Application of QUAL2K Model to Assess Ecological Purification Technology for a Polluted River
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Site
2.2. Experimental Design
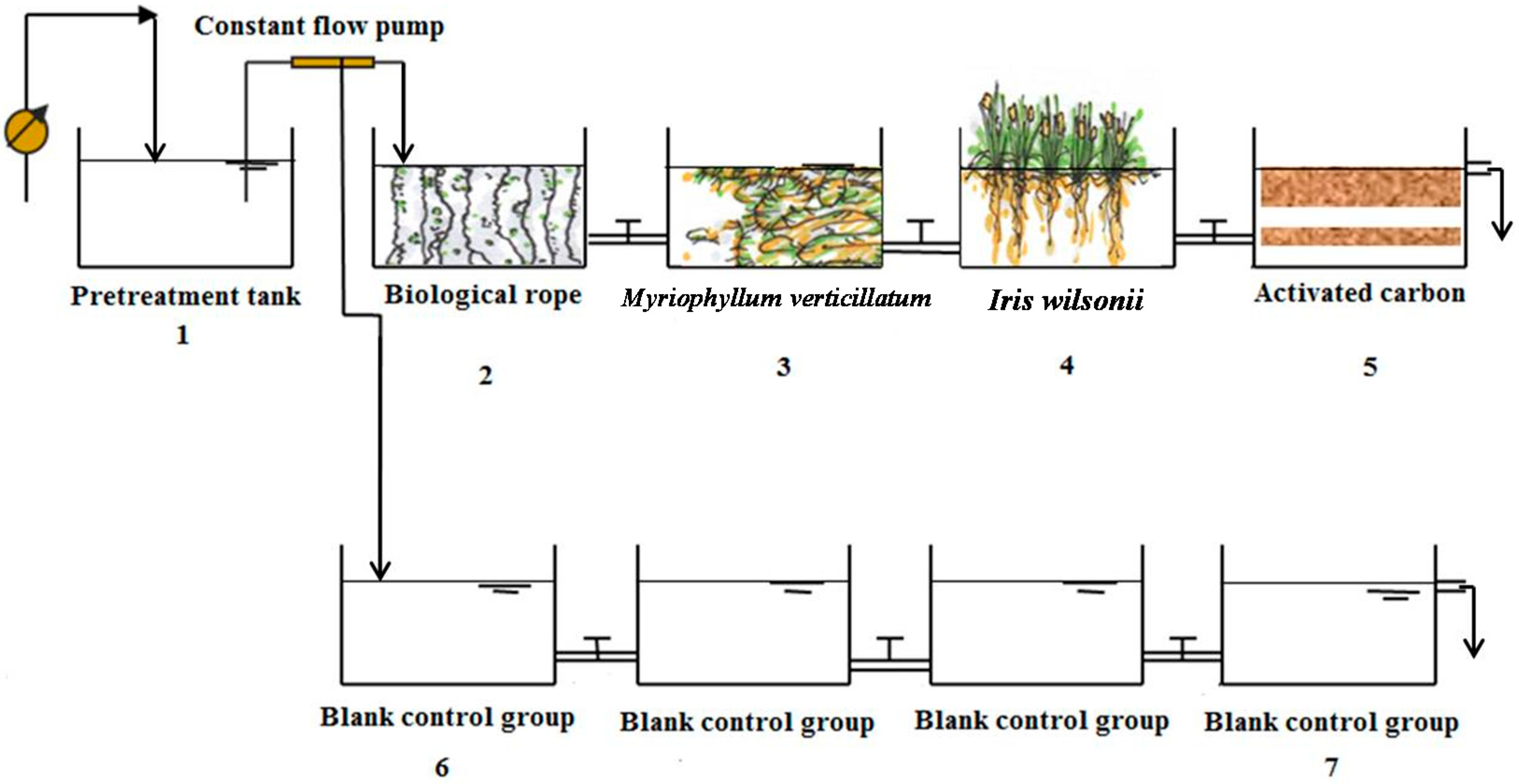
2.3. Sampling and Analysis
2.4. QUAL2K Model
3. Results and Discussion
3.1. Plant Growth and Biomass Production
3.2. Removal Rate of nitrogen (N) and phosphorus (P)
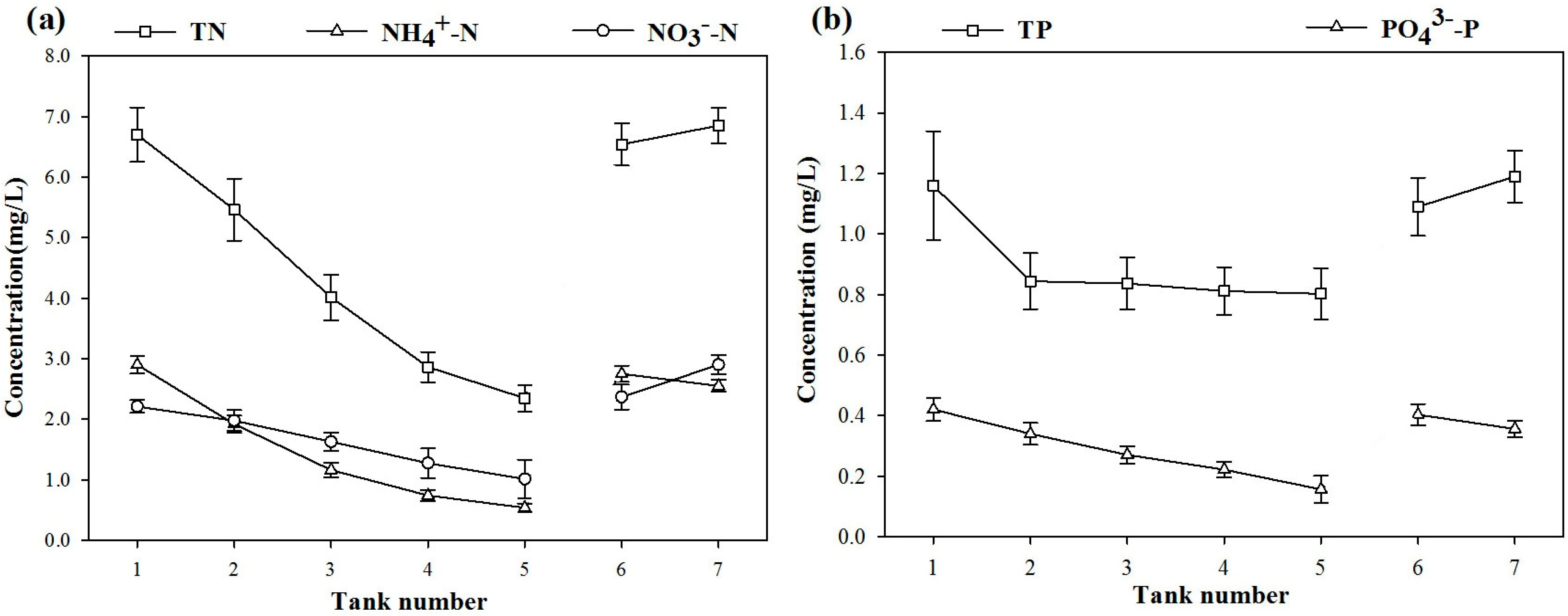
| Indexes | Biological Rope Unit | Myriophyllum verticillatum Unit | Iris wilsonii Unit | Activated Carbon Unit |
|---|---|---|---|---|
| TN | 16.07% | 28.10% | 30.33% | 19.55% |
| NO3−-N | 28.64% | 37.02% | 33.78% | 25.03% |
| NH4+-N | 17.68% | 25.12% | 29.22% | 28.04% |
| TP | 21.19% | 3.96% | 6.05% | 4.17% |
| PO43−-P | 15.05% | 16.51% | 14.23% | 25.52% |
3.3. QUAL2K Model Simulation
3.3.1. Input Data and Model Parameters
| Classification | NO3−-N | NH4+-N | TN | TP | PO43−-P | DO | SS |
|---|---|---|---|---|---|---|---|
| Concentration of upstream water quality | 2.90 | 2.21 | 6.70 | 1.16 | 0.42 | 1.3 | 30 |
| IV | -- | 1.5 | 1.5 | 0.3 | -- | 3 | -- |
| V | -- | 2 | 2 | 0.4 | -- | 2 | -- |
| Parameter | Value | Units | Symbol | Range |
|---|---|---|---|---|
| Carbon | 40 | g·C | g·C | 30–50 |
| Nitrogen | 7.2 | g·N | g·N | 3–9 |
| Phosphorus | 1 | g·P | g·P | 0.4–2 |
| Dry weight | 100 | g·D | g·D | 100 |
| Chlorophyll | 1 | g·A | g·A | 0.4–2 |
| ISS settling velocity | 1 | m/d | vi | 0–2 |
| O2 reaeration model | Internal | -- | -- | -- |
| Slow CBOD:hydrolysis rate | 0.3 | /d | khc | 0–2 |
| Fast CBOD:oxidation rate | 0.4 | /d | kdc | 0.02–4.2 |
| Organic N:hydrolysis | 0.2 | /d | khn | 0–5 |
| Organic N:Settling velocity | 0.05 | m/d | von | 0–2 |
| Ammonium:nitrification | 0.8 | /d | kna | 0–10 |
| Nitrate: denitrification | 0.1 | /d | kdn | 0–2 |
| Sed-denitrification transfer coefficient | 0.05 | m/d | vdi | 0–1 |
| Organic P: hydrolysis | 0.5 | /d | khp | 0–5 |
| Organic P: Settling velocity | 0.6 | m/d | vop | 0–2 |
| Inorganic P:settling velocity | 0.27 | m/d | vip | 0–2 |
| Bottom algae:Maximum growth rate | 10 | mg·A/m2/d or /d | Cgb | 0–500 |
| Bottom algae:First-order model carrying capacity | 1000 | mg·A/m2 | ab,max | 1000 |
| Respiration rate | 1 | /d | krb | 0.05–0.5 |
| Excretion rate | 0.5 | /d | keb | 0–0.5 |
| Death rate | 0.25 | /d | kdb | 0–0.5 |
| Light constant | 50 | langleys/d | KLb | 1–100 |
| Ammonia preference | 25 | ug·N/L | khnxb | 1–100 |
| Title Unit | Model Parameter (day−1) | ||||
|---|---|---|---|---|---|
| NO3−-N | NH4+-N | ON | OP | IP | |
| Biological rope | 0.34 | 0.19 | 0.3 | 0.06 | 0.16 |
| Myriophyllum verticillatum | 0.46 | 0.29 | 0.3 | 0.04 | 0.18 |
| Iris wilsonii | 0.41 | 0.35 | 0.16 | 0.21 | 0.15 |
| Activated carbon | 0.29 | 0.33 | 0.28 | 0.04 | 0.29 |
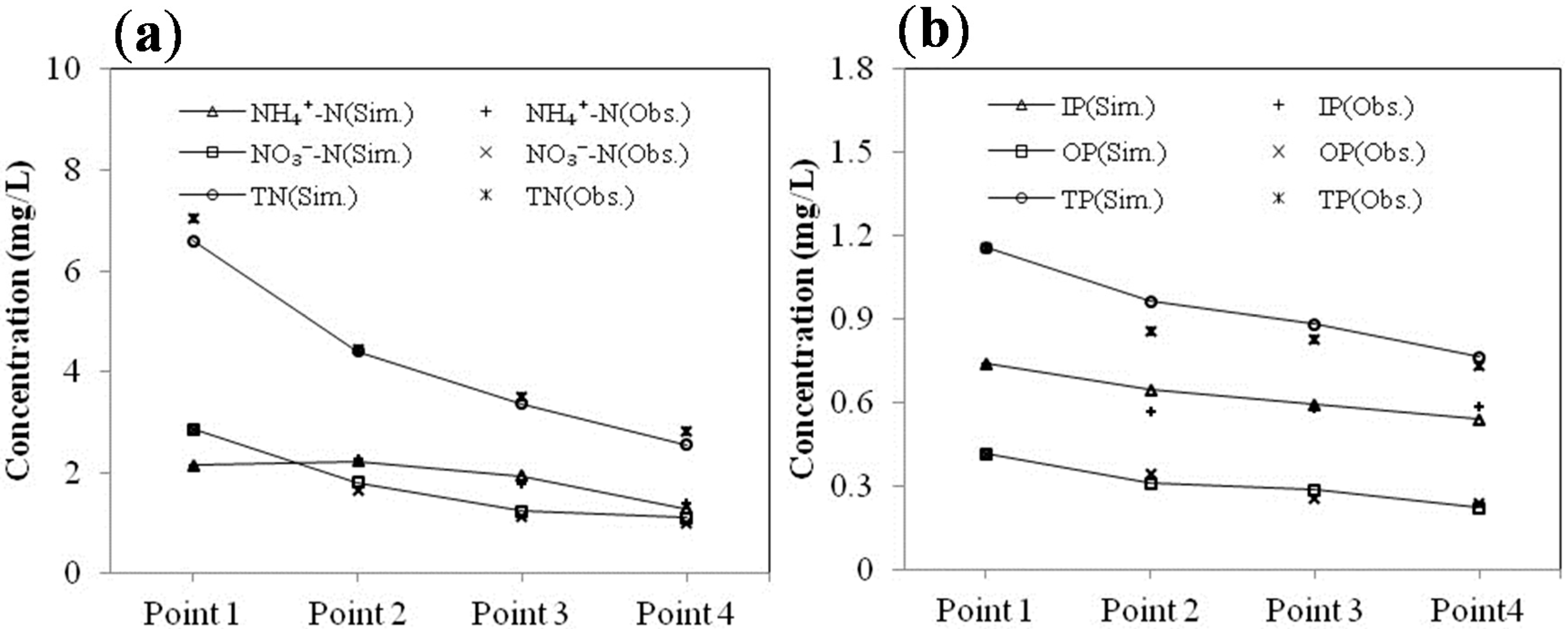
3.3.2. Model Calibration
| Sample outlet | NH4+-N | NO3−-N | TN | |||
|---|---|---|---|---|---|---|
| Sim. | Obs. | Sim. | Obs. | Sim. | Obs. | |
| Point 1 | 2.150 | 2.150 | 2.854 | 2.854 | 6.594 | 7.033 |
| Point 2 | 2.232 | 2.278 | 1.800 | 1.650 | 4.411 | 4.428 |
| Point 3 | 1.926 | 1.790 | 1.232 | 1.121 | 3.368 | 3.490 |
| Point 4 | 1.288 | 1.401 | 1.101 | 0.992 | 2.546 | 2.820 |
| Sample outlet | IP | OP | TP | |||
| Sim. | Obs. | Sim. | Obs. | Sim. | Obs. | |
| Point 1 | 0.742 | 0.742 | 0.419 | 0.419 | 1.161 | 1.161 |
| Point 2 | 0.647 | 0.570 | 0.315 | 0.347 | 0.962 | 0.857 |
| Point 3 | 0.593 | 0.580 | 0.289 | 0.259 | 0.882 | 0.829 |
| Point4 | 0.542 | 0.589 | 0.224 | 0.244 | 0.766 | 0.733 |
3.3.3. Application of the Model
A. Simulation of water quality after adding ecological purification combination at different density

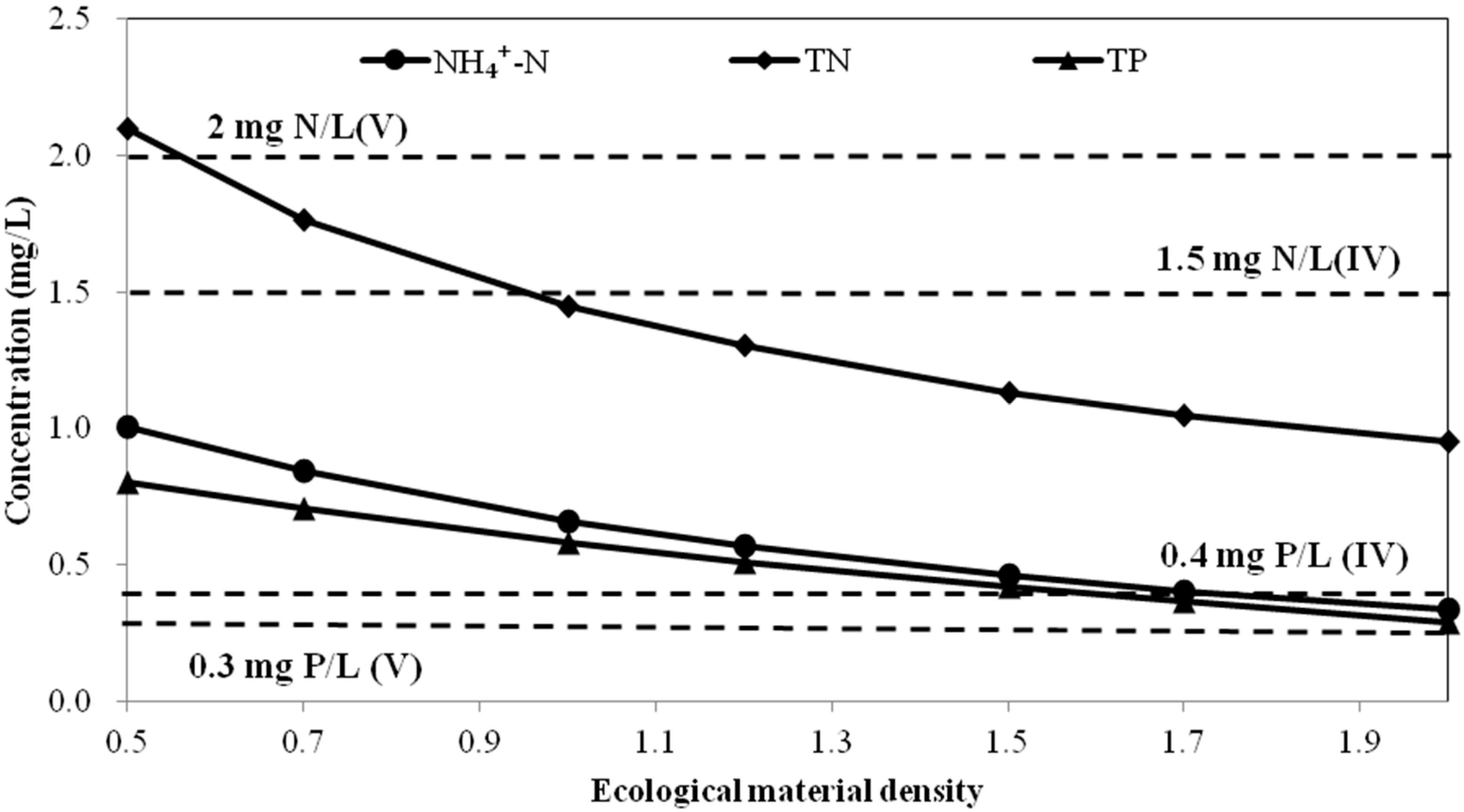
B. Simulation of water quality at different hydraulic retention time
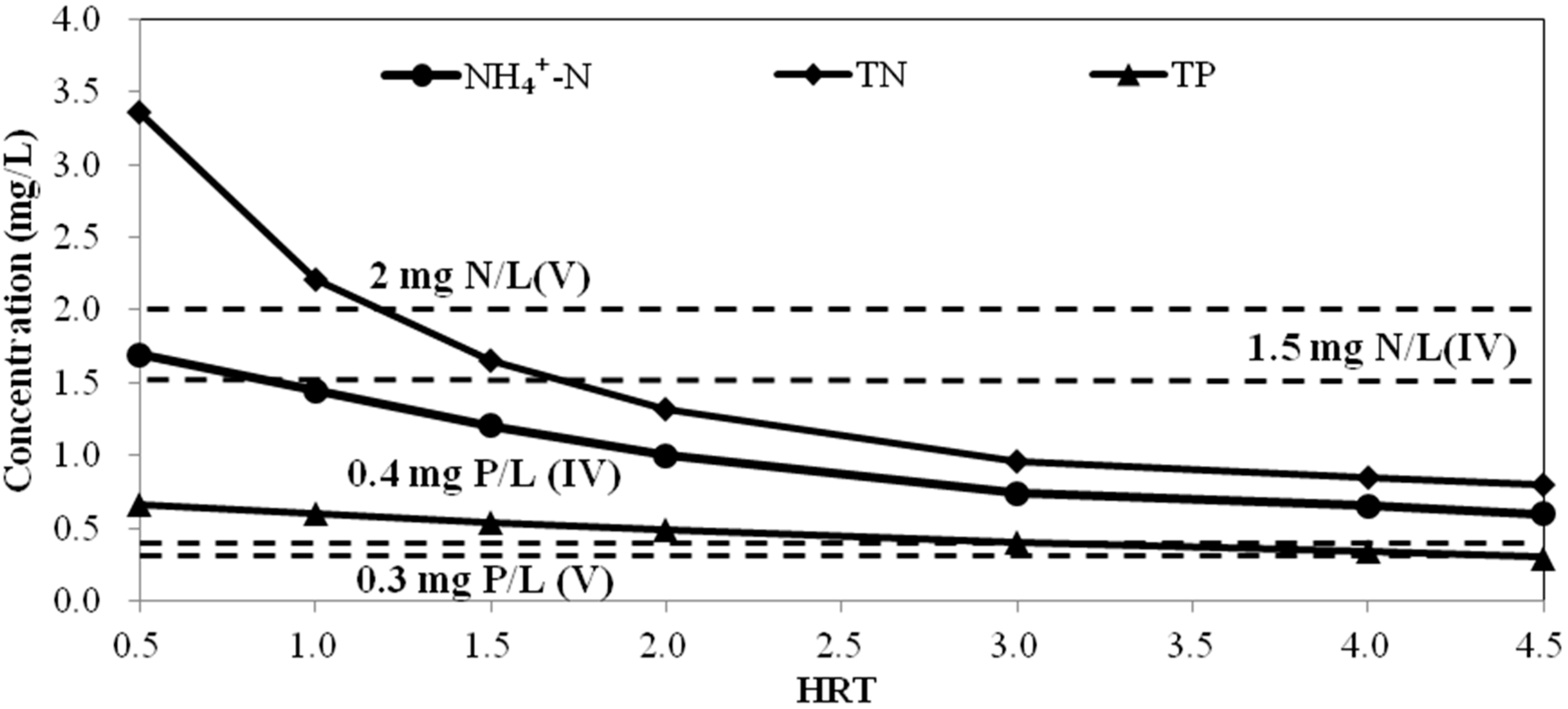
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nahlik, A.M.; Mitsch, W.J. Tropical treatment wetlands dominated by free-floating macrophytes for water quality improvement in Costa Rica. Ecol. Eng. 2006, 28, 246–257. [Google Scholar] [CrossRef]
- Jing, S.-R.; Lin, Y.-F. Seasonal effect on ammonia nitrogen removal by constructed wetlands treating polluted river water in Southern Taiwan. Environ. Pollut. 2004, 127, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.X.; Zhang, L.M.; Chua, H.; Li, X.D. A mosaic community of macrophytes for the ecological remediation of eutrophic shallow lakes. Ecol. Eng. 2009, 35, 582–590. [Google Scholar] [CrossRef]
- de-Bashan, L.E.; Bashan, Y. Recent advances in removing phosphorus from wastewater and its future use as fertilizer (1997–2003). Water Res. 2004, 38, 4222–4246. [Google Scholar] [CrossRef] [PubMed]
- Donnert, D.; Salecker, M. Elimination of phosphorus from municipal and industrial waste water. Water Sci. Technol. 1999, 40, 195–202. [Google Scholar] [CrossRef]
- Aguilar, M.I.; Sáez, J.; Lloréns, M.; Soler, A. Nutrient removal and sludge production in the coagulation-flocculation process. Water Res. 2002, 36, 2910–2919. [Google Scholar] [CrossRef]
- Huett, D.O.; Morris, S.G.; Smith, G.; Hunt, N. Nitrogen and phosphorus removal from plant nursery runoff in vegetated and unvegetated subsurface flow wetlands. Water Res. 2005, 39, 3259–3272. [Google Scholar] [CrossRef] [PubMed]
- Tanner, C.C.; Headley, T.R. Components of floating emergent macrophyte treatment wetlands influencing removal of stormwater pollutants. Ecol. Eng. 2011, 37, 474–486. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, J.; Li, P.; Zhang, J. Nutrient removal in constructed microcosm wetlands for treating polluted river water in Northern China. Ecol. Eng. 2011, 37, 560–568. [Google Scholar] [CrossRef]
- Park, Y.S.; Moon, J.H.; Kim, D.S.; Ahn, K.H. Treatment of a polluted stream by a fixed-bed biofilm reactor with sludge discharger and backwashing system. Chem. Eng. J. 2004, 99, 265–271. [Google Scholar] [CrossRef]
- Bratieres, K.; Fletcher, T.D.; Deletic, A.; Zinger, Y. Nutrient and sediment removal by stormwater biofilters: A large-scale design optimisation study. Water Res. 2008, 42, 3930–3940. [Google Scholar] [CrossRef] [PubMed]
- Vymazal, J. Plants used in constructed wetlands with horizontal subsurface flow: A review. Hydrobiologia 2011, 674, 133–156. [Google Scholar] [CrossRef]
- Fan, J.; Liang, S.; Zhang, B.; Zhang, J. Enhanced organics and nitrogen removal in batch-operated vertical flow constructed wetlands by combination of intermittent aeration and step feeding strategy. Environ. Sci. Pollut. Res. Int. 2013, 20, 2448–55. [Google Scholar] [CrossRef] [PubMed]
- Saeed, T.; Sun, G. A comparative study on the removal of nutrients and organic matter in wetland reactors employing organic media. Chem. Eng. J. 2011, 171, 439–447. [Google Scholar] [CrossRef]
- Park, S.S.; Na, Y.; Uchrin, C.G. An oxygen equivalent model for water quality dynamics in a macrophyte dominated river. Ecol. Modell. 2003, 168, 1–12. [Google Scholar] [CrossRef]
- Yu, G.; Lei, H.; Bai, T.; Li, Z. In-Situ stabilisation followed by ex-situ composting for treatment and disposal of heavy metals polluted sediments. J. Environ. Sci. 2009, 21, 877–83. [Google Scholar] [CrossRef]
- Pelletier, G.J.; Chapra, S.C.; Tao, H. QUAL2Kw—A framework for modeling water quality in streams and rivers using a genetic algorithm for calibration. Environ. Modell. Softw. 2006, 21, 419–425. [Google Scholar] [CrossRef]
- Ning, S.K.; Chang, N.B.; Yong, L. Assessing pollution prevention program by QUAL2E simulation analysis for the Kao-Ping River Basin, Taiwan. J. Environ. Manage. 2000, 60, 0–16. [Google Scholar]
- Chen, D.; Yang, K.; Wang, H. Nitrate removal from groundwater by hydrogen-fed autotrophic denitrification in a bio-ceramsite reactor. Water Sci. Technol. 2014, 69, 2417–2422. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Ha, S.R. Parameter optimization of the QUAL2K model for a multiple-reach river using an influence coefficient algorithm. Sci. Total Environ. 2010, 408, 1985–1991. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, D.P.; Schladow, S.G. Prediction of water quality in lakes and reservoirs. Part I—Model description. Ecol. Model. 1997, 96, 91–110. [Google Scholar] [CrossRef]
- Azzellino, A.; Salvetti, R.; Vismara, R.; Bonomo, L. Combined use of the EPA-QUAL2E simulation model and factor analysis to assess the source apportionment of point and non-point loads of nutrients to surface waters. Sci. Total Environ. 2006, 371, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Sims, A.; Gajaraj, S.; Hu, Z.Q. Nutrient removal and greenhouse gas emissions in duckweed treatment ponds. Water Res. 2013, 47, 1390–1398. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.; Lim, B.R.; Cho, J.; Song, K.G. Biological nitrogen and phosphorus removal and changes in microbial community structure in a membrane bioreactor: Effect of different carbon sources. Water Res. 2008, 42, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.J.; Zhang, Y.; Zhang, M.Y.; Li, W. Identification and modelling the HRT distribution in subsurface constructed wetland. J. Environ. Monitor. 2012, 14, 3037–3044. [Google Scholar] [CrossRef]
- Chen, X.; Huang, X.; He, S.; Yu, X. Pilot-scale study on preserving eutrophic landscape pond water with a combined recycling purification system. Ecol. Eng. 2013, 61, 383–389. [Google Scholar] [CrossRef]
- Tanner, C.C.; D’Eugenio, J.; McBride, G.B.; Sukias, J.P.S. Effect of water level fluctuation on nitrogen removal from constructed wetland mesocosms. Ecol. Eng. 1999, 12, 67–92. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Y.; Jin, H. Nitrogen removal from polluted river by enhanced floating bed grown canna. Ecol. Eng. 2009, 35, 135–140. [Google Scholar] [CrossRef]
- Luederitz, V.; Eckert, E.; Lange-Weber, M.; Lange, A. Nutrient removal efficiency and resource economics of vertical flow and horizontal flow constructed wetlands. Ecol. Eng. 2001, 18, 157–171. [Google Scholar] [CrossRef]
- Chou, W.-S.; Lee, T.-C.; Lin, J.-Y.; Yu, S.L. Phosphorus load reduction goals for feitsui reservoir watershed, Taiwan. Environ. Monit. Assess. 2007, 131, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Pastres, R.; Ciavatta, S. A comparison between the uncertainties in model parameters and in forcing functions: Its application to a 3D water-quality model. Environ. Modell. Softw. 2005, 20, 981–989. [Google Scholar] [CrossRef]
- Yuceer, M.; Karadurmus, E.; Berber, R. Simulation of river streams: Comparison of a new technique with QUAL2E. Math. Comp. Modell. 2007, 46, 292–305. [Google Scholar] [CrossRef]
- Zhang, R.; Qian, X.; Li, H.; Yuan, X. Selection of optimal river water quality improvement programs using QUAL2K: A case study of Taihu Lake Basin, China. Sci. Total Environ. 2012, 431, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Pu, P.; Zhang, S.; Hu, C. The purification ability of aquatic macrophytes for eutrophic lake water in winter. China Environ. Sci. 1999, 19, 106–109. (In Chinese) [Google Scholar]
- White, S.A.; Cousins, M.M. Floating treatment wetland aided remediation of nitrogen and phosphorus from simulated stormwater runoff. Eco. Eng. 2013, 61, 207–215. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Niu, Q.; Zhang, R.; Ye, R.; Qian, X.; Qian, Y. Application of QUAL2K Model to Assess Ecological Purification Technology for a Polluted River. Int. J. Environ. Res. Public Health 2015, 12, 2215-2229. https://doi.org/10.3390/ijerph120202215
Zhu W, Niu Q, Zhang R, Ye R, Qian X, Qian Y. Application of QUAL2K Model to Assess Ecological Purification Technology for a Polluted River. International Journal of Environmental Research and Public Health. 2015; 12(2):2215-2229. https://doi.org/10.3390/ijerph120202215
Chicago/Turabian StyleZhu, Wenting, Qian Niu, Ruibin Zhang, Rui Ye, Xin Qian, and Yu Qian. 2015. "Application of QUAL2K Model to Assess Ecological Purification Technology for a Polluted River" International Journal of Environmental Research and Public Health 12, no. 2: 2215-2229. https://doi.org/10.3390/ijerph120202215





