Chemical Characterisation of the Coarse and Fine Particulate Matter in the Environment of an Underground Railway System: Cytotoxic Effects and Oxidative Stress—A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling of Airborne Particulate Matter Material
2.2. Chemical Analysis: Determination of the Concentrations of Metals
2.3. Evaluation of Cytotoxicity and Oxidative Stress
2.3.1. Cell Culture
2.3.2. Toxicity Assessment
2.3.3. MTT Assay
2.3.4. Reactive Oxygen Species Assessment
2.4. Statistical Analysis
3. Results
3.1. Concentrations of Airborne Particulate Matter and of the Metallic Component
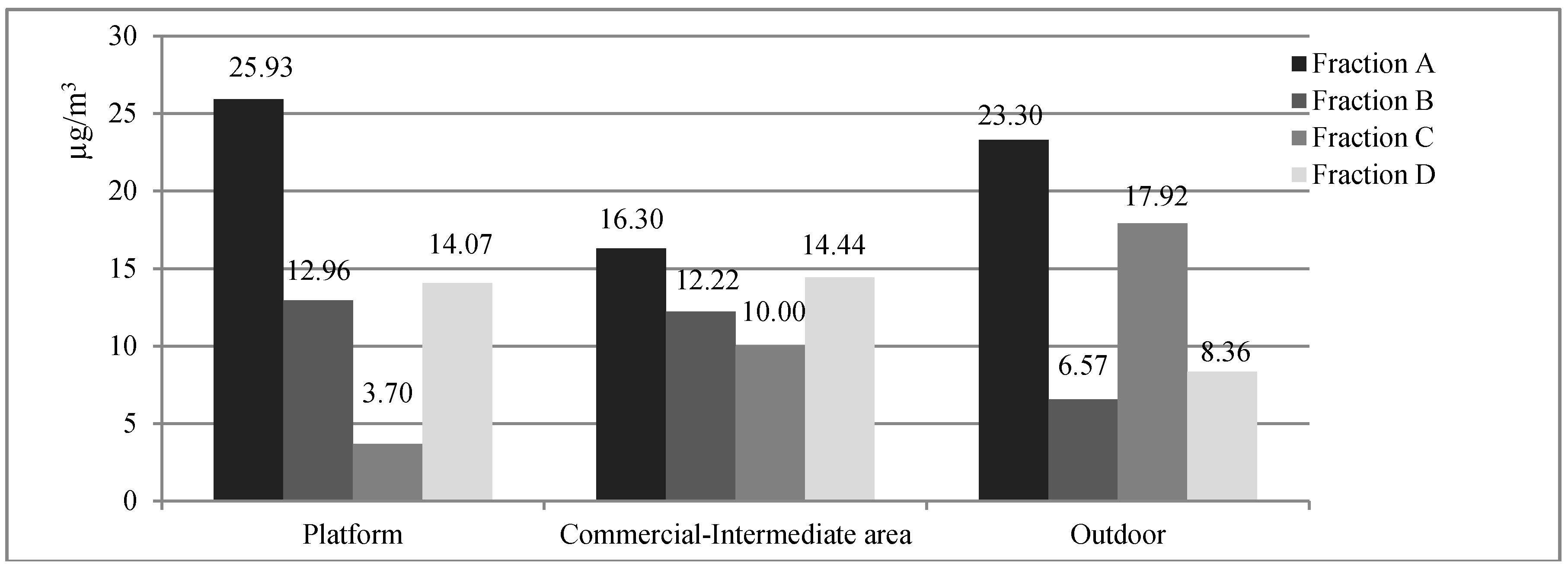
| Elements | Fraction A (2.5–10 µm) (ng/m3) | Fraction B (1–2.5 µm) (ng/m3) | Fraction C (0.5–1 µm) (ng/m3) | Fraction D (0.25–0.5 µm) (ng/m3) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Platform | Commercial-intermediate area | Outdoor | Platform | Commercial-intermediate area | Outdoor | Platform | Commercial-intermediate area | Outdoor | Platform | Commercial-intermediate area | Outdoor | ||
| Transition metals | Cr | 15.78 | 3.11 | 5.98 | 3.23 | 2.07 | 2.18 | 1.87 | 0 | 1.31 | 0 | 0 | 1.47 |
| Cu | 14.22 | 13.28 | 12.41 | 12.46 | 6.36 | 4.64 | 4.49 | 2.06 | 0.74 | 2.67 | 6.40 | 0.86 | |
| Fe | 545.34 | 244.47 | 117.15 | 212.01 | 148.99 | 115.30 | 70.79 | 33.77 | 30.94 | 30.54 | 28.65 | 32.75 | |
| Mn | 8.60 | 3.57 | 3.23 | 2.71 | 1.94 | 2.76 | 1.58 | 0 | 0 | 1.08 | 0 | 0 | |
| Mo | 10.75 | 11.00 | 0 | 9.19 | 2.90 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Ni | 2.96 | 1.74 | 0 | 2.20 | 1.12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Ti | 15.57 | 4.67 | 12.44 | 9.58 | 2.40 | 3.73 | 2.59 | 1.82 | 3.25 | 3.02 | 1.69 | 2.81 | |
| Zn | 7.73 | 6.87 | 15.15 | 6.98 | 4.98 | 6.07 | 3.49 | 3.64 | 5.64 | 3.01 | 2.93 | 5.77 | |
| Crustal elements | Al | 89.14 | 58.91 | 67.40 | 64.73 | 35.42 | 39.57 | 26.53 | 4.45 | 5.18 | 25.03 | 5.37 | 2.15 |
| Ba | 122.12 | 77.75 | 64.78 | 95.68 | 75.82 | 51.32 | 98.98 | 82.42 | 48.62 | 99.16 | 84.54 | 55.78 | |
| Ca | 1568.17 | 205.33 | 300.57 | 256.37 | 135.70 | 67.86 | 57.60 | 38.22 | 75.99 | 30.40 | 49.19 | 55.70 | |
| K | 26.29 | 73.62 | 80.19 | 10.63 | 37.43 | 68.57 | 6.97 | 27.31 | 65.62 | 5.28 | 26.54 | 59.60 | |
| Mg | 38.93 | 23.21 | 201.81 | 9.71 | 12.54 | 39.36 | 2.32 | 3.59 | 56.44 | 4.57 | 0.50 | 21.47 | |
3.2. Cytotoxic and Oxidative Stress Evaluation
3.2.1. MTT Assay

3.2.2. ROS Analysis
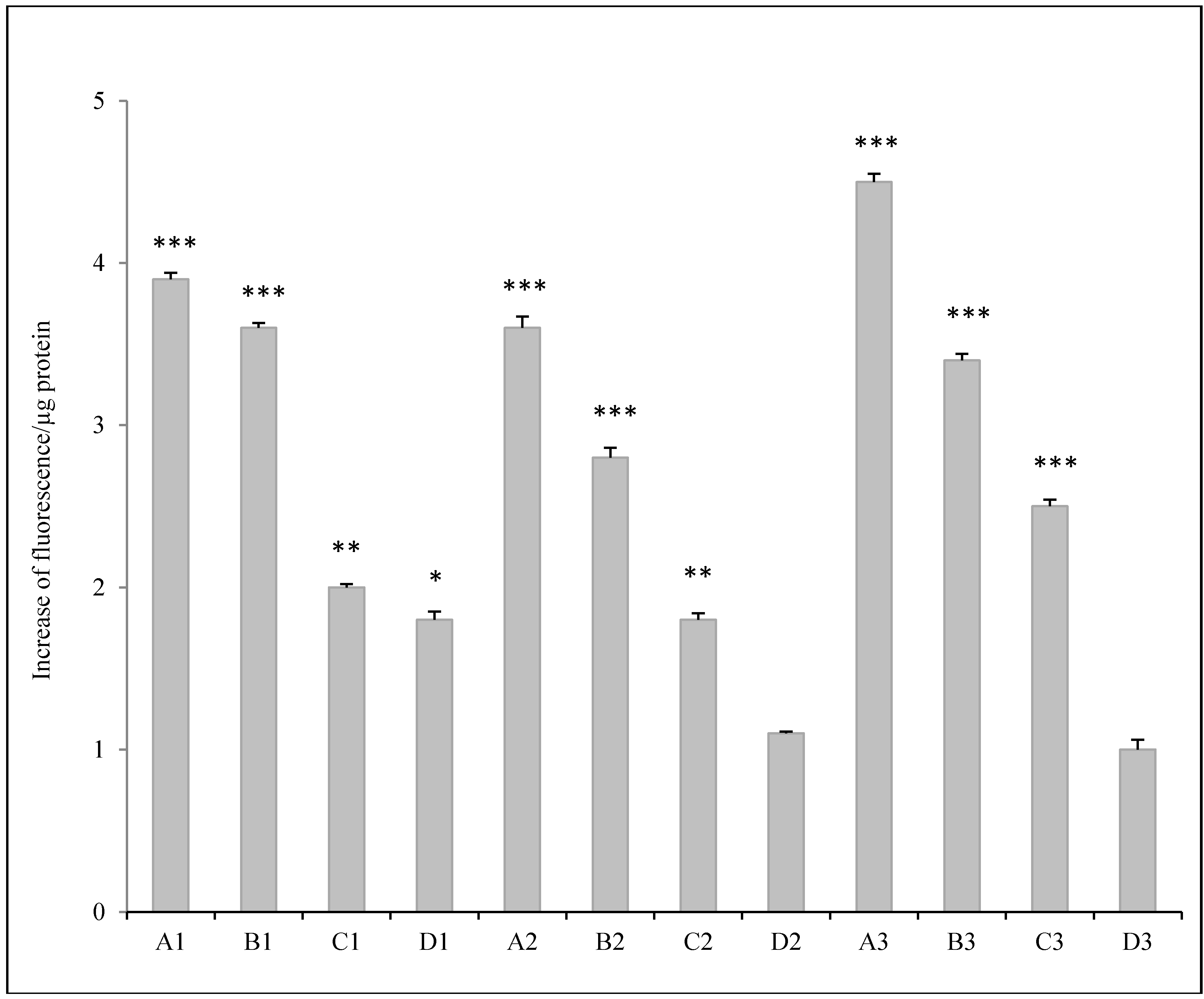
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Assimakopoulos, M.N.; Dounis, A.; Spanou, A.; Santamouris, M. Indoor air quality in a metropolitan area metro using fuzzy logic assessment system. Sci. Total. Environ. 2013, 449, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, J.; Thiering, E.; Rzehak, P.; Krämer, U.; Hochadel, M.; Rauchfuss, K.M.; Gehring, U.; Wichmann, H.-E. Long-term exposure to NO2 and PM10 and all-cause and cause-specific mortality in a prospective cohort of women. Occup. Environ. Med. 2013, 70, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Beelen, R.; Stafoggia, M.; Raaschou-Nielsen, O.; Andersen, Z.J.; Xun, W.W.; Katsouyanni, K.; Dimakopoulou, K.; Brunekreef, B.; Weinmayr, G.; Hoffmann, B.; et al. Long-term exposure to air pollution and cardiovascular mortality: An analysis of 22 European cohorts. Epidemiology 2014, 25, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A., 3rd; Burnett, R.T.; Thun, M.J.; Calle, E.E.; Krewski, K.; Brunekreef, D.; Ito, K.; Thurston, G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 2002, 287, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Langrish, J.P.; Mills, N.L. Air pollution and mortality in Europe. Lancet 2014, 383, 758–760. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.L.; Holgersson, A.; Möller, L. Mechanisms related to the genotoxicity of particles in the subway and from other sources. Chem. Res. Toxicol. 2008, 21, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Seaton, A.; Cherrie, J.; Dennekamp, M.; Donaldson, K.; Hurley, J.F.; Tran, C.L. The London underground: Dust and hazards to health. Occup. Environ. Med. 2005, 62, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Ripanucci, G.; Grana, M.; Vicentini, L.; Magrini, A.; Bergamaschi, A. Dust in the underground railway tunnels of an Italian town. J. Occup. Environm. Hyg. 2006, 3, 16–25. [Google Scholar] [CrossRef]
- Johansson, C.; Johansson, P.A. Particulate matter in the underground of Stockholm. Atmos. Environ. 2003, 37, 3–9. [Google Scholar] [CrossRef]
- Aarnio, P.; Yli-Tuomi, T.; Kousa, A.; Mäkela, T.; Hirsikko, A.; Hämeri, K.; Räisänen, M.; Hillamo, R.; Koskentalo, T.; Jantunen, M. The concentrations and composition of and exposure to fine particles (PM2.5) in the Helsinki subway system. Atmos. Environ. 2005, 39, 5059–5066. [Google Scholar] [CrossRef]
- Adams, H.S.; Nieuwenhuijsen, M.J.; Colvile, R.N. Determinants of fine particle (PM2.5) personal exposure levels in transport microenvironments, London, UK. Atmos. Environ. 2001, 35, 4557–4566. [Google Scholar]
- Salma, I.; Weidinger, T.; Maenhaut, W. Time-resolved mass concentration, composition and sources of aerosol particles in a metropolitan underground railway station. Atmos. Environ. 2007, 41, 8391–8405. [Google Scholar] [CrossRef]
- Kam, W.; Ning, Z.; Shafer, M.M.; Schauer, J.J.; Sioutas, C. Chemical characterization and redox potential of coarse and fine particulate matter (PM) in underground and ground-level rail systems of the Los Angeles Metro. Environ. Sci. Technol. 2011, 45, 6769–6776. [Google Scholar] [CrossRef] [PubMed]
- Sitzmann, B.; Kendall, M.; Watt, J.; Williams, I. Characterisation of airborne particles in London by computer-controlled scanning electron microscopy. Sci. Total Environ. 1999, 241, 63–73. [Google Scholar] [CrossRef]
- Kang, S.; Hwang, H.; Park, Y.; Kim, H.; Ro, C. Chemical compositions of subway particles in Seoul, Korea determined by a quantitative single particle analysis. Environ. Sci. Technol. 2008, 42, 9051–9057. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Kim, B.; Malek, M.A.; Koo, Y.S.; Jung, J.H.; Son, Y.S.; Kim, J.C.; Kim, H.; Ro, C.U. Chemical speciation of size-segregated floor dusts and airborne magnetic particles collected at underground subway stations in Seoul, Korea. J. Hazard. Mater. 2012, 213–214, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.H.; Kim, H.R.; Park, Y.J.; Park, D.S.; Chung, K.H.; Oh, S.M. Genotoxic effects and oxidative stress induced by organic extracts of particulate matter (PM10) collected from a subway tunnel in Seoul, Korea. Mutat. Res. 2012, 749, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.S.; Nieuwenhuijsen, M.J.; Colvile, R.N.; McMullen, M.A.; Khandelwal, P. Fine particle (PM2.5) personal exposure levels in transport microenvironments, London, UK. Sci. Total Environ. 2001, 279, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Lippmann, M. Toxicological and epidemiological studies of cardiovascular effects of ambient air fine particulate matter (PM2.5) and its chemical components: Coherence and public health implications. Crit. Rev. Toxicol. 2014, 44, 299–347. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, D.; Ursini, C.L.; Maiello, R.; Apostoli, P.; Catalani, S.; Ciervo, A.; Iavicoli, S. Genotoxic and oxidative effects induced on A549 cells by extract of PM10 collected in an electric steel plant. Acta. Biomed. 2008, 79, 87–96. [Google Scholar] [PubMed]
- Lodovici, M.; Bigagli, E. Oxidative stress and air pollution exposure. J. Toxicol. 2011. [Google Scholar] [CrossRef]
- Akhtar, U.S.; Rastogi, N.; McWhinney, R.D.; Urch, B.; Chow, C.W.; Evansa, G.J.; Scott, J.A. The combined effects of physicochemical properties of size-fractionated ambient particulate matter on in vitro toxicity in human A549 lung epithelial cells. Toxicol. Rep. 2014, 1, 145–156. [Google Scholar] [CrossRef]
- Karlsson, H.; Nilsson, L.; Möller, L. Subway particles are more genotoxic than street particles and induce oxidative stress in cultured human lung cells. Chem. Res. Toxicol. 2005, 18, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Shuka, A.; Timblin, C.; BeruBe, K.; Gordon, T.; McKinney, W.; Driscoll, K.; Vacek, P.; Mossman, B.T. Inhaled particulate matter causes expression of Nuclear Factor (NF)-kB—Related genes and oxidant-dependent NFkB activation in vitro. Am. J. Respir. Cell. Mol. Biol. 2000, 23, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Kim, B.; Ryu, J.; Maskey, S.; Kim, J.C.; Sohn, J.; Ro, C.U. Source identification of particulate matter collected at underground subway stations in Seoul, Korea using quantitative single-particle analysis. Atmos. Environ. 2010, 44, 2287–2293. [Google Scholar] [CrossRef]
- Kim, K.Y.; Kim, Y.S.; Roh, Y.M.; Lee, C.M.; Kim, C.N. Spatial distribution of particulate matter (PM10 and PM2.5) in Seoul Metropolitan Subway stations. J. Hazard. Mat. 2008, 154, 440–443. [Google Scholar] [CrossRef]
- Murruni, L.G.; Solanes, V.; Debray, M.; Kreiner, A.J.; Davidson, J.; Davidson, M.; Vázquez, M.; Ozafrán, M. Concentrations and elemental composition of particulate matter in the Buenos Aires underground system. Atm. Environ. 2009, 43, 4577–4583. [Google Scholar] [CrossRef]
- Midander, K.; Elihn, K.; Wallén, A.; Belova, L.; Karlsson, A.K.; Wallinder, I.O. Characterisation of nano- and micron-sized airborne and collected subway particles, a multi-analytical approach. Sci. Total Environ. 2012, 427–428, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Chillrud, S.N.; Grass, D.; Ross, J.M.; Coulibaly, D.; Slavkovich, V.; Epstein, D.; Sax, S.N.; Pederson, D.; Johnson, D.; Spengler, J.D.; Kinney, P.L.; Simpson, H.J.; Brandt-Rauf, P. Steel dust in the New York City subway system as a source of manganese, chromium, and iron exposures for transit workers. J. Urban. Health. 2005, 82, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Byeon, S.H.; Willis, R.; Peters, T.M. Chemical characterization of outdoor and subway fine (PM2.5–1.0) and Coarse (PM10–2.5) Particulate Matter in Seoul (Korea) by Computer-Controlled Scanning Electron Microscopy (CCSEM). Int. J. Environ. Res. Public. Health. 2015, 12, 2090–2104. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Yoo, D.C.; Kwon, Y.M.; Han, W.S.; Kim, G.S.; Park, M.J.; Kim, Y.S.; Choi, D. A study on characteristics of atmospheric heavy metals in subway station. Toxicol. Res. 2010, 26, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Alessandria, L.; Schilirò, T.; Degan, R.; Traversi, D.; Gilli, G. Cytotoxic response in human lung epithelial cells and ion characteristics of urban-air particles from Torino, a northern Italian city. Environ. Sci. Pollut. Res. Int. 2014, 21, 5554–5564. [Google Scholar] [CrossRef] [PubMed]
- Janssen, N.A.; Yang, A.; Strak, M.; Steenhof, M.; Hellack, B.; Gerlofs-Nijland, M.E.; Kuhlbusch, T.; Kelly, F.; Harrison, R.; Brunekreef, B.; Hoek, G.; Cassee, F. Oxidative potential of particulate matter collected at sites with different source characteristics. Sci. Total Environ. 2014, 472, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Risom, L.; Møller, P.; Loft, S. Oxidative stress-induced DNA damage by particulate air pollution. Mutat. Res. 2005, 592, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Mugica-Álvarez, V.; Figueroa-Lara, J.; Romero-Romo, M.; Sepúlveda-Sánchez, J.; López-Moreno, T. Concentrations and properties of airborne particles in the Mexico City subway system. Atmos. Environ. 2012, 49, 284–293. [Google Scholar] [CrossRef]
- Longhin, E.; Holme, J.A.; Gutzkow, K.B.; Arlt, V.M.; Kucab, J.E.; Camatini, M.; Gualtieri, M. Cell cycle alterations induced by urban PM2.5 in bronchial epithelial cells: Characterization of the process and possible mechanisms involved. Part. Fibre. Toxicol. 2013, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014, 10, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.; Gonzalez-Flecha, B.; Kobzik, L. Reactive oxygen species in pulmonary inflammation by ambient particulates. Free Radic. Biol. Med. 2003, 35, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Fiotakis, K.; Thomais, V. Airborne particulate matter and human health toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2008, 26, 339–362. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spagnolo, A.M.; Ottria, G.; Perdelli, F.; Cristina, M.L. Chemical Characterisation of the Coarse and Fine Particulate Matter in the Environment of an Underground Railway System: Cytotoxic Effects and Oxidative Stress—A Preliminary Study. Int. J. Environ. Res. Public Health 2015, 12, 4031-4046. https://doi.org/10.3390/ijerph120404031
Spagnolo AM, Ottria G, Perdelli F, Cristina ML. Chemical Characterisation of the Coarse and Fine Particulate Matter in the Environment of an Underground Railway System: Cytotoxic Effects and Oxidative Stress—A Preliminary Study. International Journal of Environmental Research and Public Health. 2015; 12(4):4031-4046. https://doi.org/10.3390/ijerph120404031
Chicago/Turabian StyleSpagnolo, Anna Maria, Gianluca Ottria, Fernanda Perdelli, and Maria Luisa Cristina. 2015. "Chemical Characterisation of the Coarse and Fine Particulate Matter in the Environment of an Underground Railway System: Cytotoxic Effects and Oxidative Stress—A Preliminary Study" International Journal of Environmental Research and Public Health 12, no. 4: 4031-4046. https://doi.org/10.3390/ijerph120404031





