Awareness of Cytomegalovirus Infection among Pregnant Women in Geneva, Switzerland: A Cross-sectional Study
Abstract
:1. Introduction
2. Experimental Section
2.1. Participants and Study Design
2.2. Questionnaire
2.3. Statistical Analysis
3. Results
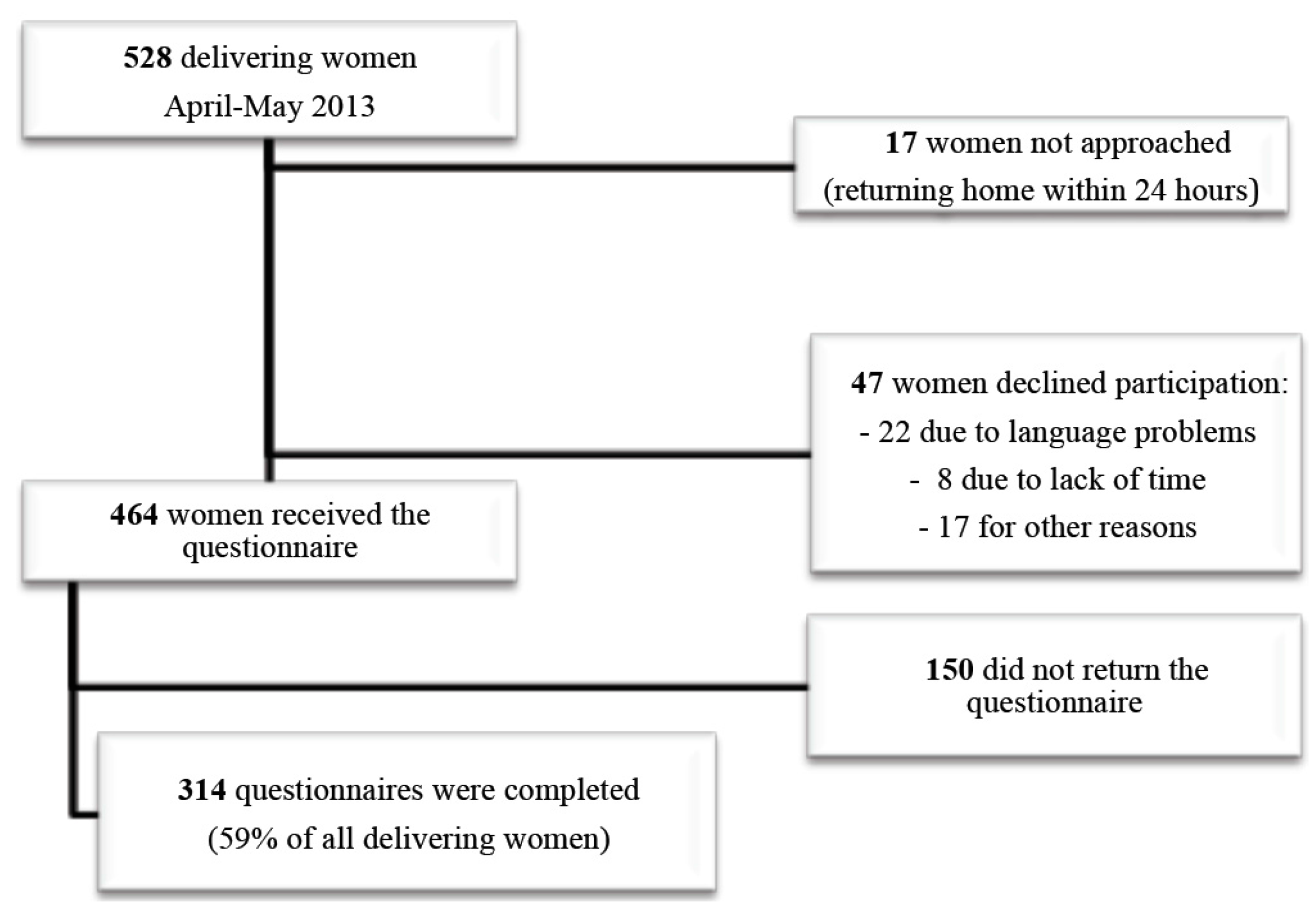
3.1. Participants
| Knowledge of CMV | Odds Ratio (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|
| All (n = 314) | No (n = 191) | Yes (n = 123) | Univariate | p-value | Adjusted * | p-value | ||
| Nationality | Swiss | 125/309 (40.5%) | 61/187 (32.6%) | 64/121 (52.5%) | 1 (reference) | 0.002 | 1 (reference) | 0.004 |
| European | 102/309 (33.3%) | 69/187 (36.9%) | 33/121 (27.0%) | 0.5 (0.3;0.8) | 0.4 (0.2;0.7) | 0.003 | ||
| Other | 82/309 (26.5%) | 57/187 (30.5%) | 25/121 (20.5%) | 0.4 (0.2;0.8) | 0.4 (0.2;0.8) | 0.008 | ||
| Language | French, German | 269/314 (85.7%) | 160/191 (83.8%) | 109/123 (88.6%) | 1 (reference) | 0.12 | ||
| English | 30/314 (9.6%) | 18/191 (9.4%) | 12/123 (9.8%) | 1.0 (0.5;2.1) | ||||
| Spanish, Portuguese | 15/314 (4.8%) | 13/191 (6.8%) | 2/123 (1.6%) | 0.2 (0.0;1.0) | ||||
| Age | ≤25 years | 34/311 (10.9%) | 25/188 (13.3%) | 9/123 (7.3%) | 1 (reference) | 0.25 | 1 (reference) | 0.38 |
| 26 to 35 years | 192/311 (61.7%) | 112/188 (59.6%) | 80/123 (65.0%) | 2.0 (0.9;4.5) | 1.4 (0.5;3.8) | 0.49 | ||
| ≥36 years | 85/311 (27.3%) | 51/188 (27.1%) | 34/123 (27.6%) | 1.9 (0.8;4.5) | 1.0 (0.3;2.8) | 0.93 | ||
| Education level | Minimal schooling | 43/306 (14.1%) | 37/184 (20.1%) | 6/122 (4.9%) | 1 (reference) | <0.001 | 1 (reference) | <0.001 |
| Apprenticeship | 53/306 (17.3%) | 38/184 (20.7%) | 15/122 (12.3%) | 2.4 (0.9;7.0) | 1.2 (0.4;4.0) | 0.74 | ||
| High school | 74/306 (24.2%) | 44/184 (23.9%) | 30/122 (24.6%) | 4.2 (1.6;11.2) | 2.9 (1.0;8.5) | 0.06 | ||
| University | 136/306 (44.4%) | 65/184 (35.3%) | 71/122 (58.2%) | 6.7 (2.7;17.0) | 6.0 (2.2;16.4) | <0.001 | ||
| Employment | Not exposed to risk | 263/304 (86.5%) | 172/184 (93.5%) | 91/120 (75.8%) | 1 (reference) | <0.001 | 1 (reference) | <0.001 |
| Healthcare-related | 25/304 (8.2%) | 6/184 (3.3%) | 19/120 (15.8%) | 6.0 (2.3;15.5) | 6.9 (2.4;19.4) | <0.001 | ||
| With children | 16/304 (5.3%) | 6/184 (3.3%) | 10/120 (8.3%) | 3.2 (1.1;8.9) | 5.7 (1.6;20.0) | 0.006 | ||
| Parity | Primiparity | 129/308 (41.9%) | 83/185 (44.9%) | 46/123 (37.4%) | 1 (reference) | 0.24 | 1 (reference) | |
| Multiparity | 179/308 (58.1%) | 102/185 (55.1%) | 77/123 (62.6%) | 1.4 (0.9;2.2) | 1.5 (0.9;2.6) | 0.13 | ||
| Follow-up during 1st | No | 8/312 (2.6%) | 5/189 (2.6%) | 3/123 (2.4%) | 1 (reference) | 1 | ||
| trimester of pregnancy | Yes | 304/312 (97.4%) | 184/189 (97.4%) | 120/123 (97.6%) | 1.0 (0.3;3.6) | |||
| Pregnancy follow-up by | General practitioner | 31/294 (10.5%) | 22/177 (12.4%) | 9/117 (7.7%) | 1 (reference) | 0.01 | ||
| Midwife | 32/294 (10.9%) | 26/177 (14.7%) | 6/117 (5.1%) | 0.6 (0.2;1.8) | ||||
| Obstetrician | 231/294 (78.6%) | 129/177 (72.9%) | 102/117 (87.2%) | 1.9 (0.9;4.4) | ||||
3.2. Knowledge of Congenital Diseases
3.3. General Knowledge of CMV and Associated Factors
3.4. Quality of Knowledge about CMV Infection and Preventive Measures
| (A) | Women Followed-Up in the First Trimester by | ||||
| GP * (n = 31) | Midwife (n = 32) | Obstetrician (n = 231) | Total (n = 314) | p-Value | |
| Patients aware of CMV | 9 (29.0%) | 6 (18.8%) | 102 (44.2%) | 123 (39.2%) | 0.009 |
| Source of information ** | |||||
| General practitioner | 3 (33.3%) | 2 (33.3%) | 5 (5.0%) | 11 (9.0%) | 0.003 |
| Obstetrician | 3 (33.3%) | 2 (33.3%) | 72 (71.3%) | 78 (63.9%) | 0.01 |
| Midwife | 2 (22.2%) | 3 (50.0%) | 8 (7.9%) | 15 (12.3%) | 0.006 |
| Pediatrician | 1 (11.1%) | 1 (16.7%) | 4 (4.0%) | 6 (4.9%) | 0.17 |
| Media | 0 (0.0%) | 1 (16.7%) | 25 (24.8%) | 28 (23.0%) | 0.27 |
| Family/friends | 1 (11.1%) | 1 (16.7%) | 15 (14.9%) | 19 (15.6%) | 1 |
| Missing data | 0 | 0 | 1 | 1 | |
| (B) | Women aware of CMV and Followed-Up in the First Trimester by | ||||
| GP * (n = 9) | Midwife (n = 6) | Obstetrician (n = 102) | Total (n = 123) | p-Value | |
| Information about CMV preventive measures | 4 (44.2%) | 2 (33.3%) | 54 (52.9%) | 62 (50.4%) | 0.59 |
| Source of information ** | |||||
| General practitioner | 4 (100.0%) | 1 (50.0%) | 1 (1.9%) | 6 (9.8%) | <0.001 |
| Obstetrician | 0 (0.0%) | 0 (0.0%) | 51 (96.2%) | 52 (85.2%) | <0.001 |
| Midwife | 0 (0.0%) | 1 (50.0%) | 3 (5.7%) | 5 (8.2%) | 0.15 |
| Pediatrician | 0 (0.0%) | 0 (0.0%) | 2 (3.8%) | 2 (3.3%) | 1 |
| Studies | 0 (0.0%) | 0 (0.0%) | 5 (9.4%) | 5 (8.2%) | 1 |
| Media | 0 (0.0%) | 0 (0.0%) | 2 (3.8%) | 2 (3.3%) | 1 |
| Family/friends | 0 (0.0%) | 0 (0.0%) | 7 (13.2%) | 7 (11.5%) | 1 |
| Missing data | 0 | 0 | 1 | 1 | |
| Which Symptoms are Related to CMV? | Answers * (n = 123) | ||
|---|---|---|---|
| No | Yes | Do Not Know | |
| Is CMV contagious? | 2 (1.6%) | 92 (74.8%) | 29 (23.6%) |
| Is CMV not dangerous? | 66 (56.9%) | 10 (8.6%) | 40 (34.5%) |
| Does CMV cause deafness? | 9 (7.6%) | 30 (25.2%) | 80 (67.2%) |
| Does CMV cause mental retardation? | 5 (4.2%) | 41 (34.5%) | 73 (61.3%) |
| Does CMV cause jaundice? | 36 (30.5%) | 9 (7.6%) | 73 (61.9%) |
| Does CMV cause convulsion? | 15 (12.8%) | 15 (12.8%) | 87 (74.4%) |
| Does CMV cause microcephaly? | 18 (15.1%) | 16 (13.4%) | 85 (71.4%) |
| Does CMV cause cardiac malformation? | 12 (10.3%) | 22 (18.8%) | 83 (70.9%) |
| Does CMV cause death? | 10 (8.5%) | 30 (25.4%) | 78 (66.1%) |
| Number of Correct Answers | |||
| 0 to 3 correct answers | 70 (63.65%) | ||
| 4 to 6 correct answers | 37 (33.65%) | ||
| 7 or more correct answers | 3 (2.7%) | ||
| Which Hygiene Measure Prevents CMV? | Answers * (n = 123) | ||
|---|---|---|---|
| No | Yes | Do Not Know | |
| Handwashing | 6 (5.0%) | 88 (72.7%) | 27 (22.3%) |
| Not sharing the same tool | 12 (9.9%) | 81 (66.95%) | 28 (23.15%) |
| Using mosquito repellent | 68 (56.2%) | 2 (1.7%) | 51 (42.1%) |
| Avoiding eating raw meat/dairy products | 80 (66.1%) | 17 (14.05%) | 24 (19.8%) |
| Drinking caffeinated drinks | 97 (80.2%) | 1 (0.8%) | 23 (19.0%) |
| Avoiding cleaning the cat litter box | 69 (57.5%) | 30 (25.0%) | 21 (17.5%) |
| Avoiding contact with urine | 12 (10.1%) | 80 (67.2%) | 27 (22.7%) |
| Exercising | 88 (72.7%) | 7 (5.8%) | 26 (21.5%) |
| Avoiding kissing on the mouth | 11.9 (9.2%) | 84 (70.6%) | 24 (20.2%) |
| Number of Correct Answers | |||
| 0 to 1 correct answer | 16 (13.55%) | ||
| 2 to 4 correct answers | 14 (11.85%) | ||
| 5 or more correct answers | 88 (74.6%) | ||
4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Walker, S.; Palmo-Dias, R.; Wood, E.M.; Shekleton, P.; Giles, M.L. Cytomegalovirus in pregnancy: To screen or not to screen. BMC Pregnancy Childbirth 2013, 13, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Dollard, S.; Grosse, S.; Ross, D. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev. Med. Virol. 2007, 17, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Boppana, S.; Fowler, K.; Britt, W.; Stagno, S.; Pass, R.F. Symptomatic congenital cyto-megalovirus infection in infants born to mothers with preexisting immunity to cytomegalovirus. Pediatrics 1999, 104, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Basha, J.; Iwasenko, J.M.; Robertson, P.; Craig, M.E.; Rawlinson, W. Congenital cytomegalovirus infection is associated with high maternal socio-economic status and corresponding low maternal cytomegalovirus seropositiviy. J. Paediatr. Child Health 2014, 50, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Kenneson, A.; Cannon, M.J. Review and meta-analysis of the epidemiology of congenital cytomegalovirus infection. Rev. Med. Virol. 2007, 17, 253–276. [Google Scholar] [CrossRef] [PubMed]
- Bate, S.; Dollard, S.; Cannon, M.J. Cytomegalovirus seroprevalence in the United States: The national health and nutrition examination surveys, 1988–2004. Clin. Infect. Dis. 2010, 50, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Mussi-Pinhata, M.M.; Boppana, S.B.; Novak, Z.; Wagatsuma, V.M.; de Oliveira, P.F.; Duarte, G.; Britt, W.J. Human cytomegalovirus reinfection is associated with intrauterine transmission in a highly cytomegalovirus-immune maternal population. Am. J. Obstet. Gynecol. 2010, 202, 297.e1–297.e8. [Google Scholar] [CrossRef] [PubMed]
- Ornoy, A.; Diav-Citrin, O. Fetal effects of primary and secondary cytomegalovirus infection in pregnancy. Reprod. Toxicol. 2006, 21, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Zalel, Y.; Gilboa, Y.; Berkenshtat, M.; Yoeli, R.; Auslander, R.; Achiron, R.; Goldberg, Y. Secondary cytomegalovirus infection can cause severe fetal sequelae despite maternal preconceptional immunity. Ultrasound Obstet. Gynecol. 2008, 31, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.; Wood, G. Perinatal outcome after maternal primary cytomegalovirus infection in the first trimestre: A practical update and counseling aid. Prenat. Diagn. 2015, 35, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nigro, G.; La Torre, R.; Anceschi, M.M.; Mazzocco, M.; Cosmi, E. Hyperimmunoglobulin therapy for a twin fetus with cytomegalovirus infection and growth restriction. Am. J. Obstet. Gynecol. 1999, 180, 1222–1226. [Google Scholar] [CrossRef]
- Adler, S.P.; Nigro, G. Findings and conclusions from CMV hyperimmune globulin treatment trials. J. Clin. Virol. 2009, 46, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Nigro, G.; Adler, S.P.; la Torre, R. Passive immunization during pregnancy for congenital cytomegalovirus infection. N. Engl. J. Med. 2005, 353, 1350–1362. [Google Scholar] [CrossRef] [PubMed]
- Buxmann, H.; Stackelberg, O.M.; Schlößer, R.L.; Enders, G.; Gonser, M.; Meyer-Wittkopf, M.; Hamprecht, K.; Enders, M. Use of cytomegalovirus hyperimmunoglobulin for prevention of congenital cytomegalovirus disease: A retrospective analysis. J. Perinatal. Med. 2012, 40, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Jückstock, J.; Rothenburger, M.; Friese, K.; Traunmüller, F. Passive immunization against congenital cytomegalovirus infection: Current state of knowledge. Pharmacology 2015, 95, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Visentin, S.; Manara, R.; Milanese, L.; da Roit, A.; Forner, G.; Salviato, E.; Citton, V.; Magno, F.M.; Orzan, E.; Morando, C.; et al. Early primary cytomegalovirus infection in pregnancy: Maternal hyperimmunoglobulin therapy improves outcomes among infants at 1 year of age. Clin. Infect. Dis. 2012, 55, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Nigro, G.; Adler, S.P.; Mascaretti, G.; Megaloikonomou, A.; la Torre, R.; Necozione, S.; Gatta, E. Fetal hyperechogenic bowel may indicate congenital cytomegalovirus disease responsive to immunoglobulin therapy. J. Matern. Fetal Neonatal Med. 2012, 25, 2202–2205. [Google Scholar] [CrossRef] [PubMed]
- Moxley, K.; Knudtson, E.J. Resolution of hydrops secondary to cytomegalovirus after maternal and fetal treatment with human cytomegalovirus hyperimmune globulin. Obstet. Gynecol. 2008, 111, 524–526. [Google Scholar] [CrossRef] [PubMed]
- Moise, K.J.; Wolfe, H. Treatment of second trimester fetal cytomegalovirus infection with maternal hyperimmune globulin. Prenat. Diagn. 2008, 28, 264–265. [Google Scholar] [CrossRef] [PubMed]
- Nigro, G.; Torre, R.; de Tejada, B.; Adler, S.P.; Lituania, M.; Pentimalli, H. Regression of fetal cerebral abnormalities by primary cytomegalovirus infection following hyperimmunoglobulin therapy. Prenat. Diagn. 2008, 28, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Revello, M.G.; Gerna, G. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N. Engl. J. Med. 2014, 70, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; Garofoli, F.; Villani, P.; Tizzoni, M.; Angelini, A.; Cusato, M.; Bollani, L.; de Silvestri, A.; Regazzi, M.; Stronati, M. Oral valganciclovir treatment in newborns with symptomatic congenital cytomegalovirus infection. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 1465–1470. [Google Scholar] [CrossRef] [PubMed]
- Del Rosal, T.; Baquero-Artigao, F.; Blazquez, D.; Noguera-Julian, A.; Moreno-Pérez, D.; Reyes, A.; Vilas, J. Treatment of symptomatic congenital cytomegalovirus infection beyond the neonatal period. J. Clin. Virol. 2012, 55, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Whitley, R.J.; Cloud, G.; Gruber, W.G.; Storch, G.; Demmler, R.; Jacobs, W.; Dankner, S.; Spector, S.; Starr, R.; Pass, S.; et al. Ganciclovir treatment of symptomatic congenital cytomegalovirus infection: Results of a phase II study. J. Infect. Dis. 1997, 175, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Michaels, M.G.; Greenberg, D.P.; Sabo, D.L.; Wald, E.R. Treatment of children with congenital cytomegalovirus infection with ganciclovir. Pediatr. Infect. Dis. J. 2003, 22, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Kimberlin, D.W.; Lin, C.Y.; Sanchez, P.J. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: A randomized, controlled trial. J. Pediatr. 2003, 143, 16–25. [Google Scholar] [CrossRef]
- Tanaka-Kitajima, N.; Futatani, T.; Sugaya, N. Ganciclovir therapy for congenital cytomegalovirus infection in six infants. Pediatr. Infect. Dis. J. 2005, 24, 782–785. [Google Scholar] [CrossRef] [PubMed]
- Lackner, A.; Acham, A; Alborno, T. Effect on hearing of ganciclovir therapy for asymptomatic congenital cytomegalovirus infection: Four to 10 year follow up. J. Laryngol. Otol. 2009, 123, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Amir, J.; Wolf, D.; Levy, I. Treatment of symptomatic congenital cytomegalovirus infection with intravenous ganciclovir followed by longterm oral valganciclovir. Eur. J. Pediatr. 2010, 169, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Miyajima, T.; Shimura, M.; Morichi, S.; Morishima, Y.; Ioi, H.; Oana, S.; Yamanaka, G.; Kawashima, H.; Hoshika, A. Successful treatment of congenital cytomegalovirus infection with valganciclovir. No to Hattatsu 2012, 44, 55–59. [Google Scholar] [PubMed]
- Dasari, V.; Smith, C.; Khanna, R. Recent advances in designing an effective vaccine to prevent cytomegalovirus associated clinical disease. Expert Rev. Vaccines 2013, 12, 661–676. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; An, Z.; Wang, D. Progress on pursuit of human cytomegalovirus vaccines for prevention of congenital infection and disease. Vaccine 2014, 32, 2525–2533. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, P.; Plotkin, S.; Mocarski, E.; Pass, R.; Schleiss, M.; Krause, P. Bialek, S. Desirability and feasibility of a vaccine against cytomegalovirus. Vaccine 2013, 31, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.; Bialek, S.R.; Boppana, S.B.; Griffiths, P.D.; Laughlin, C.A.; Ljungman, P.; Mocarski, E.S.; Pass, R.F.; Read, J.S.; Schleiss, M.R.; et al. Priorities for CMV vaccine development. Vaccine 2013, 32, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Rieder, F.; Steiniger, C. Cytomegalovirus vaccine: Phase II clinical trial results. Clin. Microbiol. Infect. 2014, 5, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S. The history of vaccination against cytomegalovirus. Med. Microbiol. Immunol. 2015, 204, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Cahill, A.G.; Odibo, A.O.; Stamilio, D.M.; Macones, G.A. Screening and treating for primary cytomegalovirus infection in pregnancy: Where do we stand? A decision-analytic and economic analysis. Am. J. Obstet. Gynecol. 2007, 201, 466.e1–466.e7. [Google Scholar] [CrossRef] [PubMed]
- Carslson, A.; Norwitz, E.; Stiller, R.J. Cytomegalovirus infection in pregnancy: Should all women be screened? Rev. Obstet. Gynecol. 2010, 3, 172–179. [Google Scholar]
- Adler, S.; Finney, J.W.; Manganello, A.M.; Best, A.M. Prevention of child-to-mother transmission of cytomegalovirus by changing behaviors: A randomized controlled trial. Pediatr. Infect. Dis. 1996, 15, 240–246. [Google Scholar] [CrossRef]
- Picone, O.; Vauloup-Fellous, C.; Cordier, A.G.; Parent-du-Châtelet, I.; Senat, M.-V.; Frydman, R.; Grangeot-Keros, L. A 2-year study on cytomegalovirus infection during pregnancy in a French hospital. BJOG 2009, 116, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Vauloup-Felloup, C.; Picone, O.; Cordier, A.G.; Parent-du-Châtelet, I.; Senat, M.-V.; Frydman, R.; Grangeot-Keros, L. Does hygiene counseling have an impact on the rate of CMV primary infection during pregnancy? Results of a 3-year prospective study in a French hospital. J. Clin. Virol. 2009, 46 (Suppl. 4), S49–S53. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.; Dennis, C. Hygiene interventions for prevention of cytomegalovirus infection among childbearing women: Systematic review. J. Adv. Nurs. 2008, 63, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Cordier, A.; Vauloup-Fellous, C.; Picone, O. Is maternal infection with cytomegalovirus prevention possible? Gynecol. Obstet. Fertil. J. 2010, 38, 620–623. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Prevention and Control. Clinical Diagnosis and Treatment, Cytomegalovirus; CDC: Atlanta, GA, USA, 2010. Available online: http://www.cdc.gov/cmv/clinical/diagnosis-treatment.html (accessed on 10 June 2015).
- Hyde, T.B.; Schmid, D.S.; Cannon, M.J. CMV seroconversion rates and risks factors: Implication for congenital CMV. Rev. Med. Virol. 2010, 20, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Cannon, M.J. Congenital cytomegalovirus epidemiology awareness. J. Clin. Virol. 2009, 46, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Pereboom, M.; Manniën, J.; Spelten, E.R.; Schellevis, F.G.; Hutton, E.K. Observational study to assess pregnant women’s knowledge and behaviour to prevent toxoplasmosis, listeriosis and cytomegalovirus. BMC Pregnancy Childbirth 2013. [Google Scholar] [CrossRef] [PubMed]
- Cannon, M.J.; Davis, K. Washing our hands of the congenital cytomegalovirus disease epidemic. BMC Public Health 2005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, D.; Victor, M.; Sumartojo, E.; Cannon, M.J. Women’s knowledge of congenital cytomegalovirus. J. Womens Health 2008, 17, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.; Schulkin, J.; Ross, D. Knowledge and practices of obstetricians and gynecologists regarding cytomegalovirus infection during pregnancy. CDC Morb. Mortal. Wkly. Rep. 2008, 57, 65–68. [Google Scholar]
- Cordier, A.; Guitton, S.; Vauloup-Fellous, C.; Grangeot-Keros, L.; Benachi, A.; Picone, O. Awareness and knowledge of congenital cytomegalovirus infection among health care providers in France. J. Clin. Virol. 2012, 55, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Korver, A.; de Vries, J.J.; de Jong, J.W.; Dekker, F.W.; Vossen, A.C.; Oudesluys-Murphy, A. Awareness of congenital cytomegalovirus among doctors in the Netherlands. J. Clin. Virol. 2009, 46, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Cordier, A.; Guitton, S.; Vauloup-Fellous, C.; Grangeot-Keros, L.; Benachi, A.; Picone, O. Awareness of cytomegalovirus infection among pregnant women in France. J. Clin. Virol. 2012, 53, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Victor, M.; Adler, S.P.; Arwady, A.; Demmler, G.; Fowler, K.; Goldfarb, J.; Keyserling, H.; Massoudi, M.; Richard, K.; Staras, S.A.; Cannon, M.J. Knowledge and awareness of congenital cytomegalovirus among women. Infect. Dis. Obstet. Gynecol. 2006. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Tan, W.C.; Tan, L.K. Awareness of and attitudes toward congenital cytomegalovirus infection among pregnant women in Singapore. Int. J. Obstet. Gynecol. 2011, 117, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Morioka, I.; Sonoyama, A.; Tairaku, S.; Ebina, Y.; Nagamata, S.; Morizane, M.; Tanimura, K.; Iijima, K.; Yamada, H. Awareness of and knowledge about mother-to-child infections in Japanese pregnant women. Congenit. Anomal. 2014, 54, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Swiss Federal Office of Public Health. Naissances en Suisse; Swiss Federal Office of Public Health: Berne, Switzerland, 2013; Available online: http://www.bag.admin.ch/themen/medizin/ 13641/index.html?lang=fr (accessed on 29 April 2015).
- Swiss Federal Office of Public Health. VIH et autres infections sexuellement transmissibles. Swiss Federal Office of Public Health: Berne, Switzerland, 2010; Available online: http://www.hiv.ch/rubriken/epidx/bag/BAG_2f_HIV_Programm_lang.pdf (accessed on 29 April 2015).
- Vaudaux, B.; Kind, C. Prise en charge de la toxoplasmose chez l’enfant. Paediatrica 2010, 21, 66–69. [Google Scholar]
- Jones, J.L.; Krueger, A.; Schulkin, J.; Schantz, P.M. Toxoplasmosis prevention and testing in pregnancy. Survey of obstetricians and gynaecologists. Zoonose Public Health 2010, 57, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Kahn, J.; Jiwani, A.; Gomez, G.B.; Hawkes, S.J.; Chesson, H.W.; Broutet, N.; Kamb, M.L.; Newman, L.M. The cost and cost-effectiveness of scaling up screening and treatment of syphilis in pregnancy: A model. PLoS ONE 2014. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.; Meyer Sauteur, P.M.; Trück, J.; Bosshard, P.P.; Tomaske, M.; Cadenas, M.F.; Lautenschlager, S.; Goetschel, P. Congenital syphilis in Switzerland: Gone, forgotten, on the return. Swiss Med. Wkly. 2012. [Google Scholar] [CrossRef]
- Newman, L.M.; Kamb, M.; Hawkes, S.; Gomez, G.; Say, L.; Seuc, A.; Broutet, N. Global estimates of syphilis in pregnancy and associated adverse outcomes: Analysis of multinational antenatal surveillance data. PLOS Med. 2013. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. STD Surveillance-Syphilis. 2011. Available online: http://www.cdc.gov/std/stats11/syphilis.htm (accessed on 3 June 2015). [Google Scholar]
- Robert-Gangneux, F.; Dardé, M.-L. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin. Microbiol. Rev. 2012, 25, 264–296. [Google Scholar] [CrossRef] [PubMed]
- Bénard, A.; Petersen, E.; Salamon, R.; Chêne, G.; Gilbert, R.; Rachid Salmi, L.; European Toxo Prevention Study Group. Survey of European programmes for the epidemiological surveillance of congenital toxoplasomosis. Euro. Surveill. 2008, 13. Available online: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2740836 (accessed on 3 June 2015). [Google Scholar]
- Vaudaux, B.; Rudin, C. Abandon du dépistage de la toxoplasmose durant la grossesse. Une brève explication. Forum Med. Suisse 2009, 9, 105–106. [Google Scholar]
- Revello, M.G.; Tibaldi, C.; Masuelli, G.; Frisina, V.; Sacchi, A.; Furione, M.; Arossa, A.; Spinillo, A.; Klersy, C.; Ceccarelli, M.; et al. Prevention of Primary Cytomegalovirus Infection in Pregnancy. EBio Med. 2015, 2, 1205–1210. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.; Boubaker, K.; Raeber, P.A.; Vaudaux, B.; Bucher, H.C.; Garweg, J.G.; Hoesli, I.; Kind, C.; Hohlfeld, P. Toxoplasmosis during pregnancy and infancy A new approach for Switzerland. Swiss Med. Wkly. 2008, 138, 1–8. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willame, A.; Blanchard-Rohner, G.; Combescure, C.; Irion, O.; Posfay-Barbe, K.; Martinez de Tejada, B. Awareness of Cytomegalovirus Infection among Pregnant Women in Geneva, Switzerland: A Cross-sectional Study. Int. J. Environ. Res. Public Health 2015, 12, 15285-15297. https://doi.org/10.3390/ijerph121214982
Willame A, Blanchard-Rohner G, Combescure C, Irion O, Posfay-Barbe K, Martinez de Tejada B. Awareness of Cytomegalovirus Infection among Pregnant Women in Geneva, Switzerland: A Cross-sectional Study. International Journal of Environmental Research and Public Health. 2015; 12(12):15285-15297. https://doi.org/10.3390/ijerph121214982
Chicago/Turabian StyleWillame, Alexia, Geraldine Blanchard-Rohner, Christophe Combescure, Olivier Irion, Klara Posfay-Barbe, and Begoña Martinez de Tejada. 2015. "Awareness of Cytomegalovirus Infection among Pregnant Women in Geneva, Switzerland: A Cross-sectional Study" International Journal of Environmental Research and Public Health 12, no. 12: 15285-15297. https://doi.org/10.3390/ijerph121214982





