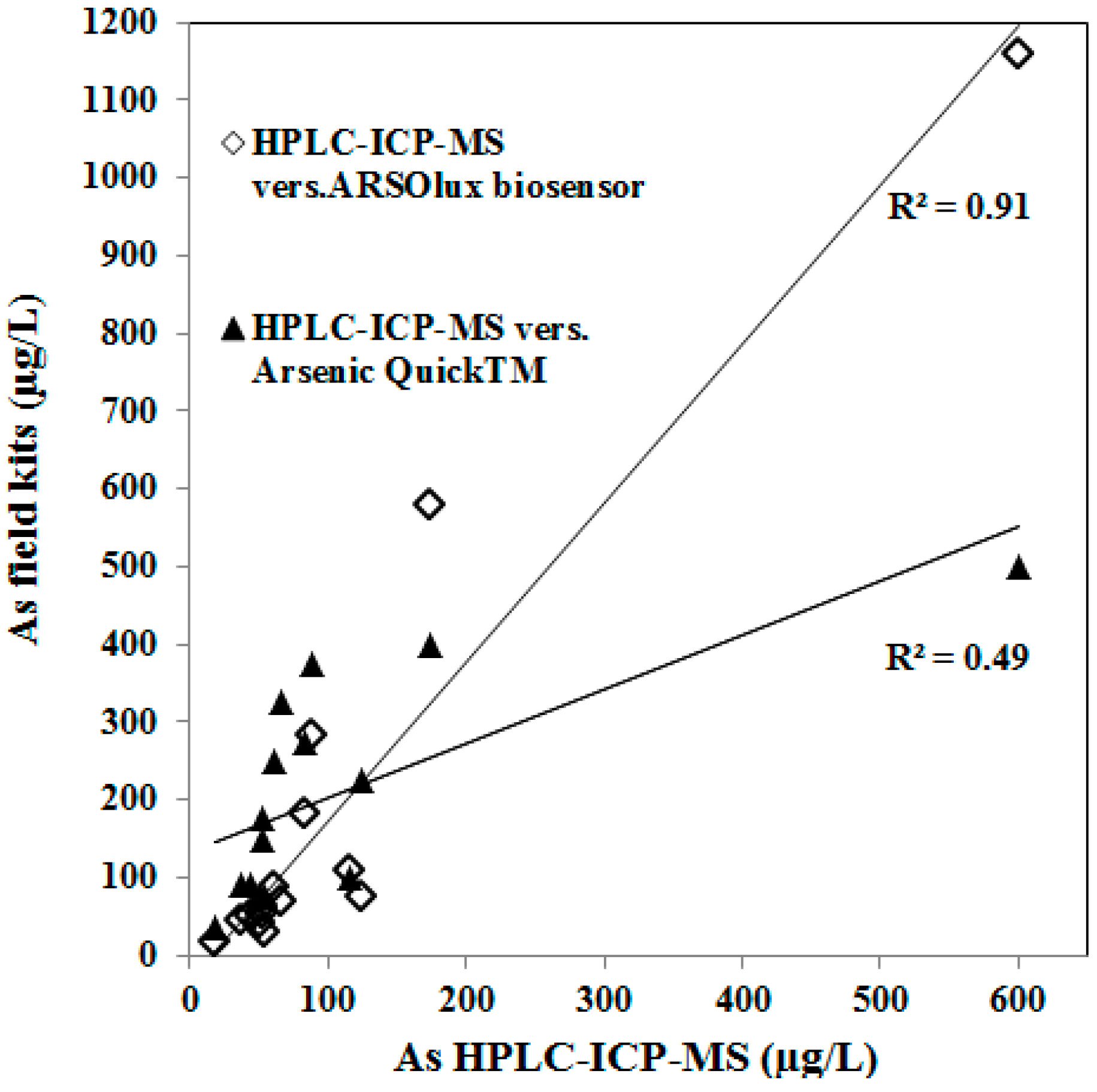
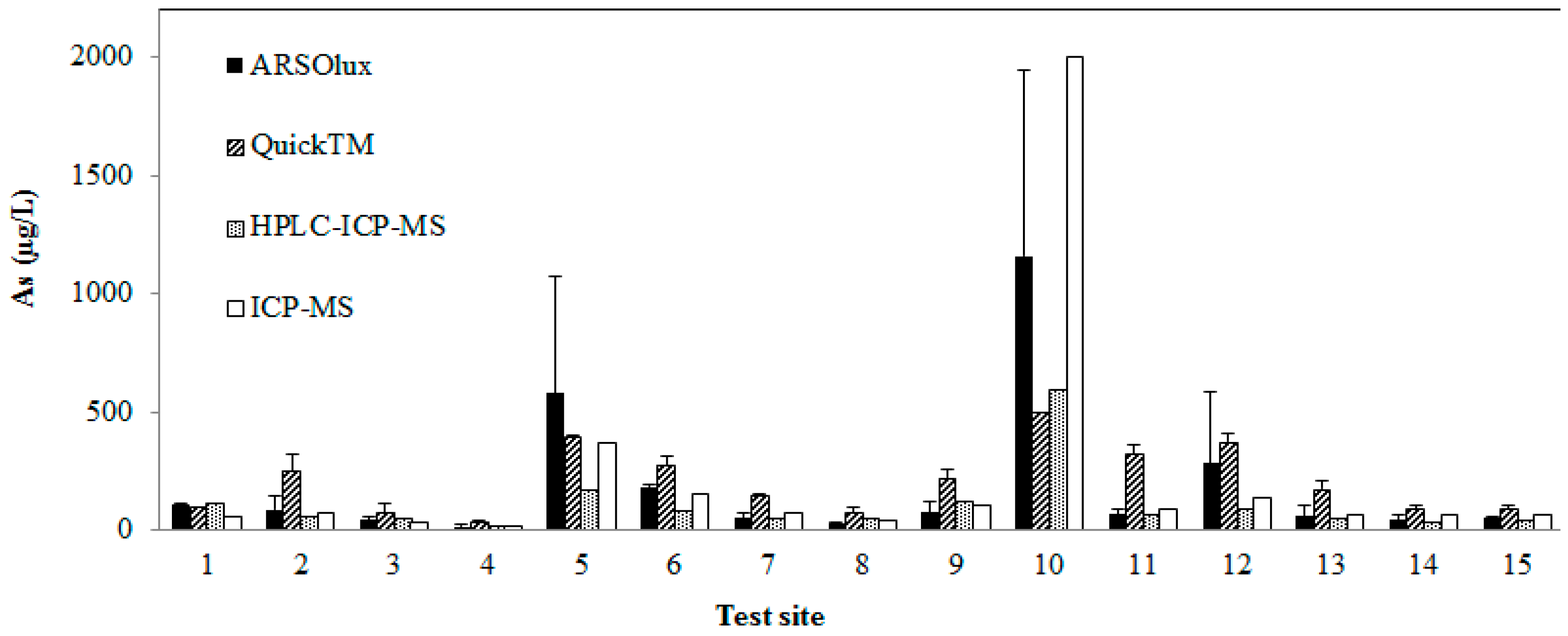
3.2. Arsenic Detection with Field Kits
| Spl. ID | As | Al | B | F | NO3− | Cl− | HCO3− | pH | EC |
|---|---|---|---|---|---|---|---|---|---|
| µg/L | µg/L | mg/L | mg/L | mg/L | mg/L | mg/L | µS/cm | ||
| 1 | 55 | 210 | 4.3 | 2.6 | 19.1 | 20.0 | 552.9 | 7.844 | 2730 |
| 2 | 73 | 123 | 5.4 | 1.3 | 131.1 | 94.2 | 967.6 | 7.991 | 3040 |
| 3 | 33 | 135 | 3.5 | 0.4 | 9.2 | 41.9 | 552.9 | 7.498 | 4040 |
| 4 | 16 | 114 | 4.3 | 0.6 | 638.5 | 304.5 | 1105.8 | 6.978 | 6900 |
| 5 | 370 | 103 | 2.7 | 1.1 | 15.4 | 127.5 | 992.7 | 8.3 | 1015 |
| 6 | 150 | 140 | 5.9 | 2.3 | 28.1 | 255.0 | 1130.9 | 7.872 | 2030 |
| 7 | 74 | 124 | 1.16 | 0.8 | 31.9 | 1208.3 | 754 | 7.69 | 906 |
| 8 | 41 | 115 | 4.5 | n/a | n/a | n/a | 1055.5 | 7.526 | 3910 |
| 9 | 103 | 98 | 4.7 | 0.7 | 31.5 | 296.8 | 716.3 | 7.984 | 2190 |
| 10 | 2000 | 114 | 8.6 | 0.5 | 39.6 | 196.0 | 791.7 | 8.436 | 3000 |
| 11 | 87 | 160 | 7.1 | 0.7 | 125.6 | 207.4 | 879.6 | 7.997 | 6950 |
| 12 | 140 | 133 | 4 | 2.6 | 19.1 | 20.0 | 552.9 | 7.872 | 1750 |
| 13 | 66 | 128 | 4.1 | 1.3 | 131.1 | 94.2 | 967.6 | 7.46 | 2880 |
| 14 | 63 | 490 | 4.9 | 0.4 | 9.2 | 41.9 | 552.9 | 7.49 | 2500 |
| 15 | 67 | 140 | 5.8 | 0.6 | 638.5 | 304.5 | 1105.8 | 7.667 | 3320 |
4. Discussion
4.1. Effects of the Chemical Composition of the Samples
4.2. Comparison of the Technical Performance of Arsenic Field Test Kits
4.3. Considerations for Deployment of the Arsenic Biosensor ARSOlux
5. Conclusions
Acknowledgments
Author Contributions
Appendix
| Spl. ID | Latitude S | Longitude W |
|---|---|---|
| 1 | 31.273594 | 61.489766 |
| 2 | 31.357068 | 61.513025 |
| 3 | 31.338499 | 61.622651 |
| 4 | 31.338839 | 61.622951 |
| 5 | 30.943634 | 61.556958 |
| 6 | 30.845649 | 61.590777 |
| 7 | 31.029629 | 61.454898 |
| 8 | 31.029501 | 61.454980 |
| 9 | 31.271950 | 61.500500 |
| 10 | 31.314193 | 61.363348 |
| 11 | 31.296873 | 61.390786 |
| 12 | 31.269692 | 61.431587 |
| 13 | 31.215263 | 61.616112 |
| 14 | 31.215168 | 61.616238 |
| 15 | 31.232784 | 61.609028 |
| Spl. ID | pH | EC µS/cm | DO mg/L | TDS mg/L | Sal | T °C |
|---|---|---|---|---|---|---|
| 1 | 7.844 | 2730 | 6.89 | 1908 | 1.4 | 20.4 |
| 2 | 7.991 | 3040 | 6.79 | 2120 | 1.6 | 18.8 |
| 3 | 7.498 | 4040 | 7.35 | 2930 | 2.1 | 18.7 |
| 4 | 6.978 | 6900 | 7.03 | 4830 | 3.8 | 20.3 |
| 5 | 8.3 | 1015 | 4.73 | 711 | 0.4 | 20.7 |
| 6 | 7.872 | 2030 | 6.25 | 1421 | 1 | 19.5 |
| 7 | 7.69 | 906 | 4 | 634 | 0.4 | 20.8 |
| 8 | 7.526 | 3910 | 6.9 | 2730 | 2 | 19 |
| 9 | 7.984 | 2190 | 8.73 | 1534 | 1.1 | 19.1 |
| 10 | 8.436 | 3000 | 3.72 | 2100 | 1.5 | 20.7 |
| 11 | 7.997 | 6950 | 3.28 | 4860 | 3.8 | 20 |
| 12 | 7.872 | 1750 | 8.3 | 1224 | 0.8 | 20 |
| 13 | 7.46 | 2880 | 5.84 | 1978 | 1.4 | 18 |
| 14 | 7.49 | 2500 | 5.66 | 1754 | 1.3 | 20 |
| 15 | 7.667 | 3320 | 8.4 | 2330 | 1.7 | 13.2 |
| Spl. ID | ARSOlux | Arsenic Quick™ | HPLC-ICP-MS | ||
|---|---|---|---|---|---|
| As tot | As tot | As tot | As III | As V | |
| µg/L | |||||
| 1 | 110.5 | 100 | 116.9 | 1.4 | 115.4 |
| 2 | 88.4 | 250 | 60.8 | 0.9 | 59.9 |
| 3 | 43.5 | 75 | 50.5 | 1.2 | 49.3 |
| 4 | 17.3 | 35 | 18.4 | 0.6 | 17.9 |
| 5 | 579.3 | 400 | 174.1 | 0.7 | 173.4 |
| 6 | 182.0 | 275 | 83.5 | 0.7 | 82.8 |
| 7 | 55.2 | 150 | 51.9 | 0.5 | 51.5 |
| 8 | 30.0 | 75 | 54.4 | 1.0 | 53.4 |
| 9 | 77.0 | 225 | 124.2 | 0.5 | 123.7 |
| 10 | 1160.8 | 500 | 599.7 | 0.6 | 599.1 |
| 11 | 68.9 | 325 | 66.0 | 1.1 | 65.0 |
| 12 | 284.0 | 375 | 89.5 | 1.2 | 88.2 |
| 13 | 61.9 | 175 | 53.4 | 1.0 | 52.4 |
| 14 | 44.4 | 90 | 38.0 | 1.3 | 36.7 |
| 15 | 51.2 | 90 | 43.9 | 1.0 | 42.9 |
| Spl. ID | As | Al | Cr | Fe | Mn | V | B | Ca | Si |
|---|---|---|---|---|---|---|---|---|---|
| µg/L | mg/L | mM | |||||||
| 1 | 55 | 210 | 10.4 | 109 | 5.7 | 160 | 4.3 | 17 | 1.21 |
| 2 | 73 | 123 | 4.3 | 50 | 1.5 | 150 | 5.4 | 45 | 1.39 |
| 3 | 33 | 135 | 3 | 34 | 2.6 | 102 | 3.5 | 123 | 1.42 |
| 4 | 16 | 114 | 4.9 | 66 | 1.6 | 76 | 4.3 | 290 | 1.50 |
| 5 | 370 | 103 | 1.3 | 4.7 | 0.25 | 550 | 2.7 | 4.2 | 1.17 |
| 6 | 150 | 140 | 1.1 | 9.2 | 0.99 | 290 | 5.9 | 22 | 1.25 |
| 7 | 74 | 124 | 1 | 21 | 0.35 | 150 | 1.16 | 39 | 1.25 |
| 8 | 41 | 115 | 3 | 29 | 6.5 | 113 | 4.5 | 98 | 1.39 |
| 9 | 103 | 98 | 8 | 12.3 | 0.21 | 180 | 4.7 | 16 | 1.25 |
| 10 | 2000 | 114 | 5.4 | 3.6 | 0.14 | 1090 | 8.6 | 4.3 | 1.10 |
| 11 | 87 | 160 | 4.3 | 44 | 3.9 | 190 | 7.1 | 48 | 1.17 |
| 12 | 140 | 133 | 5.1 | 18 | 0.61 | 230 | 4 | 14 | 1.35 |
| 13 | 66 | 128 | 5.1 | 15 | 0.126 | 133 | 4.1 | 53 | 1.39 |
| 14 | 63 | 490 | 5.3 | 160 | 23 | 160 | 4.9 | 47 | 1.46 |
| 15 | 67 | 140 | 2.7 | 132 | 5.6 | 160 | 5.8 | 62 | 1.32 |
| Spl. ID | F | NO3− | Cl− | HCO3− | SO42− | Total Carbon | Inorganic Carbon | Total Organic Carbon |
|---|---|---|---|---|---|---|---|---|
| mg/L | mg∙C/L | |||||||
| 1 | 2.6 | 19.1 | 20.0 | 552.9 | 32.6 | 200.5 | 158.4 | 42.1 |
| 2 | 1.3 | 131.1 | 94.2 | 967.6 | 115.1 | 220.3 | 195.0 | 25.4 |
| 3 | 0.4 | 9.2 | 41.9 | 552.9 | 103.2 | 184.9 | 126.5 | 58.4 |
| 4 | 0.6 | 638.5 | 304.5 | 1105.8 | 340.9 | 170.6 | 111.4 | 59.2 |
| 5 | 1.1 | 15.4 | 127.5 | 992.7 | 220.7 | 150.1 | 97.2 | 53.0 |
| 6 | 2.3 | 28.1 | 255.0 | 1130.9 | 439.4 | 200.6 | 173.7 | 26.9 |
| 7 | 0.8 | 31.9 | 1208.3 | 754 | 1091.3 | 137.4 | 96.4 | 41.1 |
| 8 | n/a | n/a | n/a | 1055.5 | 109.1 | 221.8 | 197.6 | 24.2 |
| 9 | 0.7 | 31.5 | 296.8 | 716.3 | 453.8 | 198.3 | 160.3 | 38.0 |
| 10 | 0.5 | 39.6 | 196.0 | 791.7 | 396.2 | 219.8 | 199.1 | 20.8 |
| 11 | 0.7 | 125.6 | 207.4 | 879.6 | 596.2 | 180.5 | 133.8 | 46.7 |
| 12 | 2.6 | 19.1 | 20.0 | 552.9 | 32.6 | 218.9 | 185.3 | 33.6 |
| 13 | 1.3 | 131.1 | 94.2 | 967.6 | 115.1 | 179.1 | 124.7 | 54.5 |
| 14 | 0.4 | 9.2 | 41.9 | 552.9 | 103.2 | 189.7 | 141.7 | 48.0 |
| 15 | 0.6 | 638.5 | 304.5 | 1105.8 | 340.9 | 198.3 | 150.0 | 48.3 |
Conflicts of Interest
References
- Ravenscroft, P.; Brammer, H.; Richards, K. Arsenic Pollution: A Global Synthesis; Wiley-Blackwell: Oxford, U.K., 2009. [Google Scholar]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Rahman, M.M.; Chowdhury, U.K.; Mukherjee, S.C.; Mondal, B.K.; Paul, K.; Lodh, D.; Biswas, B.K.; Chanda, C.R.; Basu, G.K.; Saha, K.C.; et al. Chronic arsenic toxicity in Bangladesh and West Bengal, India—A review and commentary. J. Toxicol.-Clin. Toxicol. 2001, 39, 683–700. [Google Scholar] [CrossRef] [PubMed]
- Murcott, S. Arsenic Contamination in the World—An International Sourcebook; IWA Publishing: London, U.K., 2012. [Google Scholar]
- Smedley, P.L.; Nicolli, H.B.; Macdonald, D.M.J.; Barros, A.J.; Tullio, J.O. Hydrogeochemistry of arsenic and other inorganic constituents in groundwaters from La Pampa, Argentina. Appl. Geochem. 2002, 17, 259–284. [Google Scholar] [CrossRef]
- Bundschuh, J.; Litter, M.I.; Parvez, F.; Roman-Ross, G.; Nicolli, H.B.; Jean, J.S.; Liu, C.W.; Lopez, D.; Armienta, M.A.; Guilherme, L.R.G.; et al. One century of arsenic exposure in Latin America: A review of history and occurrence from 14 countries. Sci. Total Environ. 2012, 429, 2–35. [Google Scholar] [CrossRef] [PubMed]
- Nicolli, H.B.; Bundschuh, J.; Blanco, M.C.; Tujchneider, O.; Panarello, H.; Dapeña, C.; Rusansky, J. Arsenic and associated trace-elements in groundwater from the Chaco-Pampean plain, Argentina: Results from 100 years of research. Sci. Total Environ. 2012, 490, 36–56. [Google Scholar] [CrossRef]
- Alarcón-Herrera, M.T.; Bundschuh, J.; Nath, B.; Nicolli, H.B.; Gutierrez, M.; Reyes-Gomez, V.M.; Nuñez, D.; Martín-Dominguez, I.R.; Sracek, O. Co-occurrence of arsenic and fluoride in groundwater of semi-arid regions in Latin America: Genesis, mobility and remediation. J. Hazard. Mat. 2013, 262, 960–969. [Google Scholar] [CrossRef]
- Gomez, M.L.; Blarasin, M.T.; Martinez, D.E. Arsenic and fluoride in a loess aquifer in the central area of Argentina. Environ. Geol. 2009, 57, 143–155. [Google Scholar] [CrossRef]
- Hung, D.Q.; Nekrassova, O.; Compton, R.G. Analytical methods for inorganic arsenic in water: A review. Talanta 2004, 64, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A. Rapid Review of Locally Available Arsenic Field Testing Kits; DFID: Dhaka, Bangladesh, 2005. [Google Scholar]
- Rahman, M.; Mukherjee, D.; Sengupta, M.K.; Chowdhury, U.K.; Lodh, D.; Chanda, C.R.; Roy, S.; Selim, M.; Quamrussaman, Q.; Milton, A.H.; et al. Effectiveness and reliability of arsenic field testing kits: Are the million dollar screening projects effective or not? Environ. Sci. Technol. 2002, 36, 5385–5394. [Google Scholar] [CrossRef] [PubMed]
- Harms, H.; Wells, M.C.; van der Meer, J.R. Whole-cell living biosensors—Are they ready for environmental application? Appl. Microbiol. Biot. 2006, 70, 273–280. [Google Scholar] [CrossRef]
- Siegfried, K.; Endes, C.; Bhuiyan, A.; Kuppardt, A.; Mattusch, J.; van der Meer, J.R.; Chatzinotas, A.; Harms, H. Field testing of arsenic in groundwater samples of Bangladesh using a test kit based on lyophilized bioreporter bacteria. Environ. Sci. Technol. 2012, 46, 3281–3287. [Google Scholar] [CrossRef] [PubMed]
- Stocker, J.; Balluch, D.; Gsell, M.; Harms, H.; Feliciano, J.S.; Daunert, S.; van der Meer, J.R.; Malik, K.A. Development of a set of simple bacterial biosensors for quantitative and rapid field measurements of arsenite and arsenate in potable water. Environ. Sci. Technol. 2003, 37, 4743–4750. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Guidelines for Drinking Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Nicolli, H.B.; Bundschuh, J.; Garcia, J.W.; Falcon, C.M.; Jean, J.S. Sources and controls for the mobility of arsenic in oxidizing groundwaters from loess-type sediments in arid/semi-arid dry climates evidence from the Chaco-Pampean Plain (Argentina). Water Res. 2010, 44, 5589–5604. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, M.; Albertengo, A.; Brusa, L.; Beldomenico, H.; Tudino, M. Distribution of inorganic arsenic species in groundwater from central-west part of Santa Fe province, Argentina. Appl. Geochem. 2013, 39, 43–48. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Handbook For Sampling And Sample Preservation Of Water And Wastewater; EPA-60014-76-049; National Service Center for Environmental Publications (NSCEP): Maryland, MD, USA, 1976. [Google Scholar]
- Mattusch, J.; Wennrich, R.; Schmidt, A.C.; Reisser, W. Determination of arsenic species in water, soils and plants. Fresenius J. Anal. Chem. 2000, 366, 200–203. [Google Scholar] [CrossRef] [PubMed]
- George, C.M.; Zheng, Y.; Graziano, J.H.; Bin Rasul, S.; Hossain, Z.; Mey, J.L.; van Geen, A. Evaluation of an arsenic test kit for rapid well screening in Bangladesh. Environ. Sci. Technol. 2012, 46, 11213–11219. [Google Scholar] [CrossRef] [PubMed]
- Standard Methods for the Examination of Water and Wastewater, Ion-Selective Electrode Method SM 4500-F−—C. 1997. Available online: http://www.mwa.co.th/download/file_upload/SMWW_4000-6000.pdf (accessed on 22 April 2015).
- Standard Methods for the Examination of Water and Wastewater, Nitrate Electrode Method SM 4500-NO3−D Nitrogen (Nitrate). 2000. Available online: http://www.mwa.co.th/download/file_upload/SMWW_4000-6000.pdf (accessed on 22 April 2015).
- Standard Methods for the Examination of Water and Wastewater, Colorimetric Method SM 4500-NO2− B Nitrogen (Nitrite). 2000. Available online: https://www.nemi.gov/methods/method_summary/7415/ (accessed on 22 April 2015).
- Jeong, H.; Park, J.; Kim, H. Determination of NH4+ in Environmental Water with Interfering Substances Using the Modified Nessler Method. Available online: http://www.hindawi.com/journals/jchem/2013/359217/ (accessed on 2 March 2015).
- Standard Methods for the Examination of Water and Wastewater, Argentometric Method—SM 4500-Cl− B− Chloride. 1997. Available online: http://www.standardmethods.org/store/ProductView.cfm?ProductID=182 (accessed on 2 March 2015).
- Standard Methods for the Examination of Water and Wastewater, Titration Method—SM 2320 B Alkalinity. 1997. Available online: http://folk.uio.no/rvogt/KJM_MEF_4010/Alkalinity.pdf (accessed on 2 March 2015).
- Standard Methods for the Examination of Water and Wastewater, Turbidimetry. SM 4500-SO42-E Sulfate. 1997. Available online: http://www.standardmethods.org/store/ProductView.cfm?ProductID=202 (accessed on 2 March 2015).
- World Health Organisation. Guidelines for Drinking Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Santa Fé Province. Ley 11.220. Available online: http://www.santafe.gov.ar/index.php/web/content/download/6361/36477/file/ley (accessed on 3 March 2015).
- US EPA. last updated on 17 November 2014. Available online: www2.epa.gov/sites/production/files/2014-09/documents/fact_sheet_final_third_ccl.pdf (accessed on 3 March 2015).
- International Agency for Research on Cancer (IARC). Cobalt in Hard Metals and Cobalt Sulfate, Gallium Arsenide, Indium Phosphide and Vanadium Pentoxide Series. Available online: http://www.monographs.iarc.fr/ENG/Monographs/vol86/mono86.pdf (accessed on 3 March 2015).
- Notification Levels—California Department Public of Health. Available online: http://www.cdph.ca.gov/certlic/drinkingwater/Documents/Notificationlevels/NotificationLevels.pdf (accessed on 2 February 2015).
- Raychowdhury, N.; Mukherjee, A.; Bhattacharya, P.; Johannesson, K.; Bundschuh, J.; Nordberg, E.; Bejarano Sifuentes, G.; Martin, R.A.; Storniolo, A.R. Provenance and fate of arsenic and other solutes in the Chaco-Pampean Plain of the Andean foreland, Argentina: From perspectives of hydrogeochemical modeling and regional tectonic setting. J. Hydrol. 2014, 518, 300–316. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G.; Macdonald, D.M.J.; Nicolli, H.B.; Barros, A.J.; Tullio, J.O.; Pearce, J.M.; Alonso, M.S. Arsenic associations in sediments from the loess aquifer of La Pampa, Argentina. Appl. Geochem. 2005, 20, 989–1016. [Google Scholar] [CrossRef]
- García, M.A.; Sracek, O.; Fernández, D.S.; del Valle Hidalgo, M. Factors affecting arsenic concentration in groundwaters from Northwestern Chaco-Pampean Plain, Argentina. Environ. Geol. 2007, 52, 1261–1275. [Google Scholar] [CrossRef]
- Ingallinella, A.M.; Fernández, R.G. Experiencia argentina en la remoción de arsénico por diversas tecnologías. In Tecnologías Económicas Para El Abatimiento De Arsénico En Aguas; Litter, M.I., Sancha, A.M., Ingallinella, A.M., Eds.; Programa Iberoamericano de Ciencia y Tecnología para el Desarrollo: Buenos Aires, Argentina, 2010; pp. 155–167. [Google Scholar]
- Cartagena Protocol on Biosafety to the Convention on Biological Diversity, Montreal. 2000. Available online: http://bch.cbd.int/protocol/publications/cartagena-protocol-en.pdf (accessed on 22 April 2015).
- ZKBS. Statement of the ZKBS on the Risk Assessment of the ARSOlux Test System. 2012. Available online: http://www.bvl.bund.de/SharedDocs/Downloads/06_Gentechnik/ZKBS/02_Allgemeine_Stellungnahmen_englisch/02_bacteria/ARSOlux.pdf?__blob=publicationFile&v=2 (accessed on 15 December 2014).
- Word Health Organization (WHO). Laboratory Biosafety Manual, 3rd ed.; WHO: Geneva, Switzerland, 2004. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siegfried, K.; Hahn-Tomer, S.; Koelsch, A.; Osterwalder, E.; Mattusch, J.; Staerk, H.-J.; Meichtry, J.M.; De Seta, G.E.; Reina, F.D.; Panigatti, C.; et al. Introducing Simple Detection of Bioavailable Arsenic at Rafaela (Santa Fe Province, Argentina) Using the ARSOlux Biosensor. Int. J. Environ. Res. Public Health 2015, 12, 5465-5482. https://doi.org/10.3390/ijerph120505465
Siegfried K, Hahn-Tomer S, Koelsch A, Osterwalder E, Mattusch J, Staerk H-J, Meichtry JM, De Seta GE, Reina FD, Panigatti C, et al. Introducing Simple Detection of Bioavailable Arsenic at Rafaela (Santa Fe Province, Argentina) Using the ARSOlux Biosensor. International Journal of Environmental Research and Public Health. 2015; 12(5):5465-5482. https://doi.org/10.3390/ijerph120505465
Chicago/Turabian StyleSiegfried, Konrad, Sonja Hahn-Tomer, Andreas Koelsch, Eva Osterwalder, Juergen Mattusch, Hans-Joachim Staerk, Jorge M. Meichtry, Graciela E. De Seta, Fernando D. Reina, Cecilia Panigatti, and et al. 2015. "Introducing Simple Detection of Bioavailable Arsenic at Rafaela (Santa Fe Province, Argentina) Using the ARSOlux Biosensor" International Journal of Environmental Research and Public Health 12, no. 5: 5465-5482. https://doi.org/10.3390/ijerph120505465





