Identification, Characterization and Antibiotic Resistance of Bacterial Isolates Obtained from Waterpipe Device Hoses
Abstract
:1. Introduction
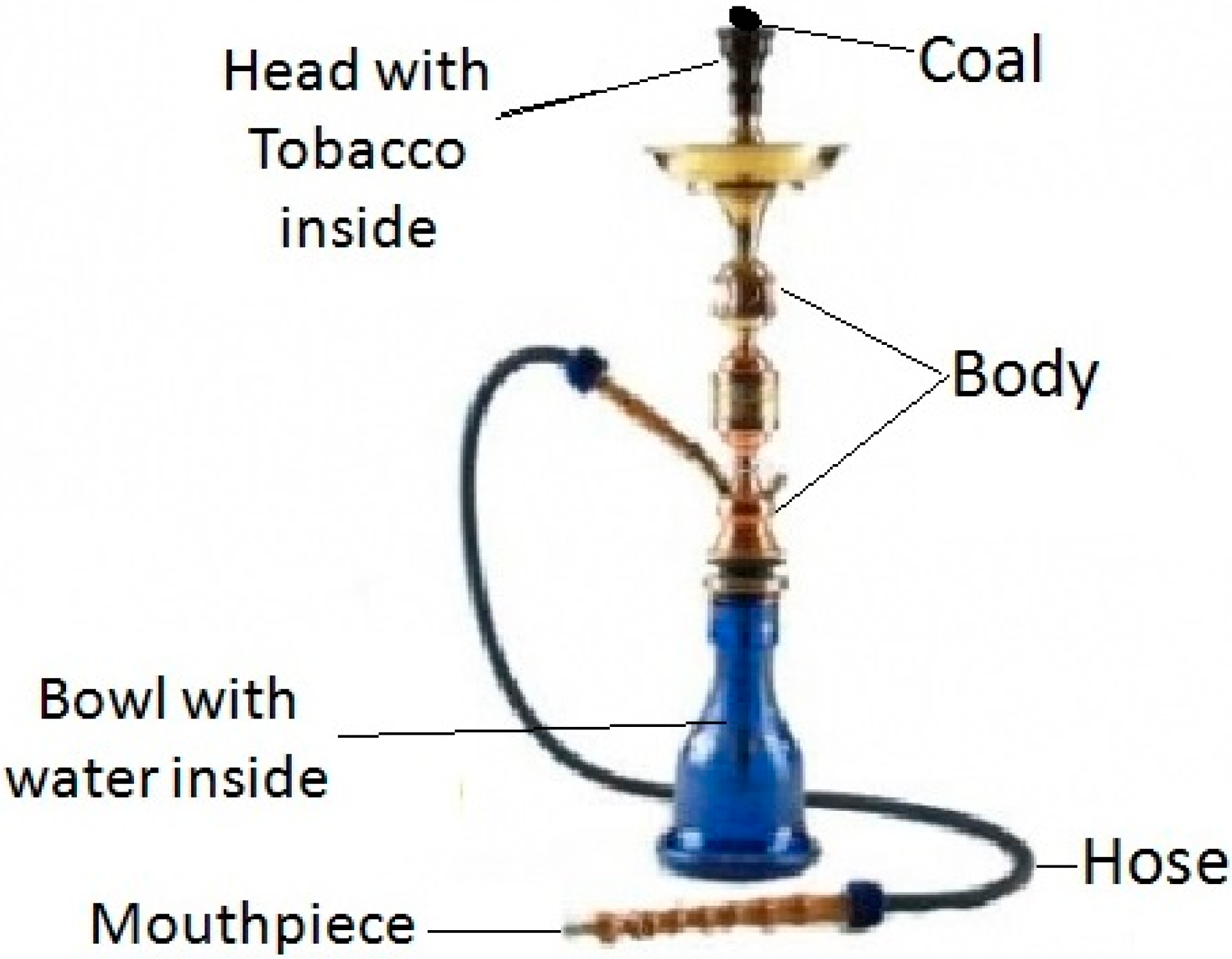
2. Experimental Section
2.1. Bacterial Isolation
2.2. Biochemical and Physiological Characterization
2.3. Antimicrobial Screening of Selected Antibiotics: Disc Diffusion Assay
2.4. Data Processing and Analysis
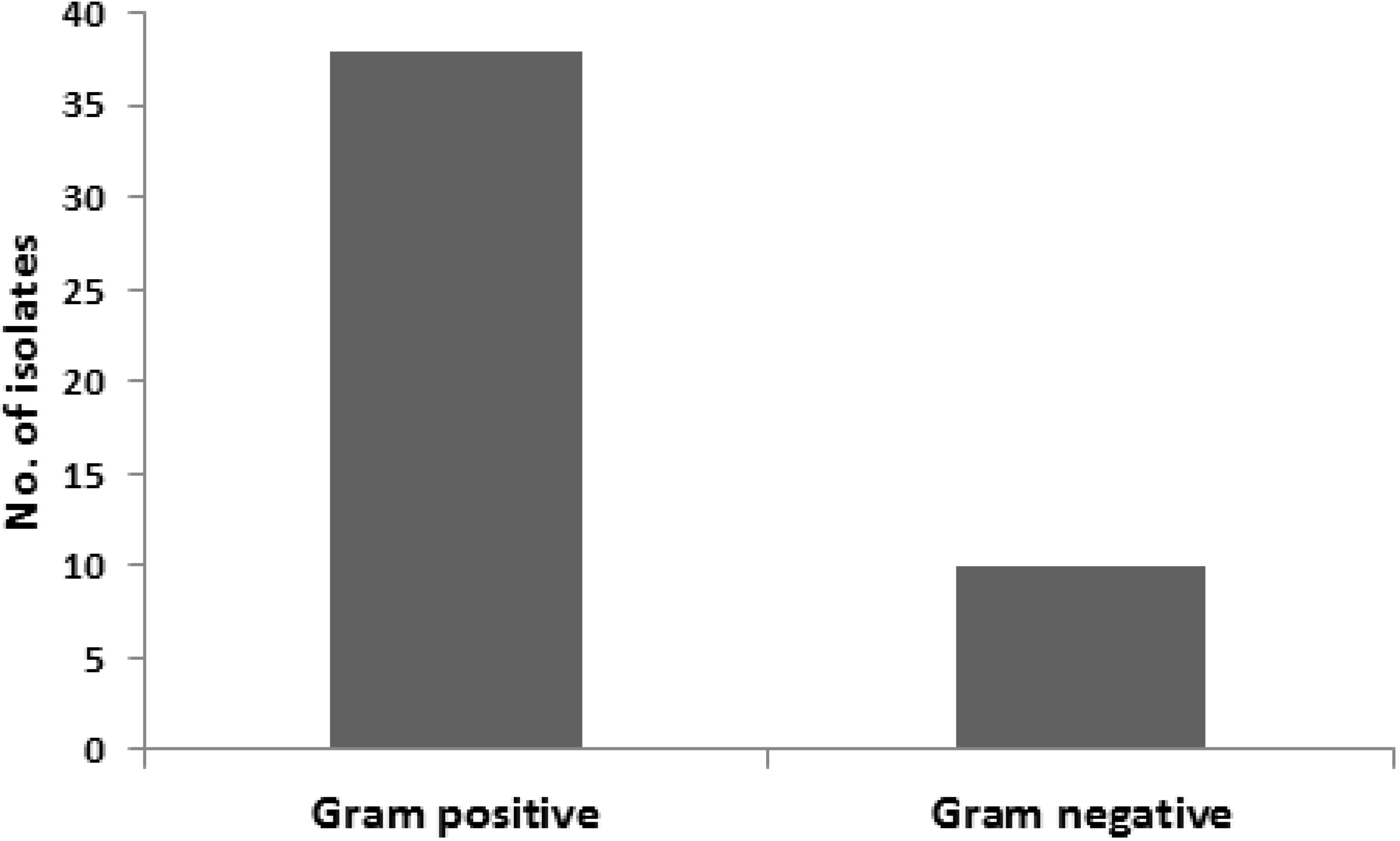
3. Results and Discussion

| Bacterial Species | Norfloxacin (mg/mL) | Amoxicillin (mg/mL) | Cefixime (mg/mL) | Tetracycline (mg/mL) | Cephalexin (mg/mL) |
|---|---|---|---|---|---|
| Microccoccus | 0.048 | 0.6 | 2.56 | 0.08 | 6.4 |
| Proteus vulgaris | 0.048 | 1.2 | 0.16 | 0.08 | 6.4 |
| Serratia fonticola | 0.384 | 0.3 | 0.64 | 0.08 | 6.4 |
| Pasedo diphtheriticum | 0.192 | 1.2 | 0.64 | 0.08 | 0.1 |
| Bacillus cereus | 0.384 | 3.2 | 1.28 | 0.06 | 3.2 |
| Bacillus subtilis | 0.024 | 0.6 | 2.56 | 0.8 | 6.4 |
| Staphylococcus aureus | 0.048 | 0.6 | 1.28 | 0.2 | 0.08 |
| Listeria monocytogenes | 0.024 | 0.6 | 0.32 | 0.08 | 0.06 |
| Antibiotic | Number of Resistant Isolates | Frequency of Resistant Isolates (out of 48) |
|---|---|---|
| Aztreonam | 38 | 79% |
| Azithromycin | 13 | 27% |
| Amoxicillin | 23 | 47% |
| Cefixime | 38 | 79% |
| Imipenem | 10 | 21% |
| Trimethoprim/sulfamethoxazole | 11 | 23% |
| Piperacillin/tazobactam | 9 | 19% |
| Clarithromycin | 22 | 46% |
| Enrofloxacin baytril | 18 | 38% |
| Norfloxacin | 6 | 13% |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jawad, M.; Abass, J.; Hariri, A.; Rajasooriar, K.G.; Salmasi, H.; Millett, C.; Hamilton, F.L. Waterpipe smoking: Prevalence and attitudes among medical students in London. Int. J. Tuberc. Lung Dis. 2013, 17, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Jradi, H.; Wewers, M.E.; Pirie, P.R.; Binkley, P.F.; Ferketich, K. Cigarette and waterpipe smoking associated knowledge and behaviour among medical students in Lebanon. East Mediterr. Health J. 2013, 19, 861–868. [Google Scholar] [PubMed]
- Khabour, O.F.; Alzoubi, K.H.; Eissenberg, T.; Mehrotra, P.; Azab, M.; Carroll, M.V.; Afifi, R.A.; Primack, B.A. Waterpipe tobacco and cigarette smoking among university students in Jordan. Int. J. Tuberc. Lung Dis. 2012, 16, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Akl, E.A.; Jawad, M.; Lam, W.Y.; Co, C.N.; Obeid, R.; Irani, J. Motives, beliefs and attitudes towards waterpipe tobacco smoking: A systematic review. Harm Reduct. J. 2013, 10. [Google Scholar] [CrossRef]
- Obeidat, S.R.; Khabour, O.F.; Alzoubi, K.H.; Mahasneh, A.M.; Bibars, A.R.; Khader, Y.S.; Alsa’di, A. Prevalence, social acceptance, and awareness of waterpipe smoking among dental university students: A cross sectional survey conducted in Jordan. BMC Res. Notes 2014, 7. [Google Scholar] [CrossRef] [PubMed]
- Shihadeh, A. Investigation of mainstream smoke aerosol of the argileh water pipe. Food Chem. Toxicol. 2003, 41, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Markowicz, P.; Londahl, J.; Wierzbicka, A.; Suleiman, R.; Shihadeh, A.; Larsson, L. A study on particles and some microbial markers in waterpipe tobacco smoke. Sci. Total Environ. 2014, 499, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Safizadeh, H.; Moradi, M.; Rad, M.R.; Nakhaee, N. Bacterial contamination of different components of the waterpipe. Int. J. Tuberc. Lung Dis. 2014, 18, 988–991. [Google Scholar] [CrossRef] [PubMed]
- Akl, E.A.; Gaddam, S.; Gunukula, S.K.; Honeine, R.; Jaoude, P.A.; Irani, J. The effects of waterpipe tobacco smoking on health outcomes: A systematic review. Int. J. Epidemiol. 2010, 39, 834–857. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Abbreviated Identification of Bacteria and Yeast, Approved Guidlines, 2nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- CLSI. Performance Sta1ndards for Antimicrobial Susceptibility Testing, 22nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Masadeh, M.M.; Mhaidat, N.M.; Alzoubi, K.H.; Al-Azzam, S.I.; Shaweesh, A.I. Ciprofloxacin-induced antibacterial activity is reversed by vitamin E and vitamin C. Curr. Microbiol. 2012, 64, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Sepetdjian, E.; Shihadeh, A.; Saliba, N.A. Measurement of 16 polycyclic aromatic hydrocarbons in narghile waterpipe tobacco smoke. Food Chem. Toxicol. 2008, 46, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Shihadeh, A.; Azar, S.; Antonios, C.; Haddad, A. Towards a topographical model of narghile water-pipe cafe smoking: A pilot study in a high socioeconomic status neighborhood of Beirut, Lebanon. Pharmacol. Biochem. Behav. 2004, 79, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Shihadeh, A.; Saleh, R. Polycyclic aromatic hydrocarbons, carbon monoxide, “Tar”, and nicotine in the mainstream smoke aerosol of the narghile water pipe. Food Chem. Toxicol. 2005, 43, 655–661. [Google Scholar] [CrossRef] [PubMed]
- El-Setouhy, M.; Loffredo, C.A.; Radwan, G.; Abdel Rahman, R.; Mahfouz, E.; Israel, E.; Mohamed, M.K.; Ayyad, S.B. Genotoxic effects of waterpipe smoking on the Buccal Mucosa cells. Mutat. Res. 2008, 655, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Yadav, J.S.; Thakur, S. Genetic risk assessment in hookah smokers. Cytobios 2000, 101, 101–113. [Google Scholar] [PubMed]
- Alsatari, E.S.; Azab, M.; Khabour, O.F.; Alzoubi, K.H.; Sadiq, M.F. Assessment of DNA damage using chromosomal aberrations assay in lymphocytes of waterpipe smokers. Int. J. Occup. Med. Environ. Health 2012, 25, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Khabour, O.F.; Alsatari, E.S.; Azab, M.; Alzoubi, K.H.; Sadiq, M.F. Assessment of genotoxicity of waterpipe and cigarette smoking in lymphocytes using the sister-chromatid exchange assay: A comparative study. Environ. Mol. Mutagen 2011, 52, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Aoun, J.; Saleh, N.; Waked, M.; Salame, J.; Salameh, P. Lung cancer correlates in Lebanese adults: A pilot case—Control study. J. Epidemiol. Glob. Health 2013, 3, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Alomari, M.A.; Khabour, O.F.; Alzoubi, K.H.; Shqair, D.M.; Eissenberg, T. Central and peripheral cardiovascular changes immediately after waterpipe smoking. Inhal. Toxicol. 2014, 26, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Hawari, F.I.; Obeidat, N.A.; Ayub, H.; Ghonimat, I.; Eissenberg, T.; Dawahrah, S.; Beano, H. The acute effects of waterpipe smoking on lung function and exercise capacity in a pilot study of healthy participants. Inhal. Toxicol. 2013, 25, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S. Waterpipe smoking, oral cancer and other oral health effects. Evid. Based Dent. 2011, 12, 44–45. [Google Scholar] [CrossRef] [PubMed]
- Bibars, A.R.; Obeidat, S.R.; Khader, Y.; Mahasneh, A.M.; Khabour, O.F. The effect of waterpipe smoking on periodontal health. Oral Health Prev. Dent. 2014. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masadeh, M.M.; Hussein, E.I.; Alzoubi, K.H.; Khabour, O.; Shakhatreh, M.A.K.; Gharaibeh, M. Identification, Characterization and Antibiotic Resistance of Bacterial Isolates Obtained from Waterpipe Device Hoses. Int. J. Environ. Res. Public Health 2015, 12, 5108-5115. https://doi.org/10.3390/ijerph120505108
Masadeh MM, Hussein EI, Alzoubi KH, Khabour O, Shakhatreh MAK, Gharaibeh M. Identification, Characterization and Antibiotic Resistance of Bacterial Isolates Obtained from Waterpipe Device Hoses. International Journal of Environmental Research and Public Health. 2015; 12(5):5108-5115. https://doi.org/10.3390/ijerph120505108
Chicago/Turabian StyleMasadeh, Majed M., Emad I. Hussein, Karem H. Alzoubi, Omar Khabour, Muhamad Ali K. Shakhatreh, and Mahmoud Gharaibeh. 2015. "Identification, Characterization and Antibiotic Resistance of Bacterial Isolates Obtained from Waterpipe Device Hoses" International Journal of Environmental Research and Public Health 12, no. 5: 5108-5115. https://doi.org/10.3390/ijerph120505108






