Drinking Water from Dug Wells in Rural Ghana — Salmonella Contamination, Environmental Factors, and Genotypes
Abstract
:1. Introduction
2. Methods

3. Results
3.1. Contamination with Gram-Negative Rod-Shaped Bacteria
3.2. Salmonella Isolates and Serovars
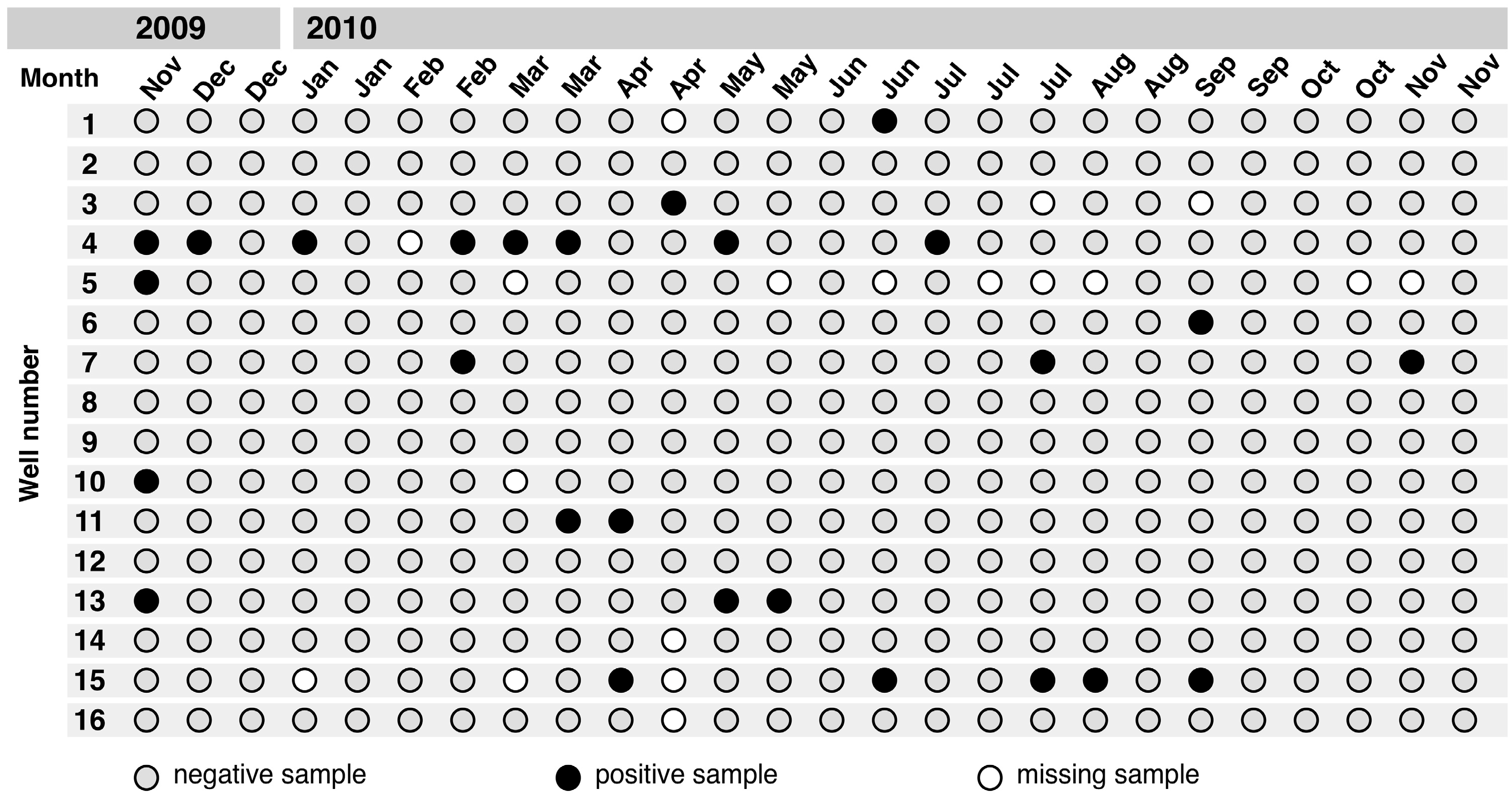
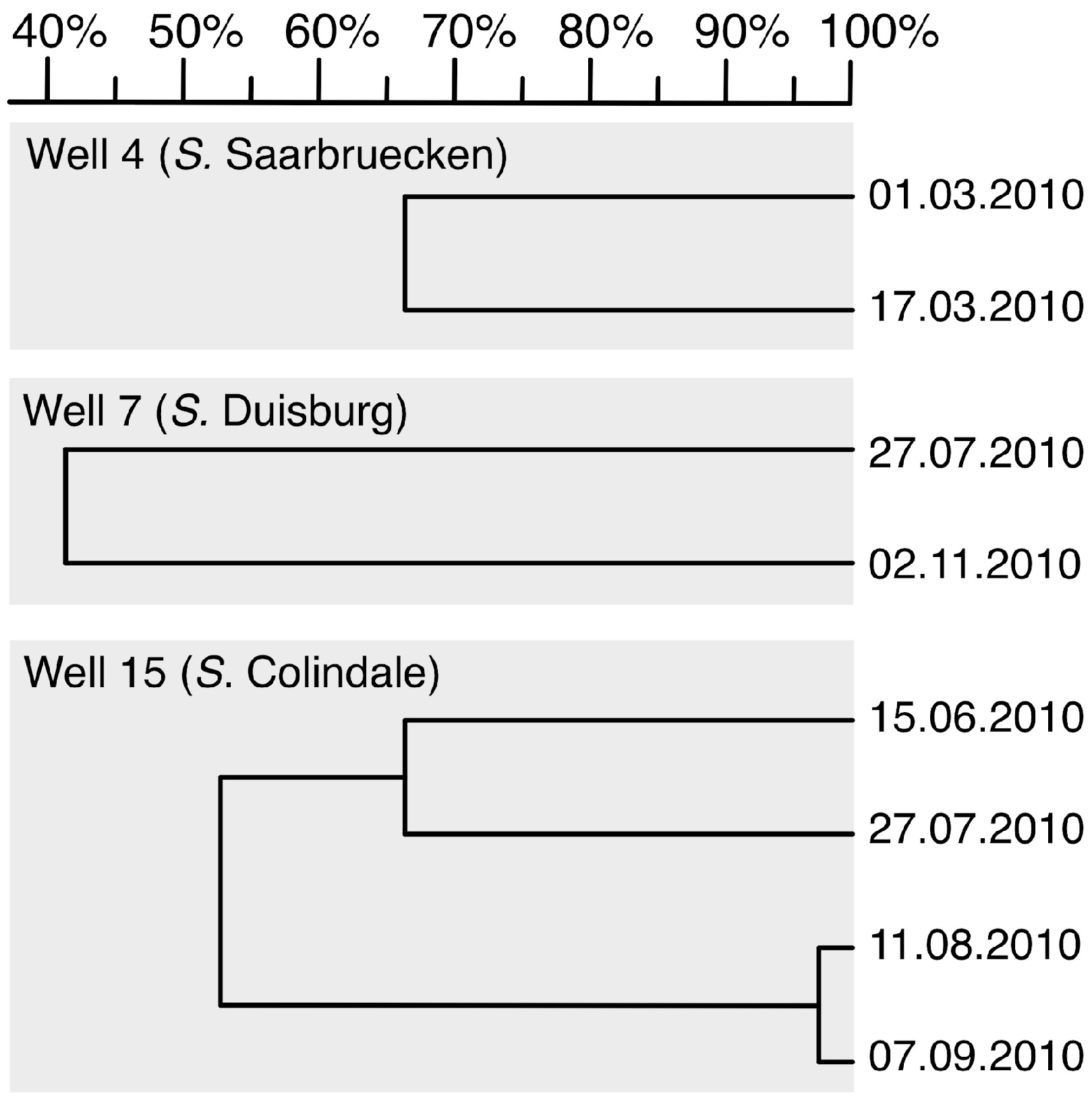
3.3. Clusters of Salmonella Isolates
3.4. Antibiotic Susceptibility
| Frequency (%) | ||
|---|---|---|
| Drug (AC μg) | Susceptible | Resistant |
| Ampicillin (10) | 24 (92.3) | 2 (7.7) |
| Ampicillin/Sulbactam (20) | 25 (96.2) | 1 (3.8) |
| Ceftriaxone (30) | 26 (100.0) | 0 (0) |
| Chloramphenicol (30) | 26 (100.0) | 0 (0) |
| Ciprofloxacin (5) | 26 (100.0) | 0 (0) |
| Cotrimoxazole (25) | 26 (100.0) | 0 (0) |
| Nalidixic acid (30) | 26 (100.0) | 0 (0) |
| Tetracycline (30) | 25 (96.2) | 1 (3.8) |
3.5. PFGE Genotyping
3.6. Well Characteristics and Salmonella Contamination
| Independent Variables | Frequency (%) | Random-Effect Models OR (95% CI) | ||||
|---|---|---|---|---|---|---|
| Model A | Model B | Model C | Model D | Model E | ||
| Presence of frame (>30 cm) | 294 (74.1) | 0.3 (0.1–1.3) | – | – | – | 0.3 (0.1–0.8) |
| Well covered | 269 (67.8) | – | 2.0 (0.3–13.6) | – | – | – |
| Rubbish in well | 75 (18.9) | – | – | 2.6 (0.9–7.4) | – | 2.9 (0.9–9.6) |
| Season (rainy versus dry) | 272 (68.5) | – | – | – | 2.6 (1.2–5.6) | 2.6 (1.2–5.5) |
| Random Intercept Variance (SE) | – | 0.89 (0.82) | 2.96 (3.21) | 0.96 (0.49) | 1.68 (0.97) | 0.76 (1.28) |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gordon, M.A. Salmonella infections in immunocompromised adults. J. Infect. 2008, 56, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Mtove, G.; Amos, B.; von Seidlein, L.; Hendriksen, I.; Mwambuli, A.; Kimera, J.; Mallahiyo, R.; Kim, D.R.; Ochiai, R.L.; Clemens, J.D.; et al. Invasive salmonellosis among children admitted to a rural Tanzanian hospital and a comparison with previous studies. PloS One 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Reddy, E.A.; Shaw, A.V.; Crump, J.A. Community-acquired bloodstream infections in Africa: A systematic review and meta-analysis. Lancet Infect. Dis. 2010, 10, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Gross, U.; Amuzu, S.K.; de Ciman, R.; Kassimova, I.; Gross, L.; Rabsch, W.; Rosenberg, U.; Schulze, M.; Stich, A.; Zimmermann, O. Bacteremia and antimicrobial drug resistance over time, Ghana. Emerg Infect. Dis. 2011, 17, 1879–1882. [Google Scholar] [CrossRef] [PubMed]
- Wilkens, J.; Newman, M.J.; Commey, J.O.; Seifert, H. Salmonella bloodstream infection in Ghanaian children. Clin. Microbiol. Infec. 1997, 3, 616–620. [Google Scholar] [CrossRef]
- Marks, F.; Adu-Sarkodie, Y.; Hunger, F.; Sarpong, N.; Ekuban, S.; Agyekum, A.; Nkrumah, B.; Schwarz, N.G.; Favorov, M.O.; Meyer, C.G.; et al. Typhoid fever among children, Ghana. Emerg. Infect. Dis. 2010, 16, 1796–1797. [Google Scholar] [CrossRef] [PubMed]
- La Qualité de l`eau de Puits dans la Commune d`Abomey-Calvi au Benin. Available online: http://gwppnebenin.org/dru/node/163 (accessed on 26 January 2015).
- Degbey, C.; Makoutode, M.; Agueh, V.; Dramaix, M.; de Brouwer, C. Factors associated with the quality of well water and the prevalence of waterborne diseases in the municipality of Abomey-Calavi in Benin. Sante 2011, 21, 47–55. [Google Scholar] [PubMed]
- UN. Goal7: Ensure Environmental Sustainability. Available online: http://www.un.org/millenniumgoals/environ.shtml (accessed 14 November 2013).
- WHO. Guidelines for Drinking-Water Quality; WHO Press: Geneva, Switzerland, 2011. [Google Scholar]
- Chan, K.E. The extended Widal test in the diagnosis of fevers due to Salmonella infection. Med. J. Malaya. 1962, 17, 18–24. [Google Scholar] [PubMed]
- Antigenic Formulas of the Salmonella Serovars. Available online: http://www.pasteur.fr/ip/portal/action/WebdriveActionEvent/oid/01s-000036-089 (accessed on 26 January 2015).
- Rabe-Hesketh, S.; Skrondal, A. Multilevel and Longitudinal Modeling using Stata; Stata Press: College Station, TX, USA, 2005. [Google Scholar]
- UNICEF; WHO. Drinking Water: Equity, Safety and Sustainability; UNICEF: New York, NY, USA, 2011. [Google Scholar]
- Baudart, J.; Lemarchand, K.; Brisabois, A.; Lebaron, P. Diversity of Salmonella strains isolated from the aquatic environment as determined by serotyping and amplification of the ribosomal DNA spacer regions. Appl. Environ. Microbiol. 2000, 66, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Arvanitidou, M.; Stathopoulos, G.A.; Constantinidis, T.C.; Katsouyannopoulos, V. The occurrence of Salmonella, Campylobacter and Yersinia spp. in river and lake waters. Microbiol. Res. 1995, 150, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Catalao Dionisio, L.P.; Joao, M.; Ferreiro, V.S.; Fidalgo, M.L.; Garcia Rosado, M.E.; Borrego, J.J. Occurrence of Salmonella spp. in estuarine and coastal waters of Portugal. Antonie Van Leeuwenhoek 2000, 78, 99–106. [Google Scholar]
- Guinee, P.A.; Kampelmacher, E.H. Four new Salmonella types (S. Gambaga, S. Kumasi, S. Pramiso and S. Ashanti isolated in Ghana. Antonie Van Leeuwenhoek 1961, 27, 203–205. [Google Scholar] [CrossRef]
- Ward, L. Fatal neonatal Salmonella Rubislaw infection in household with pet reptile in England. Euro surveillance: Bulletin Europeen sur les maladies transmissibles. Euro. Commun. Dis. Bull. 2000, 4. [Google Scholar] [CrossRef]
- Kennedy, M.E. Salmonella isolations from snakes and other reptiles. Can. J. Comp. Med. 1973, 37, 325–326. [Google Scholar] [PubMed]
- Huehn, S.; Bunge, C.; Junker, E.; Helmuth, R.; Malorny, B. Poultry-associated Salmonella enterica subsp. enterica serovar 4,12:d:—Reveals high clonality and a distinct pathogenicity gene repertoire. Appl. Environ. Microbiol. 2009, 75, 1011–1020. [Google Scholar]
- Kagambega, A.; Barro, N.; Traore, A.S.; Siitonen, A.; Haukka, K. Characterization of Salmonella enterica and detection of the virulence genes specific to diarrheagenic Escherichia coli from poultry carcasses in Ouagadougou, Burkina Faso. Foodborne Pathog. Dis. 2012, 9, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Tennant, S.M.; Diallo, S.; Levy, H.; Livio, S.; Sow, S.O.; Tapia, M.; Fields, P.I.; Mikoleit, M.; Tamboura, B.; Kotloff, K.L.; et al. Identification by PCR of non-typhoidal Salmonella enterica serovars associated with invasive infections among febrile patients in Mali. PLoS Negl. Trop. Dis. 2010, 4. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A.; Frank, C.; Prager, R.; Oppermann, H.; Stark, K. Nation-wide outbreak of Salmonella give in Germany, 2004. Z. Gastroenterol. 2005, 43, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Lebeaux, D.; Zarrouk, V.; Petrover, D.; Nicolas-Chanoine, M.H.; Fantin, B. Salmonella Colindale osteomyelitis in an immunocompetent female patient. Med. Mal. Infect. 2012, 42, 36–37. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.C.; Martinez, E.; Gay, J.M.; Rice, D.H. Survival of Salmonella enterica in freshwater and sediments and transmission by the aquatic midge Chironomus tentans (Chironomidae: Diptera). Appl. Environ Microbiol. 2003, 69, 4556–4560. [Google Scholar] [CrossRef] [PubMed]
- Barrell, R.A.; Rowland, M.G. The relationship between rainfall and well water pollution in a West African (Gambian) village. J. Hyg. 1979, 83, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Marks, S.L.; Rankin, S.C.; Byrne, B.A.; Weese, J.S. Enteropathogenic bacteria in dogs and cats: Diagnosis, epidemiology, treatment, and control. J. Vet. Intern. Med. 2011, 25, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Salmonella Infections Associated with Reptiles: The Current Situation in Europe. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=18902 (accessed on 26 January 2015).
- Prüss-Üstün, A.; Bos, R.; Gore, F.; Bartram, J. Safer Water, Better Health: Costs, Benefits and Sustainability of Interventions to Protect and Promote Health; WHO: Geneva, Swizarland, 2008. [Google Scholar]
- WHO; UNICEF. Joint Monitoring Programme (JMP) for Water Supply and Sanitation. Available online: http://www.wssinfo.org/definitions-methods/watsan-categories/ (accessed 15 November 2013).
- WHO. Progress on Sanitation and Drinking Water: 2013 Update; WHO Press: Geneva, Swizarland, 2013. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dekker, D.M.; Krumkamp, R.; Sarpong, N.; Frickmann, H.; Boahen, K.G.; Frimpong, M.; Asare, R.; Larbi, R.; Hagen, R.M.; Poppert, S.; et al. Drinking Water from Dug Wells in Rural Ghana — Salmonella Contamination, Environmental Factors, and Genotypes. Int. J. Environ. Res. Public Health 2015, 12, 3535-3546. https://doi.org/10.3390/ijerph120403535
Dekker DM, Krumkamp R, Sarpong N, Frickmann H, Boahen KG, Frimpong M, Asare R, Larbi R, Hagen RM, Poppert S, et al. Drinking Water from Dug Wells in Rural Ghana — Salmonella Contamination, Environmental Factors, and Genotypes. International Journal of Environmental Research and Public Health. 2015; 12(4):3535-3546. https://doi.org/10.3390/ijerph120403535
Chicago/Turabian StyleDekker, Denise Myriam, Ralf Krumkamp, Nimako Sarpong, Hagen Frickmann, Kennedy Gyau Boahen, Michael Frimpong, Renate Asare, Richard Larbi, Ralf Matthias Hagen, Sven Poppert, and et al. 2015. "Drinking Water from Dug Wells in Rural Ghana — Salmonella Contamination, Environmental Factors, and Genotypes" International Journal of Environmental Research and Public Health 12, no. 4: 3535-3546. https://doi.org/10.3390/ijerph120403535






