On the Road to HIV/AIDS Competence in the Household: Building a Health-Enabling Environment for People Living with HIV/AIDS
Abstract
:1. Introduction
2. Key Concepts
2.1. AIDS Competent Community
2.2. Households: Challenged, not Damaged
3. Methods
3.1.Ethical Approval
3.2.Context and Setting
3.3. Data Collection
3.4. Respondents
3.5. Data analysis
4. Results
“I feel like I can defeat it now, I am more positive. […] Because of the support that I have been getting here in the household.”(Male PLWHA, 24)
4.1. Context
“In some instances, you will find that in other families there are more than three or four people taking the same pills and then it’s easy, they always support each other. And that there is not stigma because everyone in the house is just open about it.”(Community Health Worker, Health Facility 3)
4.2. Process
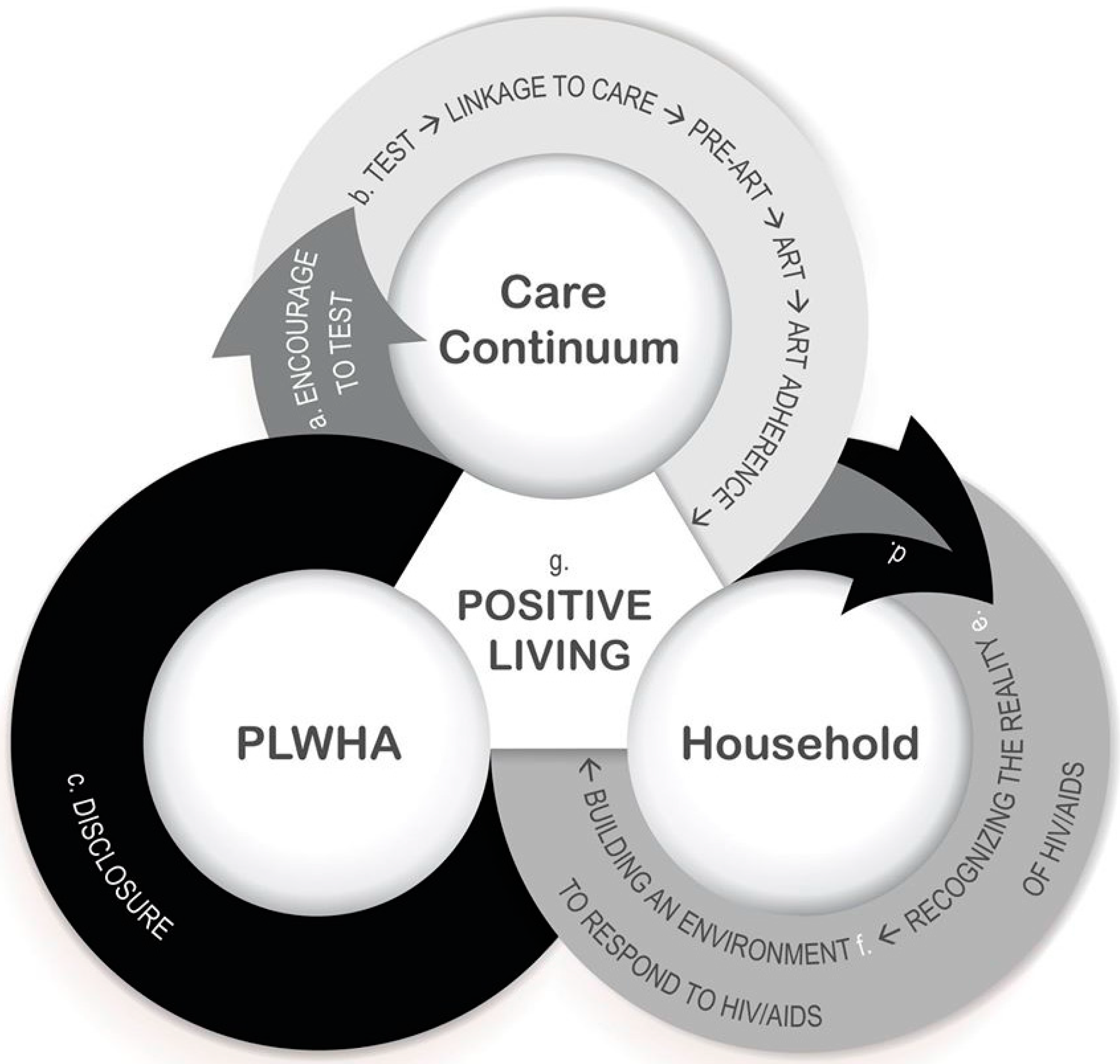
4.2.1. Recognizing the Reality of HIV/AIDS in the Household
“I am living with AIDS in my house, not in their house.”(Male PLWHA, 24)
“I decided to tell her [partner] because she asked me lots of questions about my pills because I separate other pills from ARVs. I told her she must stop interfering to my things. But the other day we were both happy and I’ve decided to tell her the truth that I’m on treatment and I am using ARVs.”(Male PLWHA, 52)
“I talk to my cousin because we are very close and I trust him a lot. Whatever I share with him, he does not spread any rumors about me or discuss it with his friends. […] My cousins are two but I share my problems with the younger one Sipho and the other one is an alcoholic, so I don’t like to talk to the older one.”(Female PLWHA, 27)
“I trust him [cousin] so much, even that day I was from the clinic he noticed that I was not well. He always comes home earlier than the other one [cousin] and he asked what was bothering me. I lied and say no I have a headache and he says whatever it is, I must tell him because I am his family, he deserves to know. And I told him.”(Female PLWHA, 27)
“There is no one [judging], because there is no one from outside who knows my status. It’s only my family that knows.”(Female PLWHA, 22)
4.2.2. Change Agents
“My mother has noticed that I lost weight and I had a skin rash; she forced me to go to the clinic and I didn’t want to go. One day she took me to the clinic and she was watching me like a hawk because she thought I was going to run away. The nurse attended me and she asked me if I was sick and I said I was suffering from a stomach ache. The nurse asked me if I was willing to do the HIV test and I agreed on that.”(Female PLWHA, 21)
4.2.3. Building a Household Environment to Respond to HIV/AIDS
4.2.4. Bridging Social Capital
“I only told her [sister] because the clinic asked me to come with her, because she had to sign for me.”(Male PLWHA, 52)
4.2.5. Exchange and Sharing of Knowledge and Prevention Skills
“I told my wife about it. The counseling was done and they told me about the same thing that she told me, that actually was not new to me.”(Male PLWHA, 46)
“I tell them [children] to use condoms. I want them to learn from my mistake. I tell them that I am HIV positive because I never used condoms. I also advise them to go and get tested for HIV.”(Female PLWHA, 56)
4.2.6. Ownership and Responsibility
“It is my responsibility, but people in the house also remind me not to forget my clinic appointments.”(Female PLWHA, 42)
4.2.7. Social Space for Dialogue and Critical Thinking
“She [partner] knows everything because she went for counseling. They told her that anything can happen. If something goes wrong with me, she says ‘no man, don’t worry about those things’. You are aware that you might get it. Otherwise, those things I already knew and [if] I have a problem I ask her, she tells me about these things, she knows about them.”(Male PLWHA, 46)
4.2.8. Solidarity and Common Purpose
“He [cousin] motivated me and said nothing will change, I am his family. He will support me right through.[cries]”(Female PLWHA, 27)
4.2.9. Positive Living
“She [partner] understood and was not angry at all. All what she did was to encourage me to go and take treatment at the clinic. She gave me her support telling me that the HIV virus doesn’t kill people if they take treatment. But it happens to be the people who kill themselves by not taking treatment.”(Male PLWHA, 45)
“Before I got tested, I used to have sex without a condom. But now I use it regularly. After I discovered that I am HIV positive, I told myself that I should stick to one partner and I am not interested to be engaged into having sex.”(Male PLWHA, 22)
4.2.10. Dynamic
“I moved here to stay here after when I was sick, because I have noticed that my son is the one who would do better to look after me.”(Female PLWHA, 56)
“No they don’t know here, but in the Eastern Cape I was open about it because even in my neighborhood in Eastern Cape they come to me and ask me how I do it and I told them. [So] they go to clinic, they tested.”(Female PLWHA, 31)
4.3. Barriers
4.3.1. Personal Barriers for PLWHA
“The thing that makes it difficult with disclosure is that one goes to the clinic and gets counseling and understanding, but [he] doesn’t accept his own status. So if he can start by accepting, it will be easy to disclose to the family first, before outside.”(Community Health Worker, Health Facility 4)
“Some families are more supportive, especially when the patient is not drinking. Because when the patient is drinking, when she will be sober she will obey them. But on weekends, she will say ‘this is my life, you are not affected by this.’”(Community Health Worker, Health Facility 2)
4.3.2. Barriers at the Household Level
“The stigma starts in the home. The people who are supposed to support this person are the ones who discriminate against her/him, you know. So it’s a big problem.”(Community Health Worker, Health Facility 4)
4.3.3. Influence of a Negative Household Context on Disclosure
“Some they have reasons [not to disclose]. They will say with their family quarrelling, they will tell other people that you are HIV positive. And some, if they are staying with people who don’t know HIV, they will put aside plates, spoons, all these utensils we you use they put aside so that they don’t want to share.”(Community Health Worker, Health Facility 2)
“We did ask why she doesn’t want the children to know. She just said ‘no they are aggressive and they are drinking too much’. She can’t tell them because they are going to swear at her. And she is scared they maybe going to abuse her.”(Community Health Worker, Health Facility 4)
4.3.4. Consequences of Non-Disclosure in the Household
“My family was so excited about my baby but I was stressing too much because I never disclosed my status to anyone at home. I was always crying and wanted to be alone at all times. You won’t believe it when I say that I woke up the other day; I went to the shop to buy poison that is meant to kill rats. I mixed the poison with water then I drank it. I collapsed and was admitted to Jooste Hospital. I was so stressed to find out that I am HIV positive.”(Female PLWHA, 36)
“I asked my boyfriend to use protection and he agreed because I lied to him and said that I have a problem in my womb. I take my medication in front of him but he doesn’t know what that medication is for. He only knows that I collect medication for high blood pressure and I have a problem in my womb.”(Female PLWHA, 27)
4.3.5. Disbelief or a Negative Response to Disclosure
“He [partner] did not believe it because I never lost weight. (…) He only believed it last year when he saw that I continued with my treatment.”(Female PLWHA, 38)
“I was under a lot of stress because there were people who were judging me, so I stopped [ART] […] They were judging me by insulting me about my HIV status. And that I am going to infect my husband, because my sisters-in-law were saying that.”(Female PLWHA, 27)
4.3.6. Poverty
“They wait for their medication, but others they don’t because they say that they want to go and drink alcohol so that their CD4 count drops so that they can be able to apply for the grant.”(Female PLWHA, 22 years)
4.4. Not a Panacea
“Sometimes when you are HIV, you lose hope, you don’t have much respect for yourself, even for others that are around you. Because the people you hurt most are those who are close to you, that care for you. You don’t want people to treat you as if like, you know, you are sick.”(Male PLWHA, 30)
5. Discussion
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Karim, S.S.A. Stigma impedes AIDS prevention. Nature 2011, 474, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Gardner, E.M.; Young, B. The HIV care cascade through time. Lancet Infect. Dis. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Mcnairy, M.L.; el-Sadr, W.M. The HIV care continuum: No partial credit given. AIDS 2012, 26, 1735–1738. [Google Scholar] [CrossRef] [PubMed]
- Hallett, T.B.; Eaton, J.W. A side door into care cascade for HIV-infected patients? J. Acquir. Immune Defic. Syndr. 2013, 63, S228–S232. [Google Scholar] [CrossRef] [PubMed]
- Doherty, M. Optimizing the HIV Treatment Continuum. In Proceedings of Controlling the HIV Epidemic with Antiretrovirals: Avoiding the Cost of Inaction, London, UK, 18–19 September 2014.
- Kilmarx, P.H.; Mutasa-Apollo, T. Patching a leaky pipe: The cascade of HIV care. Curr. Opin. HIV AIDS 2013, 8, 59–64. [Google Scholar] [PubMed]
- Russell, S.; Seeley, J. The transition to living with HIV as a chronic condition in rural Uganda: Working to create order and control when on antiretroviral therapy. Soc. Sci. Med. 2010, 70, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Wouters, E.; van Damme, W.; van Loon, F.; van Rensburg, D.; Meulemans, H. Public-sector art in the free state province, South Africa: Community support as an important determinant of outcome. Soc. Sci. Med. 2009, 69, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Wouters, E.; Meulemans, H.; van Rensburg, H.C.J. Slow to share: Social capital and its role in public HIV disclosure among public sector art patients in the free state province of South Africa. AIDS Care 2009, 21, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Lamboray, J.L.; Skevington, S.M. Defining AIDS competence: A working model for practical purposes. J. Int. Dev. 2001, 13, 513–521. [Google Scholar] [CrossRef]
- AIDS-Social Drivers Working Group. Revolutionizing the AIDS Response: Building AIDS Resilient Communities; Clark University and International Center for Research on Women (ICRW); International Development, Community and Environment (IDCE): Worcester, UK, 2010. [Google Scholar]
- Weihs, K.; Fisher, L.; Baird, M. Families, health, and behavior: A section of the commissioned report by the committee on health and behavior: Research, practice, and policy division of neuroscience and behavioral health and division of health promotion and disease prevention institute of medicine, national academy of sciences. Fam. Syst. Health 2002, 20. [Google Scholar] [CrossRef]
- Wouters, E. Life with HIV as a chronic illness: A theoretical and methodological framework for antiretroviral treatment studies in resource-limited settings. Soc. Theory Health 2012, 10, 368–391. [Google Scholar] [CrossRef]
- Waller, M.A. Resilience in ecosystemic context: Evolution of the concept. Am. J. Orthopsychiat. 2001, 71, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Latkin, C.; Knowlton, A. Micro-social structural approaches to HIV prevention: A social ecological perspective. Aids Care 2005, 17, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Celletti, F.; Wright, A.; Palen, J.; Frehywot, S.; Markus, A.; Greenberg, A.; de Aguiar, R.A.T.; Campos, F.; Buch, E.; Samb, B. Can the deployment of community health workers for the delivery of HIV services represent an effective and sustainable response to health workforce shortages? Results of a multicountry study. AIDS 2010, 24, S45–S57. [Google Scholar] [CrossRef] [PubMed]
- Tawil, O.; Verster, A.; O’Reilly, K. Enabling approaches for HIV/AIDS promotion: Can we modify the environment and minimize the risk? AIDS 1995, 9, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Alando, C. Evaluation of the Unaids/Unitar AIDS Competence Programme; UNAIDS: Geneva, Switzerland, 2005. [Google Scholar]
- Campbell, C.; Nair, Y.; Maimane, S. Building contexts that support effective community responses to HIV/AIDS: A south african case study. Am. J. Community Psychol. 2007, 39, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Nhamo, M.; Campbell, C.; Gregson, S. Obstacles to local-level AIDS competence in rural Zimbabwe: Putting HIV prevention in context. Aids Care 2010, 22, 1662–1669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, S. Building AIDS Competence in Manya Krobo and the Role of the Manya Krobo Queen Mothers Association; University of Calgary Press: Calgary, AB, Canada, 2011; p. 149. [Google Scholar]
- Rotheram-Borus, M.; Flannery, D.; Rice, E.; Lester, P. Families living with HIV. Aids Care 2005, 17, 978–987. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.W.; Ankrah, E.M.; Schumann, D.A.; Nkumbi, S.; Lubega, M. Aids and the urban family: Its impact in Kampala, Uganda. Aids Care 1993, 5, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Rehm, R.S.; Franck, L.S. Long-term goals and normalization strategies of children and families affected by HIV/AIDS. Adv. Nurs. Sci. 2000, 23, 69–82. [Google Scholar] [CrossRef]
- Du Preez, C.J.; Niehof, A. Caring for people living with AIDs: A labour of love. Med. Antropol. 2008, 20, 87–104. [Google Scholar]
- Bor, R.; du Plessis, P.; Russell, M. The impact of disclosure of HIV on the index patient’s self‐defined family. J. Fam. Ther. 2004, 26, 167–192. [Google Scholar] [CrossRef]
- Campbell, C.; Skovdal, M.; Mupambireyi, Z.; Madanhire, C.; Nyamukapa, C.; Gregson, S. Building adherence-competent communities: Factors promoting children’s adherence to anti-retroviral HIV/AIDS treatment in rural Zimbabwe. Health Place 2012, 18, 123–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SAT’s Work to Strengthen Community HIV and AIDS Competence; Southern African AIDS Trust: Johannesburg, South Africa, 2011.
- Gruber, J.; Caffrey, M. HIV/AIDS and community conflict in Nigeria: Implications and challenges. Soc. Sci. Med. 2005, 60, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Russel, M.; Schneider, H. A Rapid Appraisal of Community-Based HIV/AIDS Care and Support Programs in South Africa. Centre for Health Policy; University of Witwatersrand: Witwatersrand, South Africa, 2000. [Google Scholar]
- Mathiot, D. Experience of the un Country Team in Eritrea, in Using the Self-Assessment Framework for Aids Competence; UNAIDS: Geneva, Switzerland, 2004. [Google Scholar]
- Campbell, C. Building Aids Competent Communities: Possibilities and Challenges. In Proceedings of AIDS2031 Meeting, Salzburg, Austria, March 2009.
- Ryan, P.; Sawin, K.J. The individual and family self-management theory: Background and perspectives on context, process, and outcomes. Nurs. Outlook 2009, 57, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Innovative Care for Chronic Conditions: Building Blocks for Action; WHO: Geneva, Switzerland, 2002.
- Greeff, A.P.; de Villiers, M. Optimism in family resilience. Soc. Work Pract. Res. 2008, 20, 21–34. [Google Scholar]
- Walsh, F. A family resilience framework: Innovative practice applications. Fam. Relat. 2002, 51, 130–137. [Google Scholar] [CrossRef]
- Wallace, C. Household strategies: Their conceptual relevance and analytical scope in social research. Sociology 2002, 36, 275–292. [Google Scholar] [CrossRef]
- Niehof, A. Conceptualizing the household as an object of study. Int. J. Consum. Stud. 2011, 35, 488–497. [Google Scholar] [CrossRef]
- Nombo, C.I. When AIDS Meets Poverty: Implications for Social Capital in a Village in Tanzania; Wageningen Academic Publisher: Wageningen, The Netherlands, 2007. [Google Scholar]
- Iwelunmor, J.; Airhihenbuwa, C.; Okoror, T.; Brown, D.; BeLue, R. Family systems and HIV/AIDS in South Africa. Int. Q. Community Health Educ. 2006, 27, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Du Preez, C.J. Living and Care Arrangements of Non-Urban Households in Kwazulu-Natal, South Africa, in the Context of HIV and AIDS. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2011. [Google Scholar]
- Ferlander, S. The importance of different forms of social capital for health. Acta Sociol. 2007, 50, 115–128. [Google Scholar] [CrossRef]
- Wakefield, S.E.; Poland, B. Family, friend or foe? Critical reflections on the relevance and role of social capital in health promotion and community development. Soc. Sci. Med. 2005, 60, 2819–2832. [Google Scholar] [CrossRef] [PubMed]
- Makoae, L.N.; Portillo, C.J.; Uys, L.R.; Dlamini, P.S.; Greeff, M.; Chirwa, M.; Kohi, T.W.; Naidoo, J.; Mullan, J.; Wantland, D. The impact of taking or not taking arvs on HIV stigma as reported by persons living with HIV infection in five African countries. Aids Care 2009, 21, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Elliott, T.R.; Kurylo, M. Hope over acquired disability: Lessons of a young woman’s triumph. In Handbook of Hope: Theory, Measures, and Applications; Academic Press: Waltham, MA, USA, 2000; p. 373. [Google Scholar]
- Beeker, C.; Guenther-Grey, C.; Raj, A. Community empowerment paradigm drift and the primary prevention of HIV/AIDS. Soc. Sci. Med. 1998, 46, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J. Long Life: Positive HIV Stories; Double Storey Books: Lansdowne, South Africa, 2003. [Google Scholar]
- Holzemer, W.L.; Uys, L.; Makoae, L.; Stewart, A.; Phetlhu, R.; Dlamini, P.S.; Greeff, M.; Kohi, T.W.; Chirwa, M.; Cuca, Y. A conceptual model of HIV/AIDS stigma from five African countries. J. Adv. Nurs. 2007, 58, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Remien, R.H.; Stirratt, M.J.; Dognin, J.; Day, E.; el-Bassel, N.; Warne, P. Moving from theory to research to practice: Implementing an effective dyadic intervention to improve antiretroviral adherence for clinic patients. J. Acquir. Immune Defic. Syndr. 2006, 43, S69–S78. [Google Scholar] [CrossRef] [PubMed]
- Odendaal, W.A.; Lewin, S. The provision of TB and HIV/AIDS treatment support by lay health workers in South Africa: A time-and-motion study. Hum. Resourc. Health 2014, 12. [Google Scholar] [CrossRef]
- Mortelmans, D. Handboek Kwalitatieve Onderzoeksmethoden; ACCO Uitgeverij: Leuven, Belgium, 2013. [Google Scholar]
- Corbin, J.; Strauss, A. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory; Sage: Thousand Oaks, CA, USA, 2008. [Google Scholar]
- Eriksson, M.; Emmelin, M. What constitutes a health-enabling neighborhood? A grounded theory situational analysis addressing the significance of social capital and gender. Soc. Sci. Med. 2013, 97, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Aids by the Numbers; UNAIDS: Geneva, Switzerland, 2013.
- AIDS2031 Consortium. Aids: Taking a Long-Term View; Financial Times Science Press: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Factsheet 2014: Global Statistics; UNAIDS: Geneva, Switzerland.
- Grey, M.; Knafl, K.; McCorkle, R. A framework for the study of self-and family management of chronic conditions. Nurs. Outlook 2006, 54, 278–286. [Google Scholar] [CrossRef] [PubMed]
- National Strategic Plan on HIV, STIs and TB, 2012–2016; South African National AIDS Council: Pretoria, South Africa, 2011.
- Akintola, O. Gendered home-based care in South Africa: More trouble for the troubled. Afr. J. AIDS Res. 2006, 5, 237–247. [Google Scholar] [CrossRef]
- Chaudoir, S.R.; Fisher, J.D.; Simoni, J.M. Understanding HIV disclosure: A review and application of the disclosure processes model. Soc. Sci. Med. 2011, 72, 1618–1629. [Google Scholar] [CrossRef] [PubMed]
- Gausset, Q.; Mogensen, H.O.; Yameogo, W.M.E.; Berthé, A.; Konaté, B. The ambivalence of stigma and the double-edged sword of HIV/AIDS intervention in Burkina Faso. Soc. Sci. Med. 2012, 74, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, T.; Bernays, S.; Terzić, K.J. Medical promise and the recalibration of expectation: Hope and HIV treatment engagement in a transitional setting. Soc. Sci. Med. 2009, 68, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Niehof, A. Why should households care? A rejoinder. Med. Antropol. 2004, 16, 282–289. [Google Scholar]
- The Impact of Aids; Department of Economic and Social Affairs: Population Division: New York, NY, USA, 2004.
- Hosegood, V.; McGrath, N.; Herbst, K.; Timaeus, I.M. The impact of adult mortality on household dissolution and migration in rural South Africa. AIDS 2004, 18, 1585–1590. [Google Scholar] [CrossRef] [PubMed]
- Heuveline, P. Impact of the HIV epidemic on population and household structure: The dynamics and evidence to date. AIDS 2004, 18, S45–S53. [Google Scholar] [CrossRef] [PubMed]
- Locke, C.; Seeley, J.; Rao, N. Migration, reconfigurations of family relations and social (in) security: An introduction. Third World Quart. 2013, 34, 1872–1880. [Google Scholar] [CrossRef]
- Simoni, J.M.; Yang, J.P.; Porricolo, M. Families and HIV medication adherence. In Family and HIV/AIDs: Cultural and Contextual Issues in Prevention and Treatment; Pecquegnat, W., Bell, C.C., Eds.; Springer: New York, NY, USA, 2012; pp. 209–228. [Google Scholar]
- Rotheram-Borus, M.; Stein, J.; Jiraphongsa, C.; Khumtong, S.; Lee, S.-J.; Li, L. Benefits of family and social relationships for thai parents living with HIV. Prev. Sci. 2010, 11, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Dyer, T.; Stein, J.; Rice, E.; Rotheram-Borus, M. Predicting depression in mothers with and without HIV: The role of social support and family dynamics. AIDS Behav. 2012, 16, 2198–2208. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ji, G.; Liang, L.-J.; Ding, Y.; Tian, J.; Xiao, Y. A multilevel intervention for HIV-affected families in China: Together for empowerment activities (TEA). Soc. Sci. Med. 2011, 73, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Cross, C. Sinking deeper down: HIV/AIDS as an economic shock to rural households. Soc. Transit. 2001, 32, 133–147. [Google Scholar] [CrossRef]
- Bärnighausen, T.; Chaiyachati, K.; Chimbindi, N.; Peoples, A.; Haberer, J.; Newell, M.-L. Interventions to increase antiretroviral adherence in sub-saharan Africa: A systematic review of evaluation studies. Lancet Infect. Dis. 2011, 11, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Van Praag, E.; Swai, R. In VImplementing the Continuum of Care for HIV: Lessons Learned from Tanzania; Marlink, R., Teitelman, S., Eds.; From the Ground Up: Building Comprehensive HIV/AIDS Care Programs in Resource-Limited Settings. Elizabeth Glaser Pediatric AIDS Foundation: Washington, DC, USA, 2009; pp. 29–42. [Google Scholar]
- Knodel, J.; Kespichayawattana, J.; Saengtienchai, C.; Wiwatwanich, S. The role of parents and family members in art treatment adherence: Evidence from Thailand. Res. Aging 2010, 32, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Kasedde, S.; Doyle, A.M.; Seeley, J.A.; Ross, D.A. They are not always a burden: Older people and child fostering in Uganda during the HIV epidemic. Soc. Sci. Med. 2014, 113, 161–168. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masquillier, C.; Wouters, E.; Mortelmans, D.; Van Wyk, B. On the Road to HIV/AIDS Competence in the Household: Building a Health-Enabling Environment for People Living with HIV/AIDS. Int. J. Environ. Res. Public Health 2015, 12, 3264-3292. https://doi.org/10.3390/ijerph120303264
Masquillier C, Wouters E, Mortelmans D, Van Wyk B. On the Road to HIV/AIDS Competence in the Household: Building a Health-Enabling Environment for People Living with HIV/AIDS. International Journal of Environmental Research and Public Health. 2015; 12(3):3264-3292. https://doi.org/10.3390/ijerph120303264
Chicago/Turabian StyleMasquillier, Caroline, Edwin Wouters, Dimitri Mortelmans, and Brian Van Wyk. 2015. "On the Road to HIV/AIDS Competence in the Household: Building a Health-Enabling Environment for People Living with HIV/AIDS" International Journal of Environmental Research and Public Health 12, no. 3: 3264-3292. https://doi.org/10.3390/ijerph120303264






