Circadian Rhythmicity of Antioxidant Markers in Rats Exposed to 1.8 GHz Radiofrequency Fields
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Handling
2.2. RF Exposure Facility and Dosimetry


2.3. Measurements of Antioxidants
2.4. Statistical Analysis
3. Results
3.1. Circadian Rhythm in Sham Exposed Rats
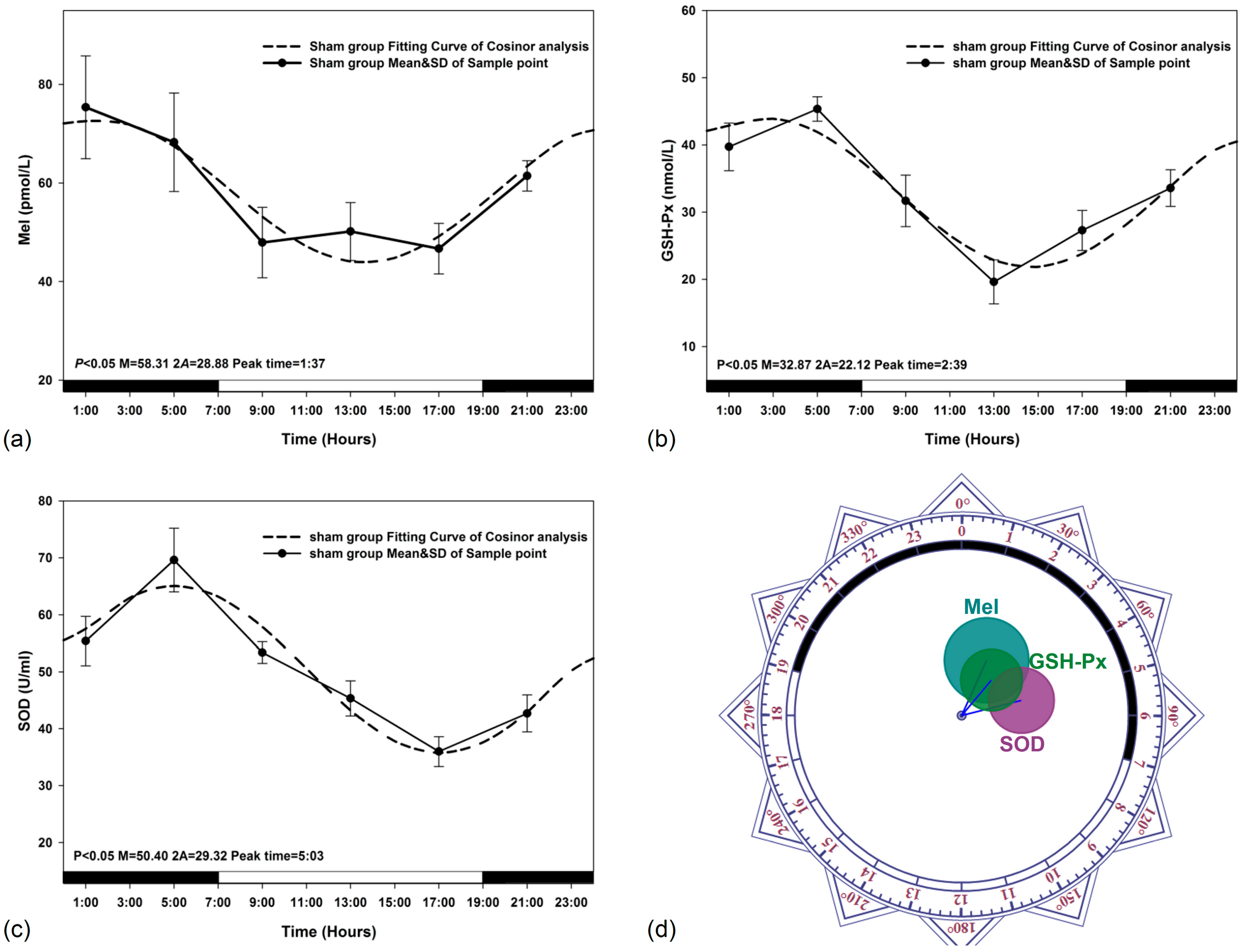
3.2. Alterations in the Circadian Rhythms of Anti-Oxidant Markers in RF-Exposed Rats
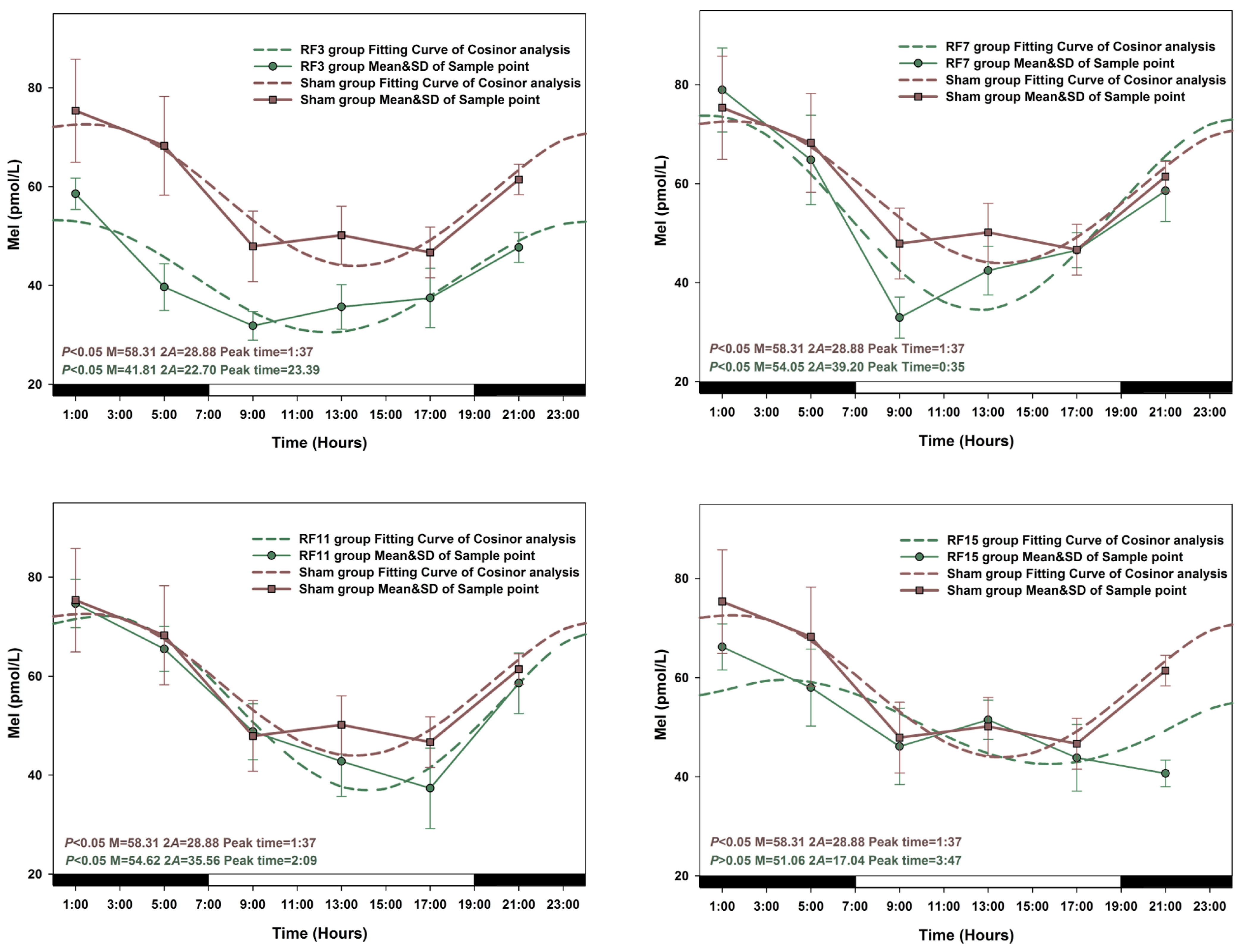
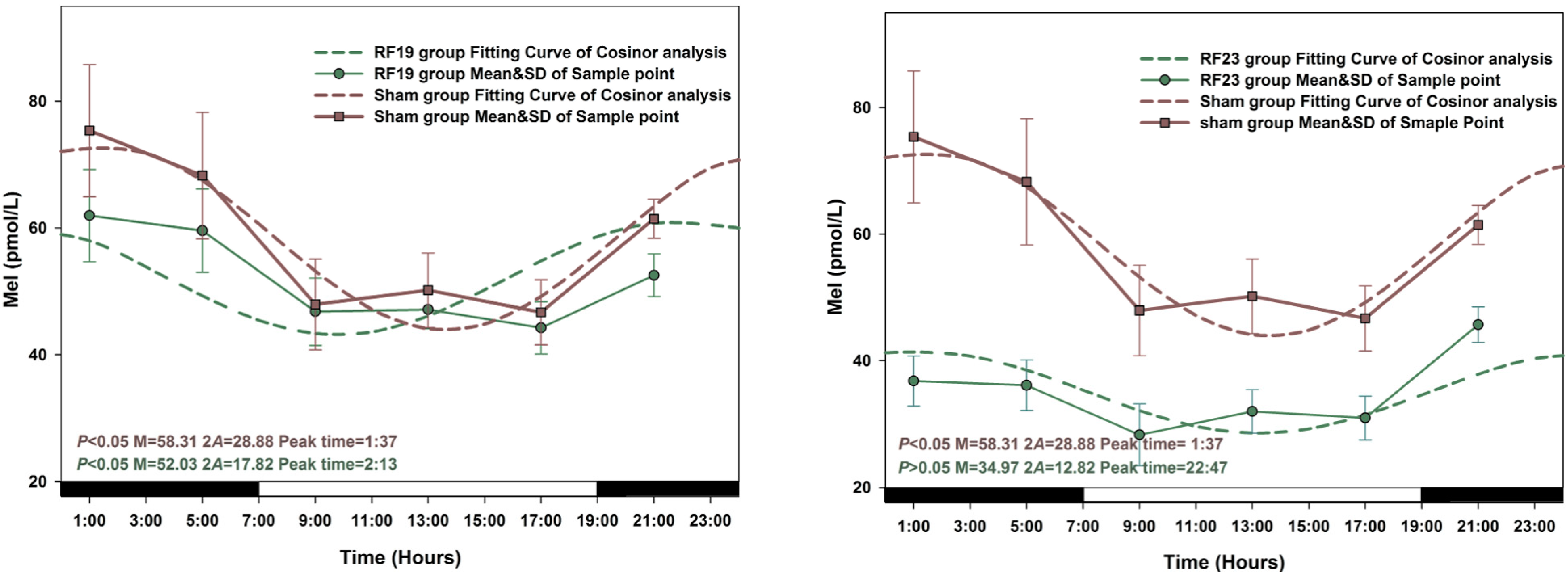
3.3. Alterations in Level of Anti-Oxidant Markers Markers in RF Groups
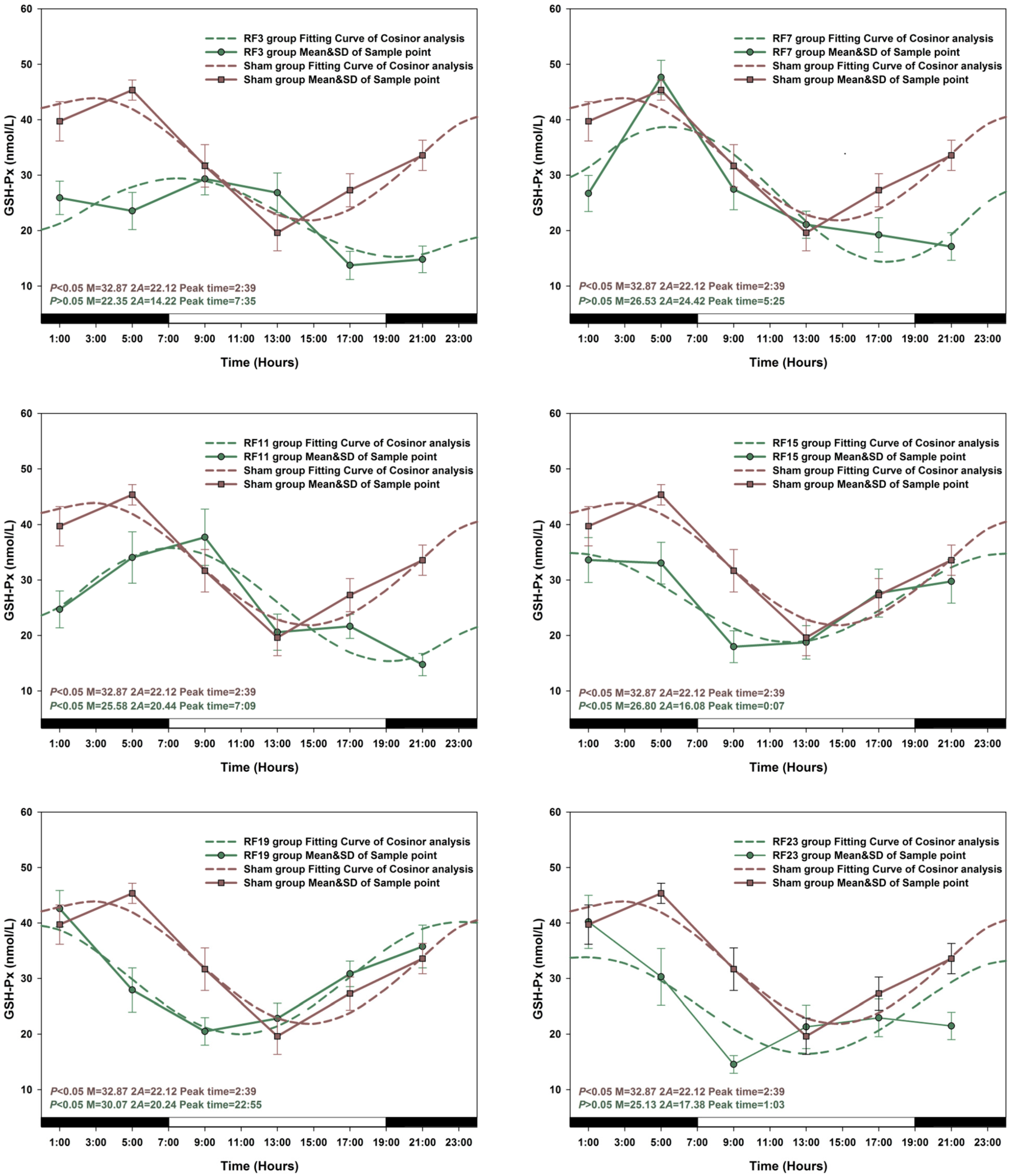

3.4. Sensitive to RF for Plasma Anti-Oxidant Functional Markers in Circadian Rhythm

4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Croft, R.J.; Chandler, J.S.; Burgess, A.P.; Barry, R.J.; Williams, J.D.; Clarke, A.R. Acute mobile phone operation affects neural function in humans. Clin. Neurophysiol. 2002, 113, 1623–1632. [Google Scholar] [CrossRef] [PubMed]
- Hossmann, K.A.; Hermann, D.M. Effects of electromagnetic radiation of mobile phones on the central nervous system. Bioelectromagnetics 2013, 24, 49–62. [Google Scholar] [CrossRef]
- Verschaeve, L. Genetic damage in subjects exposed to radiofrequency radiation. Mutat. Res. 2009, 681, 259–270. [Google Scholar] [CrossRef] [PubMed]
- De Iuliis, G.N.; Newey, R.J.; King, B.V.; Aitken, R.J. Mobile phone radiation induces reactive oxygen species production and DNA damage in human spermatozoa in vitro. PLoS One 2009, 4. [Google Scholar] [CrossRef]
- Makker, K.; Varghese, A.; Desai, N.R.; Mouradi, R.; Agarwal, A. Cell phones: Modern man’s nemesis? Reprod. Biomed. Online 2009, 18, 148–157. [Google Scholar] [PubMed]
- Van Deventer, E.; van Rongen, E.; Saunders, R. WHO research agenda for radiofrequency fields. Bioelectromagnetics 2011, 32, 417–421. [Google Scholar]
- Azadi Oskouyi, E.; Rajaei, F.; Safari Variani, A.; Sarokhani, M.R.; Javadi, A. Effects of microwaves (950MHZ mobile phone) on morphometric and apoptotic changes of rabbit epididymis. Andrologia 2014, 25. [Google Scholar] [CrossRef]
- ICNIRP. Guidelines for limiting exposure to time-varying electric, magnetic and electromagnetic fields (up to 300 GHz). Health Phys. 1998, 74, 494–522. [Google Scholar]
- Chia, S.E.; Tay, S.K. Occupational risk for male infertility: A case-control study of 218 infertile and 227 fertile men. J. Occup. Environ. Med. 2001, 43, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Gracia, C.R.; Sammel, M.D.; Coutifaris, C.; Guzick, D.S.; Barnhart, K.T. Occupational exposures and male infertility. Amer. J. Epidemiol. 2005, 162, 729–733. [Google Scholar] [CrossRef]
- Lerchl, A.; Krüger, H.; Niehaus, M.; Streckert, J.R.; Bitz, A.K.; Hansen, V. Effects of mobile phone electromagnetic fields at nonthermal SAR values on melatonin and body weight of Djungarian hamsters (Phodopus sungorus). J. Pineal Res. 2008, 44, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Zhang, J.; Cao, H.; Guo, W.; Chen, L.; Shen, O.; Sun, J.; Yi, C.; Wang, J.; Tong, J. Circadian alterations of reproductive functional markers in male rats exposed to 1800 MHz radiofrequency field. Chronobiol. Int. 2014, 31, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Luukkonen, J.; Hakulinen, P.; Mäki-Paakkanen, J.; Juutilainen, J.; Naarala, J. Enhancement of chemically induced reactive oxygen species production and DNA damage in human SH-SY5Y neuroblastoma cells by 872 MHz radiofrequency radiation. Mutat. Res. 2009, 662, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Ergüder, I.B.; Ergüder, T.; Ozkan, C.; Bozkurt, N.; Soylu, K.; Devrim, E.; Durak, I. Short-term effects of smoking cessation on blood antioxidant parameters and paraoxonase activity in healthy asymptomatic long-term cigarette smokers. Inhal. Toxicol. 2006, 18, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Madrid, J.A.; Tan, D.X.; Reiter, R.J. Melatonin, the circadian multioscillator system and health: The need for detailed analyses of peripheral melatonin signaling. J. Pineal. Res. 2012, 52, 139–166. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Reiter, R.J.; Manchester, L.C.; Yan, M.T.; El-Sawi, M.; Sainz, R.M.; Mayo, J.C.; Kohen, R.; Allegra, M.; Hardeland, R. Chemical and physical properties and potential mechanisms: Melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2002, 2, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Videnovic, A.; Noble, C.; Reid, K.J.; Peng, J.; Turek, F.W.; Marconi, A.; Rademaker, A.W.; Simuni, T.; Zadikoff, C.; Zee, P.C. Circadian melatonin rhythm and excessive daytime sleepiness in Parkinson disease. JAMA Neurol. 2014, 71, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Akakin, D.; Kiran, D.; Ozkan, N.; Erşahin, M.; Ozdemir-Kumral, Z.N.; Yeğen, B.; Sener, G. Protective effects of melatonin against spinal cord injury induced oxidative damage in rat kidney: A morphological and biochemical study. Acta Histochem. 2013, 115, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal. Res. 2014, 57, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Juutilainen, J.; Kumlin, T. Occupational magnetic field exposure and melatonin: Interaction with light-at-night. Bioelectromagnetics 2006, 27, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Brendel, H.; Niehaus, M.; Lerchl, A. Direct suppressive effects of weak magnetic fields (50 Hz and 16 2/3 Hz) on melatonin synthesis in the pineal gland of Djungarian hamsters (Phodopus sungorus). J. Pineal. Res. 2000, 29, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.B.; Choi, H.D.; Kim, B.C.; Pack, J.K.; Kim, N.; Lee, Y.S. Effects of simultaneous combined exposure to CDMA and WCDMA electromagnetic fields on serum hormone levels in rats. J. Radiat. Res. 2013, 54, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Sokolovic, D.; Djindjic, B.; Nikolic, J.; Bjelakovic, G.; Pavlovic, D.; Kocic, G.; Cvetkovic, T.; Pavlovic, V. Melatonin reduces oxidative stress induced by chronic exposure of microwave radiation from mobile phones in rat brain. J. Radiat. Res. 2008, 49, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Seifirad, S.; Farzampour, S.; Nourbakhsh, M.; Amoli, M.M.; Razzaghy-Azar, M.; Larijani, B. Effects of extremely low frequency electromagnetic fields on paraoxonase serum activity and lipid peroxidation metabolites in rat. J. Diabetes Metab. Disord. 2014, 13. [Google Scholar] [CrossRef]
- Hajhosseini, L.; Khaki, A.; Merat, E.; Ainehchi, N. Effect of rosmarinic acid on sertoli cells apoptosis and serum antioxidant levels in rats after exposure to electromagnetic fields. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Marjanović, A.M.; Pavičić, I.; Trošić, I. Biological indicators in response to radiofrequency/microwave exposure. Arh. Hig. Rada. Toksikol. 2012, 63, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Marjanovic, A.M.; Pavicic, I.; Trosic, I. Cell oxidation-reduction imbalance after modulated radiofrequency radiation. Electromagn. Biol. Med. 2014, 13, 1–6. [Google Scholar] [CrossRef]
- Portaluppi, F.; Smolensky, M.H.; Touitou, Y. Ethics and methods for biological rhythm research on animals and human beings. Chronobiol. Int. 2010, 27, 1911–1929. [Google Scholar] [CrossRef] [PubMed]
- Oktem, F.; Ozguner, F.; Mollaoglu, H.; Koyu, A.; Uz, E. Oxidative damage in the kidney induced by 900-MHz-emitted mobile phone: Protection by melatonin. Arch. Med. Res. 2005, 36, 350–355. [Google Scholar] [CrossRef] [PubMed]
- López-Martin, E.; Bregains, J.C.; Jorge-Barreiro, F.J.; Sebastian Franco, J.L.; Moreno-Piquero, E.; Ares-Pena, F.J. An experimental set-up for measurement of the power absorbed from 900 MHz GSM standing waves by small animals, illustrated by application to picrotoxin-treated rats. Progress Electromagn. Res. 2008, 87, 149–165. [Google Scholar] [CrossRef]
- Jaworek, J.; Zwirska-Korczala, K.; Szklarczyk, J.; Nawrot-Porąbka, K.; Leja-Szpak, A.; Jaworek, A.K.; Tomaszewska, R. Pinealectomy aggravates acute pancreatitis in the rat. Pharmacol. Rep. 2010, 62, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, Y.; Feng, J.; Jiang, X.F.; Xiao, W.F.; Chen, X.X. Antioxidant and anti-inflammatory effects of Schisandra and Paeonia extracts in the treatment of asthma. Exp. Ther. Med. 2014, 8, 1479–1483. [Google Scholar] [PubMed]
- Nelson, W.; Tong, Y.L.; Lee, J.-.K.; Halberg, F. Methods for cosinor-rhythmometry. Chronobiologia 1979, 6, 305–323. [Google Scholar] [PubMed]
- Kundi, M.; Mild, K.; Hardell, L.; Mattsson, M.O. Mobile telephones and cancer—A review of epidemiological evidence. J. Toxicol. Environ. Health B Crit. Rev. 2004, 7, 351–384. [Google Scholar] [CrossRef] [PubMed]
- Hardell, L.; Carlberg, M.; Söderqvist, F.; Hansson Mild, K. Meta-analysis of long-term mobile phone use and the association with brain tumours. Int. J. Oncol. 2008, 32, 1097–1103. [Google Scholar] [PubMed]
- Lerchl, A. Electromagnetic pollution: Another risk factor for infertility, or a red herring? Asian J. Androl. 2013, 15, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Radi, E.; Formichi, P.; Battisti, C.; Federico, A. Apoptosis and oxidative stress in neurodegenerative diseases. J. Alzheimers Dis. 2014, 42, S125–S152. [Google Scholar] [PubMed]
- Zhu, J.; Wu, Y.; Tang, Q.; Leng, Y.; Cai, W. The effects of choline on hepatic lipid metabolism, mitochondrial function and antioxidative status in human hepatic C3A cells exposed to excessive energy substrates. Nutrients 2014, 6, 2552–2571. [Google Scholar] [CrossRef] [PubMed]
- Manikonda, P.K.; Jagota, A. Melatonin administration differentially affects age-induced alterations in daily rhythms of lipid peroxidation and antioxidant enzymes in male rat liver. Biogerontology 2012, 13, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, Y.; Yi, W.; Li, Y.; Fan, C.; Xin, Z.; Jiang, S.; Di, S.; Qu, Y.; Reiter, R.J.; et al. A review of melatonin as a suitable antioxidant against myocardial ischemia-reperfusion injury and clinical heart diseases. J. Pineal. Res. 2014. [Google Scholar] [CrossRef]
- Baydas, G.; Gursu, M.F.; Yilmaz, S.; Canpolat, S.; Yasar, A.; Cikim, G.; Canatan, H. Daily rhythm of glutathione peroxidase activity, lipid peroxidation and glutathione levels in tissues of pinealectomized rats. Neurosci. Lett. 2002, 323, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Sae-Teaw, M.; Johns, J.; Johns, N.P.; Subongkot, S. Serum melatonin levels and antioxidant capacities after consumption of pineapple, orange, or banana by healthy male volunteers. J. Pineal. Res. 2013, 55, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Zhang, J.; Cao, H.; Yi, C.; Li, J.X.; Nie, J.; Chen, L.L.; Wang, J.; Tong, J. Effects of 1800-MHz radiofrequency fields on circadian rhythm of plasma melatonin and testosterone in male rats. J. Toxicol. Environ. Health A 2012, 75, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.W.; Loughran, S.P.; Stough, C. Does evening exposure to mobile phone radiation affect subsequent melatonin production? Int. J. Radiat. Biol. 2006, 82, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Burch, J.B.; Reif, J.S.; Noonan, C.W.; Ichinose, T.; Bachand, A.M.; Koleber, T.L.; Yost, M.G. Melatonin metabolite excretion among cellular telephone users. Int. J. Radiat. Biol. 2002, 78, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Radon, K.; Parera, D.; Rose, D.M.; Jung, D.; Vollrath, L. No effects of pulsed radio frequency electromagnetic fields on melatonin, cortisol, and selected markers of the immune system in man. Bioelectromagnetics 2001, 22, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Koyu, A.; Ozguner, F.; Cesur, G.; Gokalp, O.; Mollaoglu, H.; Caliskan, S.; Delibas, N. No effects of 900 MHz and 1800 MHz electromagnetic field emitted from cellular phone on nocturnal serum melatonin levels in rats. Toxicol. Ind. Health 2005, 21, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Ozgur, E.; Güler, G.; Seyhan, N. Mobile phone radiation-induced free radical damage in the liver is inhibited by the antioxidants N-acetyl cysteine and epigallocatechin-gallate. Int. J. Radiat. Biol. 2010, 86, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Kesari, K.K.; Behari, J. Fifty-gigahertz microwave exposure effect of radiations on rat brain. Appl. Biochem. Biotechnol. 2009, 158, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Zhang, Y.; Yang, X.; Jiang, R.; Guo, D.; Cui, X. The influence of microwave radiation from cellular phone on fetal rat brain. Electromagn. Biol. Med. 2012, 31, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Yurekli, A.I.; Ozkan, M.; Kalkan, T.; Saybasili, H.; Tuncel, H.; Atukeren, P.; Gumustas, K.; Seker, S. GSM base station electromagnetic radiation and oxidative stress in rats. Electromagn. Biol. Med. 2006, 25, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolín, I.; Herrera, F.; Martín, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal. Res. 2004, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, D.; Nakamura, M.; Iigo, M.; Okamura, H. Bimodal circadian secretion of melatonin from the pineal gland in a living CBA mouse. Proc. Natl. Acad. Sci. USA 2003, 100, 9584–9589. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Q.; Du, Y.Z.; Tong, J. Daily oscillation and photoresponses of clock gene, Clock, and clock-associated gene, arylalkylamine N-acetyltransferase gene transcriptions in the rat pineal gland. Chronobiol. Int. 2007, 24, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Simonneaux, V.; Poirel, V.J.; Garidou, M.L.; Nguyen, D.; Diaz-Rodriguez, E.; Pe’vet, P. Daily rhythm and regulation of clock gene expression in the rat pineal gland. Mol. Brain Res. 2004, 120, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Jetten, A.M. Retinoid-related orphan receptors (RORs): Critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl. Recept. Signal. 2009, 7. [Google Scholar] [CrossRef]
- Fedele, G.; Green, E.W.; Rosato, E.; Kyriacou, C.P. An electromagnetic field disrupts negative geotaxis in Drosophila via a CRY-dependent pathway. Nat. Commun. 2014, 14. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, H.; Qin, F.; Liu, X.; Wang, J.; Cao, Y.; Tong, J.; Zhao, H. Circadian Rhythmicity of Antioxidant Markers in Rats Exposed to 1.8 GHz Radiofrequency Fields. Int. J. Environ. Res. Public Health 2015, 12, 2071-2087. https://doi.org/10.3390/ijerph120202071
Cao H, Qin F, Liu X, Wang J, Cao Y, Tong J, Zhao H. Circadian Rhythmicity of Antioxidant Markers in Rats Exposed to 1.8 GHz Radiofrequency Fields. International Journal of Environmental Research and Public Health. 2015; 12(2):2071-2087. https://doi.org/10.3390/ijerph120202071
Chicago/Turabian StyleCao, Honglong, Fenju Qin, Xueguan Liu, Jiajun Wang, Yi Cao, Jian Tong, and Heming Zhao. 2015. "Circadian Rhythmicity of Antioxidant Markers in Rats Exposed to 1.8 GHz Radiofrequency Fields" International Journal of Environmental Research and Public Health 12, no. 2: 2071-2087. https://doi.org/10.3390/ijerph120202071




