Mobile Phone-Based Lifestyle Intervention for Reducing Overall Cardiovascular Disease Risk in Guangzhou, China: A Pilot Study
Abstract
:1. Introduction
2. Method
2.1. Study Overview
2.2. Ethics Statement
2.3. Participants and Enrollment
2.4. Randomization and Masking
2.5. Intervention
| 10-Year Risk of CVD | Risk Classification | Frequency of Phone Calls | Frequency of Text Message Sending |
|---|---|---|---|
| <5% | Very low risk | Twice per month | once per month |
| 5%≤ & <10% | Low risk | Twice per month | once per month |
| 10%≤ & <20% | Moderate risk | Twice per month | Twice per month |
| 20% ≤& <40% | High risk | Three times per month | Three times per month |
| ≥40% | Very high risk | Once per week | Once per week |
2.6. Control Group
2.7. Outcomes and Measures
2.8. Sample Size
2.9. Statistical Analysis
3. Results
3.1. Baseline Characteristics and Follow-Up
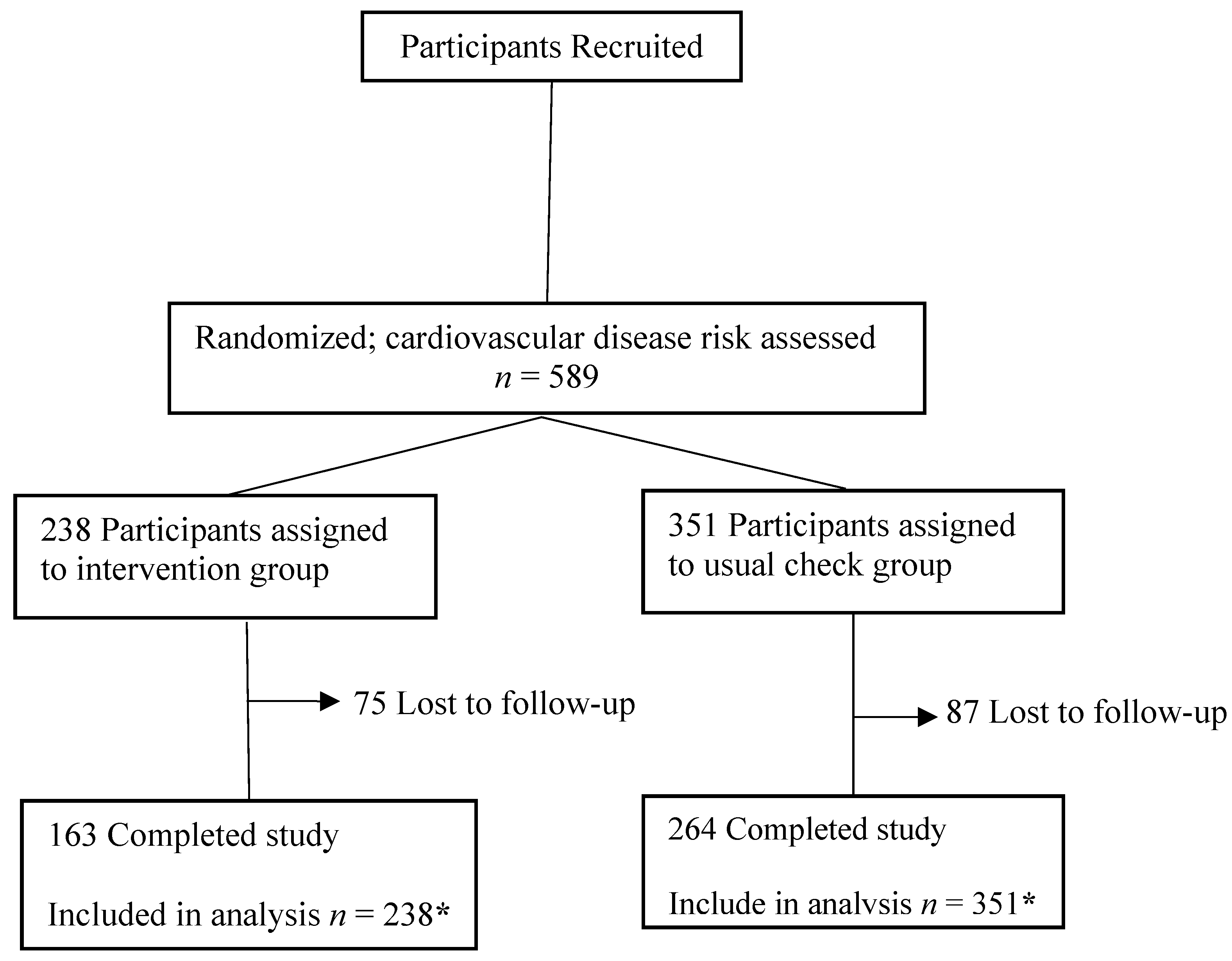
3.2. Primary Study Outcome: 10-Year Risk of CVD
| Characteristic | Total Sample | Intervention Group | Control Group | p |
|---|---|---|---|---|
| Age | 60.57 ± 8.97 | 58.72 ± 8.92 | 61.82 ± 8.80 | <0.001 |
| Female | 246 (41.8) | 99 (41.6) | 147 (41.9) | 0.945 |
| Married | 569 (96.6) | 233 (97.9) | 336 (95.3) | 0.153 |
| Education | ||||
| Middle School Or Lower | 136 (23.1) | 47 (19.8) | 89 (25.4) | 0.268 |
| Senior High School | 143 (24.3) | 62 (26.1) | 81 (23.1) | |
| College Or Above | 310 (52.6) | 129 (54.2) | 181 (51.6) | |
| Personal Monthly Income | ||||
| <¥3000 | 176 (29.9) | 83 (34.9) | 93 (26.5) | 0.001 |
| ¥3000~ | 159 (27.0) | 75 (31.5) | 84 (23.9) | |
| ¥5000~ | 254 (43.1) | 80 (33.6) | 174 (49.6) | |
| Current Smoker | 130 (22.1) | 57 (24.0) | 73 (20.8) | 0.365 |
| Alcohol Use | 154 (26.2) | 69 (29.0) | 85 (24.2) | 0.196 |
| BMI, kg/m2 | 24.05 ± 3.12 | 23.77 ± 3.21 | 24.24 ± 3.05 | 0.076 |
| WHR | 0.89 ± 0.05 | 0.89 ± 0.06 | 0.89 ± 0.05 | 0.540 |
| SBP, mmHg | 128.16 ± 13.40 | 128.60 ± 14.10 | 127.90 ± 12.92 | 0.536 |
| DBP, mmHg | 78.25 ± 11.00 | 78.54 ± 10.25 | 78.06 ± 11.49 | 0.602 |
| FPG, mmol/L | 5.55 ± 1.31 | 5.57 ± 1.50 | 5.54 ± 1.16 | 0.769 |
| TC, mmol/L | 5.63 ± 1.03 | 5.63 ± 1.02 | 5.63 ± 1.04 | 0.954 |
| triglyceride, mmol/L | 1.80 ± 1.20 | 1.83 ± 1.37 | 1.77 ± 1.07 | 0.565 |
| LDL, mmol/L | 3.61 ± 0.89 | 3.61 ± 0.84 | 3.61 ± 0.92 | 0.999 |
| HDL, mmol/L | 1.71 ± 0.37 | 1.68 ± 0.35 | 1.72 ± 0.38 | 0.154 |
| Hypertensive | 145 (24.6) | 49 (20.6) | 96 (27.4) | 0.062 |
| Diabetic | 46 (7.8) | 13 (5.5) | 33 (9.4) | 0.080 |
| Outcome | Intervention Group | Control Group | Crude Effect Size a | Adjusted Effect Size b | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Year 1 | Change | Baseline | Year 1 | Change | |||
| 10-year risk of CVD, % | 5.82 | 4.76 | −1.05 | 7.22 | 9.00 | 1.77 | −2.83 | −2.83 |
| (4.93 to 6.69) | (3.41 to 6.11) | (−2.32 to 0.22) | (6.39 to 8.08) | (7.81 to 10.19) | (0.62 to 2.92) | (−4.52 to −1.13) | (−4.47 to −1.18) | |
| Components of Risk Score | ||||||||
| SBP, mmHg | 128.58 | 123.02 | −5.55 | 127.88 | 134.77 | 6.89 | −12.45 | −12.45 |
| (126.78 to 130.37) | (120.67 to 125.37) | (−7.70 to −3.41) | (126.53 to 129.23) | (132.97 to 136.57) | (5.17 to 8.61) | (−15.09 to −9.80) | (−15.02 to −9.87) | |
| TC, mmol/L | 5.63 | 5.27 | −0.36 | 5.63 | 5.52 | −0.10 | −0.26 | −0.26 |
| (5.50 to 5.76) | (5.12 to 5.42) | (−0.51 to −0.21) | (5.52 to 5.74) | (5.37 to 5.67) | (−0.25 to 0.04) | (−0.45 to −0.07) | (−0.44 to −0.08) | |
| BMI, kg/m2 | 23.77 | 23.20 | −0.57 | 24.24 | 24.52 | 0.29 | −0.86 | −0.86 |
| (23.37 to 24.18) | (22.73 to 23.68) | (−1.00 to −0.14) | (23.92 to 24.56) | (24.10 to 24.94) | (−0.08 to 0.66) | (−1.34 to −0.38) | (−1.32 to −0.39) | |
| Other Outcomes | ||||||||
| DBP, mmHg | 78.54 | 71.94 | −6.61 | 78.06 | 83.68 | 5.62 | −12.23 | −12.23 |
| (77.24 to 79.84) | (70.34 to 73.53) | (−8.14 to −5.07) | (76.86 to 79.26) | (82.41 to 84.95) | (4.39 to 6.84) | (−14.12 to −10.33) | (−14.03 to −10.43) | |
| FPG, mmol/L | 5.57 | 5.28 | −0.31 | 5.54 | 5.55 | 0.02 | −0.32 | −0.32 |
| (5.38 to 5.76) | (5.10 to 5.45) | (−0.49 to −0.12) | (5.41 to 5.66) | (5.42 to 5.69) | (−0.13 to 0.16) | (−0.52 to −0.12) | (−0.51 to −0.13) | |
| TG, mmol/L | 1.83 | 1.74 | −0.10 | 1.77 | 1.64 | −0.13 | 0.04 | 0.04 |
| (1.66 to 2.01) | (1.52 to 1.95) | (−0.31 to 0.12) | (1.60 to 1.89) | (1.51 to 1.78) | (−0.28 to 0.01) | (−0.20 to 0.27) | (−0.19 to 0.26) | |
| HDL, mmol/L | 1.68 | 1.52 | −0.16 | 1.72 | 1.53 | −0.19 | 0.03 | 0.03 |
| (1.64 to 1.72) | (1.45 to 1.59) | (−0.23 to −0.09) | (1.68 to 1.76) | (1.49 to 1.58) | (−0.23 to −0.14) | (−0.05 to 0.11) | (−0.04 to 0.11) | |
| LDL, mmol/L | 3.61 | 3.20 | −0.41 | 3.61 | 3.17 | −0.43 | 0.02 | 0.02 |
| (3.50 to 3.71) | (3.08 to 3.32) | (−0.54 to −0.28) | (3.51 to 3.70) | (3.06 to 3.29) | (−0.55 to −0.32) | (−0.13 to 0.18) | (−0.12 to 0.17) | |
| WHR | 0.89 | 0.87 | −0.02 | 0.89 | 0.89 | 0.01 | −0.02 | −0.02 |
| (0.88 to 0.90) | (0.86 to 0.88) | (−0.03 to −0.01) | (0.88 to 0.89) | (0.89 to 0.90) | (0.00 to 0.02) | (−0.04 to −0.01) | (−0.03 to −0.01) | |
3.3. Secondary Study Outcomes
3.4. Subgroup Analysis and Sensitivity Analysis
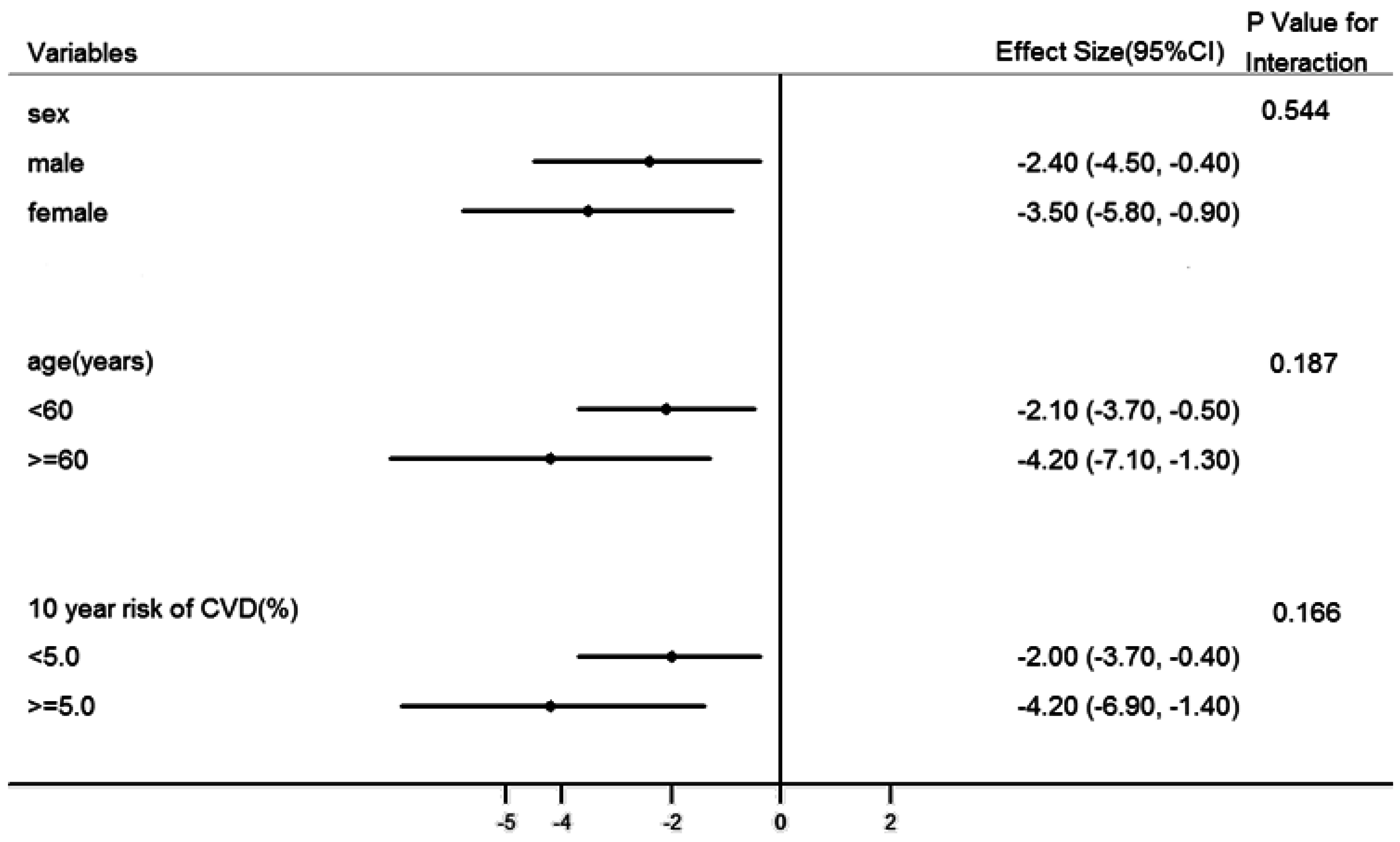
4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the global burden of disease study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Xi, B.; Liu, F.; Hao, Y.; Dong, H.; Mi, J. The growing burden of cardiovascular diseases in China. Int. J. Cardiol. 2014, 174, 736–737. [Google Scholar] [CrossRef] [PubMed]
- Gordon, N.F.; Salmon, R.D.; Franklin, B.A.; Sperling, L.S.; Hall, L.; Leighton, R.F.; Haskell, W.L. Effectiveness of therapeutic lifestyle changes in patients with hypertension, hyperlipidemia, and/or hyperglycemia. Am. J. Cardiol. 2004, 94, 1558–1561. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.; Coupal, L.; Kouache, M.; Lowensteyn, I.; Marchand, S.; Campbell, N. Estimating the benefits of patient and physician adherence to cardiovascular prevention guidelines: The MyHealthCheckup Survey. Can. J. Cardiol. 2011, 27, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, J.A.; Babyak, M.A.; Hinderliter, A.; Watkins, L.L.; Craighead, L.; Lin, P.; Caccia, C.; Johnson, J.; Waugh, R.; Sherwood, A. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: The ENCORE study. Arch. Intern. Med. 2010, 170, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Elmer, P.J.; Obarzanek, E.; Vollmer, W.M.; Simons-Morton, D.; Stevens, V.J.; Young, D.R.; Lin, P.; Champagne, C.; Harsha, D.W.; Svetkey, L.P. Effects of comprehensive lifestyle modification on diet, weight, physical fitness, and blood pressure control: 18-month results of a randomized trial. Ann. Intern. Med. 2006, 144, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Åkesson, A.; Weismayer, C.; Newby, P.K.; Wolk, A. Combined effect of low-risk dietary and lifestyle behaviors in primary prevention of myocardial infarction in women. Arch. Intern. Med. 2007, 167, 2122–2127. [Google Scholar] [CrossRef] [PubMed]
- Fineberg, H.V. The paradox of disease prevention: Celebrated in principle, resisted in practice. JAMA 2013, 310, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Wister, A.; Loewen, N.; Kennedy-Symonds, H.; McGowan, B.; McCoy, B.; Singer, J. One-year follow-up of a therapeutic lifestyle intervention targeting cardiovascular disease risk. Can. Med. Assoc. J. 2007, 177, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Beratarrechea, A.; Lee, A.G.; Willner, J.M.; Jahangir, E.; Ciapponi, A.; Rubinstein, A. The impact of mobile health interventions on chronic disease outcomes in developing countries: A systematic review. Telemed. E-Health 2014, 20, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Cole-Lewis, H.; Kershaw, T. Text messaging as a tool for behavior change in disease prevention and management. Epidemiol. Rev. 2010, 32, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Kamis, K.; Janevic, M.R.; Marinec, N.; Jantz, R.; Valverde, H.; Piette, J.D. A study of mobile phone use among patients with noncommunicable diseases in La Paz, Bolivia: Implications for mHealth research and development. Global. Health 2015, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Piette, J.D.; Mendoza-Avelares, M.O.; Milton, E.C.; Lange, I.; Fajardo, R. Access to mobile communication technology and willingness to participate in automated telemedicine calls among chronically III patients in Honduras. Telemed. E-Health 2010, 16, 1030–1041. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, L.; Moller, R.; Puig, J.G. Comprehensive cardiovascular risk management—What does it mean in practice? Vascular Health Risk Manag. 2007, 3, 587–603. [Google Scholar]
- Grover, S.A.; Lowensteyn, I.; Joseph, L.; Kaouache, M.; Marchand, S.; Coupal, L.; Boudreau, G. Discussing coronary risk with patients to improve blood pressure treatment: Secondary results from the CHECK-UP study. J. Gen. Intern. Med. 2009, 24, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Persell, S.D.; Lloyd-Jones, D.M.; Friesema, E.M.; Cooper, A.J.; Baker, D.W. Electronic health record-based patient identification and individualized mailed outreach for primary cardiovascular disease prevention: A cluster randomized trial. J. Gen. Intern. Med. 2013, 28, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Benner, J.S.; Erhardt, L.; Flammer, M.; Moller, R.A.; Rajicic, N.; Changela, K.; Yunis, C.; Cherry, S.B.; Gaciong, Z.; Johnson, E.S. A novel programme to evaluate and communicate 10-year risk of CHD reduces predicted risk and improves patients’ modifiable risk factor profile. Int. J. Clin. Pract. 2008, 62, 1484–1498. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, S.L.; Viera, A.J.; Krantz, M.J.; Ice, C.L.; Steinman, L.E.; Peters, K.E.; Kopin, L.A.; Lungelow, D. The effect of giving global coronary risk information to adults: A systematic review. Arch. Intern. Med. 2010, 170, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Cooney, M.T.; Dudina, A.; D’Agostino, R.; Graham, I.M. Cardiovascular risk-estimation systems in primary prevention do they differ? Do they make a difference? Can we see the future? Circulation 2010, 122, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, X.; Li, X.; Li, Y.; Zhao, L.; Chen, Z.; Li, Y.; Rao, X.; Zhou, B.; Detrano, R.; et al. Estimation of 10-year risk of fatal and nonfatal ischemic cardiovascular diseases in Chinese adults. Circulation 2006, 114, 2217–2225. [Google Scholar] [CrossRef] [PubMed]
- Chinese Society of Cardiology of Chines Medical Association. Chinese guidelines for prevention of cardiovascular disease. Chin. J. Cardiol. 2011, 39, 3–22. [Google Scholar]
- Maruthur, N.M.; Wang, N.Y.; Appel, L.J. Lifestyle interventions reduce coronary heart disease risk: Results from the PREMIER trial. Circulation 2009, 119, 2026–2031. [Google Scholar] [CrossRef] [PubMed]
- Peiris, D.; Praveen, D.; Johnson, C.; Mogulluru, K. Use of mHealth systems and tools for non-communicable diseases in low- and middle-income countries: A systematic review. J. Cardiovasc. Transl. 2014, 7, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, M.; Ebrahimzadeh, I.; Rabi, A.; Saedipoor, B.; Jafarabadi, M.A. Impact of distance education via mobile phone text messaging on knowledge, attitude, practice and self efficacy of patients with type 2 diabetes mellitus in Iran. J. Diabetes Metab. Disord. 2012, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kingue, S.; Angandji, P.; Menanga, A.P.; Ashuntantang, G.; Sobngwi, E.; Dossou-Yovo, R.A.; Kaze, F.F.; Kengne, A.P.; Dzudie, A.; Ndobo, P.; et al. Efficiency of an intervention package for arterial hypertension comprising telemanagement in a Cameroonian rural setting: The TELEMED-CAM study. Pan. Afr. Med. J. 2013, 15, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.; Tong, S.; Lee, V.K.M.; Ng, C.J.; Leong, K.C.; Teng, C.L. Text messaging reminders to reduce non-attendance in chronic disease follow-up: A clinical trial. Brit. J. Gen. Pract. 2009, 59, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.R.; Bauer, S.; Hamer, R.M.; Kordy, H.; Ward, D.; Bulik, C.M. Use of text messaging for monitoring sugar-sweetened beverages, physical activity, and screen time in children: A pilot study. J. Nutr. Educ. Behav. 2008, 40, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Haug, S.; Meyer, C.; Schorr, G.; Bauer, S.; John, U. Continuous individual support of smoking cessation using text messaging: A pilot experimental study. Nicotine Tob. Res. 2009, 11, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, A.; Corbett, T.; Bramley, D.; Riddell, T.; Wills, M.; Lin, R.; Jones, M. Do u smoke after txt? Results of a randomised trial of smoking cessation using mobile phone text messaging. Tob. Control 2005, 14, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, E.L.; Fallows, S.; Morris, M. A text message based weight management intervention for overweight adults. J. Hum. Nutr. Diet. 2014, 272, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Haapala, I.; Barengo, N.C.; Biggs, S.; Surakka, L.; Manninen, P. Weight loss by mobile phone: A 1-year effectiveness study. Public Health Nutr. 2009, 12, 2382–2391. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, S.L.; Behrend, L.; Vu, M.B.; Meier, A.; Griffith, J.M.; Pignone, M.P. Individuals’ responses to global CHD risk: A focus group study. Patient Educ. Couns. 2009, 76, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Alm-Roijer, C.; Fridlund, B.; Stagmo, M.; Erhardt, L. Knowing your risk factors for coronary heart disease improves adherence to advice on lifestyle changes and medication. J. Cardiovascul. Nurs. 2006, 21, 24–31. [Google Scholar] [CrossRef]
- Keyserling, T.C.; Sheridan, S.L.; Draeger, L.B.; Finkelstein, E.A.; Gizlice, Z.; Kruger, E.; Johnston, L.F.; Sloane, P.D.; Samuel-Hodge, C.; Evenson, K.R.; et al. A comparison of live counseling with a web-based lifestyle and medication intervention to reduce coronary heart disease risk. JAMA Intern. Med. 2014, 174, 1144–1157. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Chen, S.; Zhang, G.; Lin, A. Mobile Phone-Based Lifestyle Intervention for Reducing Overall Cardiovascular Disease Risk in Guangzhou, China: A Pilot Study. Int. J. Environ. Res. Public Health 2015, 12, 15993-16004. https://doi.org/10.3390/ijerph121215037
Liu Z, Chen S, Zhang G, Lin A. Mobile Phone-Based Lifestyle Intervention for Reducing Overall Cardiovascular Disease Risk in Guangzhou, China: A Pilot Study. International Journal of Environmental Research and Public Health. 2015; 12(12):15993-16004. https://doi.org/10.3390/ijerph121215037
Chicago/Turabian StyleLiu, Zhiting, Songting Chen, Guanrong Zhang, and Aihua Lin. 2015. "Mobile Phone-Based Lifestyle Intervention for Reducing Overall Cardiovascular Disease Risk in Guangzhou, China: A Pilot Study" International Journal of Environmental Research and Public Health 12, no. 12: 15993-16004. https://doi.org/10.3390/ijerph121215037






